Abstract
Rationale
The developing heart requires both mechanical load and vascularization to reach its proper size, yet the regulation of human heart growth by these processes is poorly understood.
Objective
We seek to elucidate the responses of immature human myocardium to mechanical load and vascularization using tissue engineering approaches.
Methods and Results
Using human embryonic stem cell and human induced pluripotent stem cell-derived cardiomyocytes in a three dimensional collagen matrix, we show that uniaxial mechanical stress conditioning promotes 2-fold increases in cardiomyocyte and matrix fiber alignment and enhances myofibrillogenesis and sarcomeric banding. Furthermore, cyclic strain markedly increases cardiomyocyte hypertrophy (2.2-fold) and proliferation rates (21%) vs. unstrained constructs. Addition of endothelial cells enhances cardiomyocyte proliferation under all stress conditions (14% to 19%), and addition of stromal supporting cells enhances formation of vessel-like structures by ~10-fold. Furthermore, these optimized human cardiac tissue constructs generate Starling curves, increasing their active force in response to increased resting length. When transplanted onto hearts of athymic rats, the human myocardium survives and forms grafts closely apposed to host myocardium. The grafts contain human microvessels that are perfused by the host coronary circulation.
Conclusions
Our results indicate that both mechanical load and vascular cell co-culture control cardiomyocyte proliferation, and that mechanical load further controls the hypertrophy and architecture of engineered human myocardium. Such constructs may be useful for studying human cardiac development as well as for regenerative therapy.
Keywords: cardiac tissue engineering, mechanical conditioning, vascularization, human cardiomyocyte, stem cell
Introduction
The developing heart is exquisitely sensitive to its mechanical environment, and studies in model organisms such as chick or mouse indicate that mechanical loading is required for cardiac growth and morphogenesis.1-4 Similarly, the heart requires a rich coronary vascular supply for its normal growth, with the coronaries providing both nutrient exchange and paracrine growth signals.5-7 While model organism studies shed light onto general mechanisms of vertebrate development, they do not perfectly model human cardiac growth. For example, the human heart is more than a thousand times larger than that of the mouse and requires the cardiomyocytes to remain in the cell cycle much longer to achieve this mass. Similarly, the human heart beats nearly 10 times slower than the mouse, necessitating different systems for excitation, contraction and relaxation, and these differences likely impart different responses to external mechanical stress. It therefore seems probable that the growth responses of immature human myocardium to mechanical load and vascular ingrowth will differ in some aspects from common laboratory models.
Early growth and maturation processes have been difficult to study in humans, due in part to difficulties in obtaining sufficient human cells. Cardiomyocytes in the postnatal human heart are essentially post-mitotic,8 precluding their expansion in vitro. Fetal human tissue is difficult to obtain, and endogenous adult stem cells have not, to date, shown a robust ability to generate cardiomyocytes. In contrast, pluripotent stem cells such as human embryonic stem cells (hESCs)9 or induced pluripotent stem cells (iPSCs)10 can now be used to generate large-scale cultures of human cardiomyocytes at purities >50%.11-14 These cardiomyocytes resemble fetal human cardiomyocytes in terms of their cardiac-specific transcription factors and contractile proteins, and they exhibit excitation-contraction coupling and synchronous contraction in culture.15-18 This creates opportunities for studying human cardiac growth pathways.
Most in vitro studies of myocardial growth have relied upon cardiomyocyte monocultures and 2-dimensional culture conditions. Monolayer growth on a rigid substrate is clearly not reproducing the heart’s native environment, however, and this has led us and other groups to explore tissue engineering. Tissue engineering can provide a more natural 3-D environment with appropriate stiffness, can improve intercellular organization, and can facilitate intercellular crosstalk which modulates cardiomyocyte differentiation and growth. A variety of 3-D scaffolds have been used with non-human cardiomyocytes, including various synthetic polymers,19-21 as well as natural ones such as alginate,22 fibrin,23 fibronectin,24 and collagen.25, 26 Type I collagen is attractive for cardiac tissue engineering because it is the major endogenous constituent of the heart’s extracellular matrix.27 Furthermore, collagen is self-polymerizing and can be uniformly seeded with cells as a liquid gel, molded into desirable shapes, and subjected to mechanical forces to promote cellular organization. It has been reported that rat neonatal cardiomyocytes cultured in a 3-D collagen matrix and subjected to cyclic stress are able to align in the direction of stress, express organized sarcomeres, and electrically couple by gap junctions.25, 26
In this study, we have generated 3-dimensional human cardiac tissue constructs using collagen type I and human ESC-derived cardiomyocytes to assess cardiomyocyte proliferation, maturation, and architecture under different conditions of stress. Additionally, we have examined co-culture with vascular and stromal cells within the matrix as a means to further recreate cardiomyocyte and vascular organization within the cardiac construct.
Materials and Methods
Cell Culture
Undifferentiated human ESCs of the H7 line (James A. Thomson, U.Wisconson-Madison) 28-31 and IMR90-iPS cells (James A. Thomson, U.Wisconsin-Madison)32 were maintained as previously described and differentiated into cardiomyocytes in monolayer culture with activin-A and BMP4;11 for further cardiomyocyte enrichment, Percoll gradient centrifugation11 or suspension culture33 was used. Preparations averaged 53% human cardiomyocytes (hCMs) based on β-myosin heavy chain (βMHC) immunostaining (Online Table I); this preparation is referred to as “cardiomyocytes” through the remainder of the manuscript. Rat neonatal cardiomyocytes (rNCs) were isolated34 from 1-3 day old Fisher-344 rats; human umbilical vein endothelial cells (HUVEC, passage 4-8), human marrow stromal cells (MSCs, passage 2-4), and mouse embryonic fibroblasts (MEFs, passage 5-7) were maintained using standard culture conditions described within the online expanded methods section.
Cardiac Construct Generation and Mechanical Conditioning
Engineered heart tissue constructs were generated using collagen type I, basement membrane extract, medium, and cardiomyocytes25 (see online supplement for expanded methods). Unless otherwise noted, each 100 μL construct contained 2 million cardiomyocytes; in bi- and tri-culture experiments, 2 million cardiomyocytes were mixed with 1 million HUVEC and 1 million MSCs or MEFs. The gel-cell mixture was cast in a 20 mm by 3 mm trough such that the ends of the construct impregnated into nylon mesh tabs attached to the deformable silicon floor of the well (tissue train, Flexcell International Corp.), providing a means to transmit uniaxial tension to the construct (Online Figure I). To investigate the effects of uniaxial cyclic stress, constructs were subjected to a sinusoidal waveform setting generated with a FlexCell FX-4000T system beginning the day following construct generation (see Online Figure I). Static stress was achieved by allowing cells to contract the collagen gel against the fixed ends of the construct. For unstressed conditions, one end of the construct was cut free of the mesh tab. To measure DNA synthesis rates, 10 μM BrdU was added to the medium for the last 24 hours before fixation.
Cardiac Engraftment
Animal procedures used in this study were reviewed and approved by the University of Washington Institutional Animal Care and Use Committee, and conform to federal guidelines for laboratory animal care. Male Sprague Dawley athymic nude rats were anesthetized, their chest opened and the pericardium incised to expose the anterior surface of the heart. A construct that had been cultured for one week was sutured directly onto the epicardium.
Immunostaining, Light, Fluorescent, and Electron Microscopy, Quantitative PCR
Immunohistochemistry, light microscopy, confocal microscopy, transmission electron microscopy, and quantitative PCR were performed using standard procedures described within the online expanded methods section. Circularly polarized light microscopy was conducted for collagen matrix visualization with picrosirius red, and circular polarization was adjusted to obtain a deep uniform dark background. Images were collected with a Photometrics Coolsnap camera in color mode and METAMORPH software. Illumination intensity, condenser aperture and exposure time were maintained uniformly for all images.
Histological and Statistical Analysis
To quantify cardiomyocyte axis alignment within constructs, we analyzed 100× micrographs of slides stained for desmin (rNC constructs) or βMHC (hCM constructs) using a custom fiber orientation analysis program (developed by Dr. Michael Regnier’s lab, University of Washington). Briefly, this Matlab program divides a user-defined region of interest into small subimages and, by edge detection, obtains the major axis angle of each subimage. For the whole image, an average angle and the angle dispersion (standard deviation of angles of cell edges) is determined. Cellular alignment is quantified by magnitude of angle dispersion, such that low angle dispersion (low standard deviation of cell axis angles) indicates a high degree of alignment, which is graphed as the inverse of angle dispersion, expressed as a percentage of the mean (i.e. the reciprocal of the standard deviation). Adult rat myocardium was used as a positive control; sections were stained for desmin and quantification was performed only on regions with linear, longitudinal fiber orientation.
To assess cardiomyocyte proliferation, slides double-stained for BrdU and βMHC were counted in a blinded fashion. Cardiomyocyte area was assessed by quantifying βMHC-positive area within each construct as described previously,29, 35 normalizing to the cardiomyocyte nuclear number. To quantify vascular-like structure within the constructs, slides were analyzed in a blinded fashion for multicellular CD31+ structures scored as either cord-like structures or structures with lumens and normalized per area counted, over 4 fields per section. Unless otherwise noted, n=3-6 for each experiment. Error bars represent standard error of the mean (SEM); significance was determined using single factor ANOVA followed by Student’s t-test with 95% or greater confidence level.
Results
Rat Neonatal Cardiomyocyte Constructs
To establish a baseline against which to compare our human constructs, we first optimized production of engineered heart tissue using primary rat neonatal cardiomyocytes (rNC). These studies determined that 1.10-1.25 mg/mL of type I collagen is required to prevent construct failure under stress and that a density of at least 2 million cells per 100 μL of construct is necessary for adequate survival of cardiomyocytes within the collagen matrix (data not shown). Constructs generated by this process have dimensions of 20 mm in length and 0.5 mm in thickness. As the cells remodel and contract the collagen gel, nylon tabs hold the construct under static tension or allow the application of controlled cyclic stress (See Online Figure I).
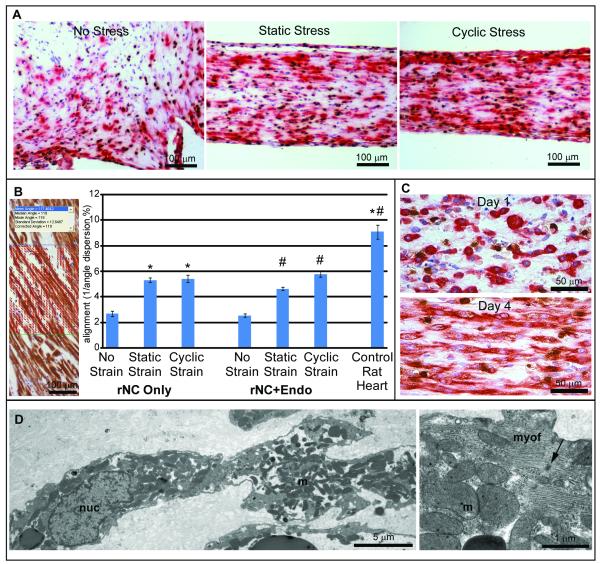
Cardiomyocyte constructs subjected to uniaxial static stress or cyclic stress conditioning (4 days of 1 Hz, 5% elongation) developed cell alignment not observed in 2-D cell culture (Online Figure II, A) or unstressed 3-D gels (Figure 1A). Intercellular alignment was quantified from the reciprocal of the cell axis angle dispersion, where a low standard deviation of angles indicates a high degree of alignment (Figure 1B). We found that, in comparison to no stress (alignment value of 2.68), cardiomyocyte alignment increased by 2-fold with either cyclic or static stress conditioning (to 5.30 and 5.41, respectively; p<0.001 for each versus no stress). However, no significant difference was found between static and cyclic stress (p=0.65). While improved, this degree of cell alignment did not reach the level observed in longitudinal fibers within adult rat heart (alignment value of 9.08). Cardiomyocyte alignment within the collagen matrix developed between 1 day and 4 days of stress conditioning (Figure 1C); further increases in alignment were not observed between 4 and 7 days (data not shown). Addition of endothelial cells resulted in the formation of cord structures within the constructs (Online Figure IV, A), but endothelial cells did not impact cardiomyocyte alignment in either unstressed or stressed cultures (Figure 1B).
Figure 1. Rat cardiac tissue constructs organize under strain.
Desmin (red) and BrdU (brown) immunostain. A) Rat neonatal cardiomyocyte (rNC) constructs conditioned under static or cyclic stress contained elongated, aligned cardiomyocytes, in comparison to constructs under no stress. B) Increased alignment was noted in static and cyclic stress groups, and co-culture of endothelial cells did not inhibit the development of cardiomyocyte alignment. Error bars = SEM; * = p<0.001 compared to rNC Only, No Stress; # = p<0.001 compared to rNC+Endo, No Stress. Inset, alignment analysis vectors depicted on micrograph of rat heart with desmin staining. C) Cardiomyocyte alignment within the constructs developed between 1 and 4 days of static stress conditioning. D) Transmission electron microscopy of cardiac constructs revealed elongated cardiomyocytes within the collagen matrix, with nuclei (nuc), numerous mitochondria (m), and contractile filaments. Higher magnification revealed mitochondria among myofibrils (myof) with scattered nascent Z-disks (arrow).
In addition to intercellular alignment within the construct, cells within this 3-D matrix also demonstrated signs of maturation, such as bi-nucleation (Online Figure II, C) and organized sarcomeric banding (Online Figure II, D and E) perpendicular to the direction of stress, which is similar to native cardiac tissue. Some cardiomyocytes also continued to undergo DNA replication (by BrdU incorporation, Figure 1A, C) and nuclear division (Online Figure II, B). After several days in culture, these constructs beat spontaneously and synchronously (Online Video I). By transmission electron microscopy, rat neonatal cardiomyocytes within constructs demonstrated internal organization with active myofibrillogenesis, occasional Z-lines, numerous mitochondria, and nascent intercalated disks containing desmosomes, intermediate junctions and occasional gap junctions (Figure 1D).
Generating Human Cardiac Tissue Constructs
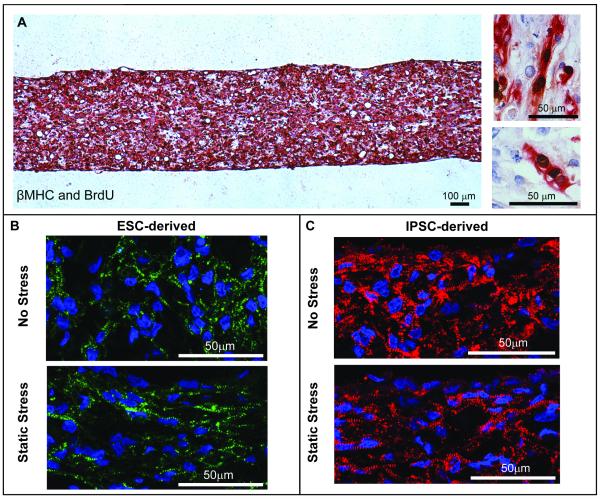
Cardiomyocytes derived from pluripotent human stem cells were used make 3-D tissue constructs using conditions optimized with rNCs (Figure 2). When cultured under static stress, human tissue constructs generated from either ESC-derived cardiomyocytes or iPSC-derived cardiomyocytes began to beat synchronously and spontaneously between 1 and 4 days, indicating that these cells were capable of electromechanical coupling within the collagen matrix (Online Videos II and III, respectively). Immunostaining showed that cells throughout the construct strongly expressed the cardiac contractile proteins β-myosin heavy chain (βMHC) (Figure 2A) and α-actinin (Figure 2B, C), as well as cardiac troponin T (cTnT), and the cardiomyocyte transcription factor Nkx2.5 (not shown). The human cardiomyocytes underwent frequent DNA synthesis, with 15-45% of nuclei incorporating BrdU after an overnight pulse (Figure 2A). Furthermore, these cells demonstrated sarcomeric banding of the contractile apparatus by α-actinin immunostaining (Figure 2B). Constructs generated using cardiomyocytes differentiated from iPSCs also showed strong sarcomeric binding by α-actinin immunostaining (Figure 2C). The iPSC constructs appeared indistinguishable from the ESC-derived constructs of similar conditioning. In both cases, sarcomeres appear more aligned in the static stress-conditioned constructs than in the unstressed constructs. Human ESC-derived cardiac constructs also were examined by transmission electron microscopy. The contractile cytoskeleton of the human cardiomyocytes was generally less well developed than that of the rat, indicating a less mature phenotype. Nevertheless, similar to rNC constructs, hCM constructs contained cardiomyocytes demonstrating ongoing myofibrillogenesis, including partially organized myofilament bundles associated with polyribosomes and nascent Z-lines (Figure 3C, D). The human cardiomyocytes contained numerous mitochondria, and the extracellular space contained readily identifiable collagen fibrils (not shown). Thus, spontaneously beating human myocardial tissue can be created in collagen gels using techniques similar to that for rat tissue engineering.
Figure 2. Characterization of ESC- and iPSC-derived human cardiac tissue constructs.
A) Constructs generated from human ESC-derived cardiomyocytes stained strongly for the cardiomyocyte marker βMHC (red) and the proliferation marker BrdU (brown). High magnification (right): human cardiomyocytes were observed undergoing nuclear division within the collagen matrix. B) Constructs generated from ESC-derived cardiomyocytes subjected to static stress conditioning (lower) or no stress conditioning (upper) stained strongly for the sarcomeric protein α-actinin (green). C) Constructs generated from iPSC-derived cardiomyocytes also stained strongly for α-actinin (red). As in B, the construct edges and vector of stress conditioning are horizontal. These constructs appeared indistinguishable from the ESC-derived constructs of similar conditioning. In both cases, myofibrils appear more aligned in the static stress conditioned constructs.
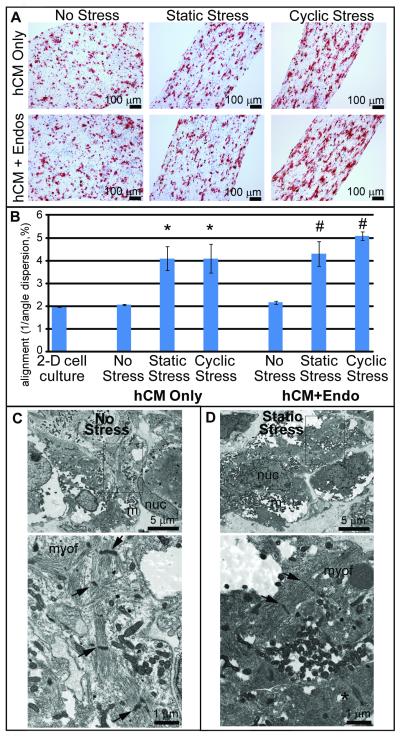
Figure 3. Stress conditioning modulates human cardiomyocyte self-organization.
A) hCM constructs derived from hESCs with or without endothelial cells were placed under conditions of no stress, static stress or cyclic stress and stained for βMHC (red) and BrdU (brown). Under static and cyclic stress conditions, cardiomyocytes aligned with each other in parallel to the direction of stress. B) Quantitative alignment assessment. Unstressed constructs did not have significantly different cell alignment versus 2-D cell culture. Static and cyclic stress significantly increased cell axis alignment (* = p<0.005 versus hCM only, No Stress). Co-culture of endothelial cells within the construct did not inhibit development of cardiomyocyte alignment (# = p<0.005 versus hCM+Endo, No Stress). C) By electron microscopy, human cardiac constructs with no stess conditioning contained, cardiomyocytes with numerous mitochondria (m). Nucleus, nuc. Higher magnification (below): showed relatively disorganized nascent myofibrillar bundles (myof) in the cytoplasm and scattered Z-disks (arrows) associated. D) Static stress conditioned constructs had more regular contractile filaments with interspersed Z-disks. Occasional desmosomal junctions were observed (lower right, *).
Human Cardiomyocyte Alignment with Stress
To test whether exogenous stress promotes human cardiomyocyte self-organization, human cardiac constructs were generated both with and without endothelial cells and subjected to no stress, static stress, and 1 Hz cyclic stress conditioning for 4 days. Constructs were immunostained for βMHC (Figure 3A), and cardiomyocyte alignment was quantified as described above (Figure 3B). Unstressed human constructs did not have significantly different cell alignment than 2-dimensional cell culture (alignment values of 1.96 versus 2.05, respectively). However, static and cyclic stress conditioning strongly increased cell alignment (4.09 for each, p<0.005 versus unstressed conditions). As with rat constructs, there were no significant differences in human cardiomyocyte alignment in static vs. cyclic stress (p=1.00).
Matrix Structure Organization with Stress
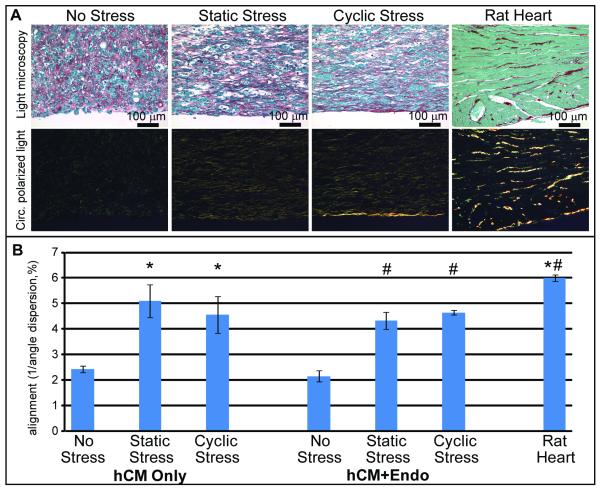
We also investigated the effect of stress conditioning on the organization of the extracellular matrix within the bioengineered constructs. To analyze the collagen fiber architecture within the cardiac constructs, we combined picrosirius red staining (to determine total collagen) and polarized light (to assess the presence of organized collagen fibers).36-38 Circularly polarized light was utilized to avoid the orientation-dependence of birefringence associated with linearly polarized light.36 In the absence of cells, no large collagen fibers were seen following static stress conditioning (Online Figure III, A). In human cardiac constructs, we observed large collagen fiber bundles by yellow birefringence (Figure 4A). The collagen fiber bundles were disarrayed in unstressed constructs, but fiber architecture was much more orderly following static and cyclic stress conditioning (Figure 4A). Rat neonatal cardiomyocyte constructs in the same conditions showed even more distinct collagen fiber organization (Online Figure III, A). The absence of large collagen fibers in cell-free collagen constructs indicated that these structures require cells for their synthesis and alignment within the tissue construct. Quantitative analyses (Figure 4B) showed a 2-fold increase in collagen alignment when comparing no stress conditions (alignment value of 2.42) to static and cyclic stress conditioning (5.10 and 4.56, respectively, p<0.01). Thus, in the context of bioengineered cardiac tissue, stress facilitates the cell-driven development of matrix architecture as well as self-organization of the cardiomyocytes within the construct.
Figure 4. Stress conditioning facilitates the cell-driven development of matrix architecture.
A) Assessment of collagen fiber bundle organization using picrosirius red and polarized light. Large bundle collagen fibers fluoresced yellow in the interstitium of rat myocardium and between cells in constructs generated from human ESC-derived cardiomyocytes. These fibers appeared disarrayed in no stress conditions but closely aligned under stress conditioning. B) Quantitative alignment assessment of extracellular matrix in human cardiac constructs indicated a 2-fold increase with stress conditioning. The presence or absence of human endothelium in co-culture did not affect matrix organization. * = p<0.01 versus hCM only, No Stress, # = p<0.005 versus hCM+Endo, No Stress.
Effects of Mechanical Load on Human Cardiomyocyte Proliferation and Hypertrophy
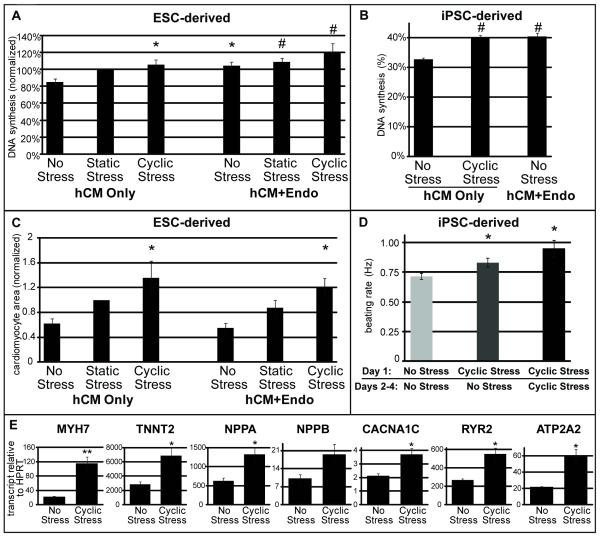
Next, we tested the hypothesis that human cardiac constructs would proliferate in response to mechanical stress. Myocardial constructs were pulsed with BrdU for 24 hours before fixation, and cardiomyocyte DNA synthesis rates were determined by measuring βMHC and BrdU double labeling (Figure 5A, B). Absolute BrdU incorporation rates ranged from 15-45%, indicating high baseline rates of proliferation. Due to run-to-run variation in baseline BrdU incorporation rates, however,39 measurements were normalized to the basal DNA synthesis rates in static stress conditions. Consistent with our hypothesis, both static and cyclic stress increased human cardiomyocyte BrdU incorporation within the ESC-derived construct by 15-21% over conditions of no stress (85%, 100%, and 106% for no stress, static, and cyclic, respectively; Figure 5A). The difference between no stress and cyclic stress was highly significant (p=0.01). To further characterize the effect of stress on human cardiomyocyte proliferation, we analyzed the incorporation of BrdU in iPSC-derived cardiac constructs (Figure 5B). In this experiment, cyclic stress conditioning increased DNA synthesis by 7.4% over a basal rate of 32.8% (p<0.005).
Figure 5. Stress and co-culture modulate human cardiomyocyte proliferation and hypertrophy.
A) Cardiomyocyte DNA synthesis was measured by βMHC and BrdU double staining in hESC-derived cardiac constructs. Data are given as fold over basal rate in the hCM-only, static stress condition. Static and cyclic stress markedly increased hESC-derived cardiomyocyte BrdU incorporation over no stress (15% and 21% increases, respectively) as did the addition of endothelial cells (19%). B) Cardiomyocyte DNA synthesis within a single experiment (n=4 per group) of iPSC-derived cardiac constructs. Co-culture and cyclic stress conditioning both significantly increase iPSC-derived cardiomyocyte DNA synthesis. C) Cardiomyocyte hypertrophy within hESC-derived cardiac constructs was assessed by βMHC immunostaining, measuring stained area within each construct, and normalizing to number of cardiomyocyte nuclei. Due to variability in input purity, data are given as fold over the hCM only, static stress condition. Cardiomyocyte area increased 2.2-fold in response to cyclic stress conditioning. D) Spontaneous beating frequency with stress conditioning in iPSC-derived cardiac constructs. The following experimental conditions were used: no stress for 4 days, 1Hz, 5% elongation cyclic stress for 1 day followed by 3 days of no stress, or 4 days under the cyclic stress condition. Afterward, beating rate was visually assessed and a time-dependent effect due to stress conditioning was observed. E) Quantitative RT-PCR was performed on iPSC-derived cardiac constructs conditioned with no or cyclic stress for 4 days to determine the mRNA transcript levels of the following contractile and hypertrophy related genes: MYH7 (βMHC), TNNT2 (cTnT), NPPA (ANP), NPPB (BNP), CACNA1C (L-type calcium channel subunit 1Cα), RYR2 (sarcoplasmic calcium channel/ryanodine receptor) and ATP2A2 (SERCA2, sarcoplasmic calcium transporter). Significance was determined by Single Factor Anova followed by Student’s t-test in comparison to the hCM only, no stress condition. * = p<0.05; ** = p<0.01; # = p<0.005 compared to the hCM Only, No Stress condition; error bars represent standard error.
Cyclic stress conditioning also increased the spontaneous beating rate of iPSC-derived constructs, from 0.71Hz to 0.83hz to 0.95Hz, respectively, for no stress, 1 day cyclic stress, 4 days cyclic stress (p<0.05 compared to no stress; Figure 5D), indicating changes in electromechanical structure of the cardiomyocytes within the construct in response to conditioning. To assess if mechanical stress promoted cardiomyocyte hypertrophy, we quantified the βMHC+ area within each ESC-derived construct and divided this by total cardiomyocyte nuclear number (Figure 5C). Cardiomyocyte area within the construct markedly increased with both static and cyclic stress, more than doubling in response to cyclic stress from 62% to 136% of static stress-normalized area (p=0.05). We assessed the effect of stress conditioning on hypertrophy in iPSC-derived cardiac constructs by quantitative RT-PCR (Figure 5E). The data showed that transcripts for βMHC, cTnT, the L-type Ca2+ channel, the ryanodine receptor, atrial and B-type natriuretic factors, and SERCA2A increased by 2-6 fold in response to cyclic stress conditioning. Thus, mechanical stress induces both DNA synthesis and hypertrophy in human myocardial tissue constructs.
Pre-Vascularizing Human Cardiac Constructs
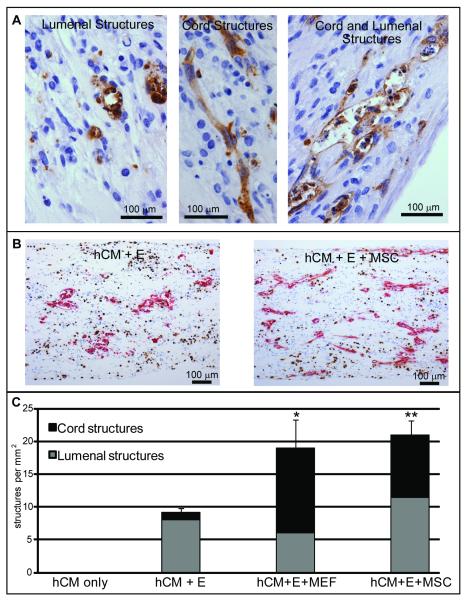
Myocardium is a highly vascular tissue, and the coronary circulation supports myocardial development through both perfusion and paracrine signaling pathways.5, 6, 40 We therefore explored the effects of adding a vascular cell network to our engineered human heart tissues. In constructs generated from preparations of hESC-derived cardiomyocytes, endothelial cells are occasionally detected by CD31 immunostaining, but they very rarely form any sort of multicellular structure (data not shown). However, when exogenous endothelial cells are added to cardiac constructs, both cord structures and structures containing lumens are observed (Figure 6A). The total number of endothelial structures increased markedly when support cells were added into the constructs in tri-culture with human cardiomyocytes and endothelial cells (Figure 6B), approximately doubling in the presence of either mouse embryonic fibroblasts (MEFs) or human marrow stromal cells (MSCs) (Figure 6C). Compared to cardiomyocyte + endothelial cell constructs, the addition of MEFs increased the prevalence of endothelial cord structures by 10-fold, while the presence of human adult MSCs increased these structures by 8-fold (Figure 6C) (p<0.05 for MEFs, p<0.01 for MSCs). The number of lumenal structures did not significantly change with either stromal cell population (p=0.76 for MEFs, p=0.56 for MSCs). Similar results were found when endothelial and stromal cells were added to neonatal rat cardiomyocyte constructs (Online Figure IV, B). Thus, in both human and rodent cardiac tissue constructs, the addition of a stromal cell population strongly augmented endothelial structure formation.
Figure 6. Stromal cells affect vascular organization within the human cardiac construct.
A) ESC-derived cardiac constructs were generated by co-culture with endothelial cells with and without stromal cells and immunostained for the endothelial marker CD31. Shown at high magnification, endothelial cells organized into cord networks, structures with lumens, or structures with characteristics of both (left, hCM+Endo; middle, hCM+Endo+MEF; right hCM+Endo+MSC). B) Shown at low magnification, the prevalence of endothelial structures (CD31, red, and BrdU, brown) in the constructs increased with the addition of stromal cells (left, without MSCs; right, with MSCs). C) Quantitation of endothelial structures. The total number of structures (cord and lumenal) doubled with either MSC or MEF co-culture. The number of cord structures increased by approximately 10-fold. *, p<0.05; **, p<0.01 for cord structures compared to hCM+Endo.
Despite formation of relatively large vascular-like structures, addition of endothelial cells had no effect on cardiomyocyte or matrix alignment in constructs, regardless of stress conditioning (Figure 3, 4). Similarly, cardiomyocyte hypertrophy, although increased more than 2-fold with cyclic stress, did not change with endothelial cell co-culture (Figure 5B). Interestingly, addition of endothelial cells increased hESC-derived cardiomyocyte DNA synthesis rates in all stress conditions (up to 19%, p=0.01), implying endothelial-derived mitogens were stimulating cardiomyocyte proliferation (Figure 5A). The mitogenic properties of co-culture with endothelial cells was also seen in human iPSC-derived cardiomyocyte constructs (Figure 5B). Cyclic stress and co-culture together increased cardiomyocyte DNA synthesis by 35% (p=0.004). Thus, mitogenic pathways are induced in human cardiomyocytes by both endothelium and cyclic stress.
Frank-Starling Relation in Bioengineered Human Cardiac Tissue
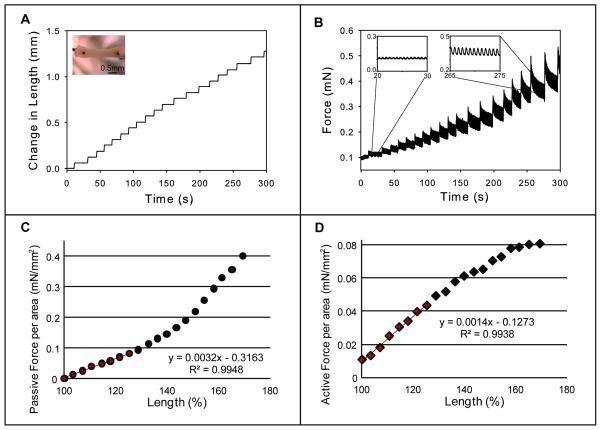
We next studied contractile function in bioengineered human cardiac tissue that had been subjected to 3 weeks of static stress conditioning. hESC-derived constructs were mounted between a force transducer and a post whose position was controlled by a motor, thereby varying resting tension. A representative trace and analysis is presented in Figure 7 (n=9). The tissue was subjected to a series of 4% length increases (Figure 7a) while continuously measuring force (Figure 7B). Active force transients (twitch force) were apparent starting from just above slack length and were increased in amplitude at higher magnitude stretches (Figure 7B, left and right insets, respectively). Passive force (preload) recorded 15 seconds after each stretch was normalized to cross-sectional area and graphed against change in length to determine the Young’s Modulus (Figure 7C). This yielded a curvilinear relationship with modest increases in tension at stretches up to 45% over slack length, and higher increases thereafter. Contractility was assessed by plotting the amplitude of active force against change in construct length (Figure 7D). This yielded a linear relationship, in which active force increased 8-fold over the first 60% of stretch over slack length and plateaued thereafter. Linear regression over the first 25% of this curve (the most physiologically relevant) yielded an R2 value of 0.99. This active force/length relationship is analogous to Frank-Starling curves in the intact heart and indicates our engineered tissue recapitulates a fundamental property of native cardiac muscle.
Figure 7. Force/length dependence in bioengineered cardiac constructs.
Constructs generated from human ESC-derived cardiomyocytes were subjected to static stress conditioning for three weeks before assessment of active force development at different lengths with a force transducer and a high speed length controller. A) Steps of 4% length increase were made starting from the slack length of the bioengineered cardiac tissue construct. B) Force was continuously measured, and an increase in magnitude (insets) occurred at greater lengths. C) Passive force (baseline) recorded 15 seconds after each acute stretch was normalized to cross-sectional area, graphed against change in length, and the slope of the first 25% length change (Young’s Modulus) was determined. D) Active force twitch height at 15 seconds was graphed against construct length. The magnitude of active force increased 8-fold in a linear manner over increasing preparation lengths before leveling off at large magnitude stretches, and the slope of the first 25% length change was calculated with an R2 value of 0.9938. This Force/Length Relationship is analogous to Starling curves generated in the intact heart.
Cardiac Engraftment
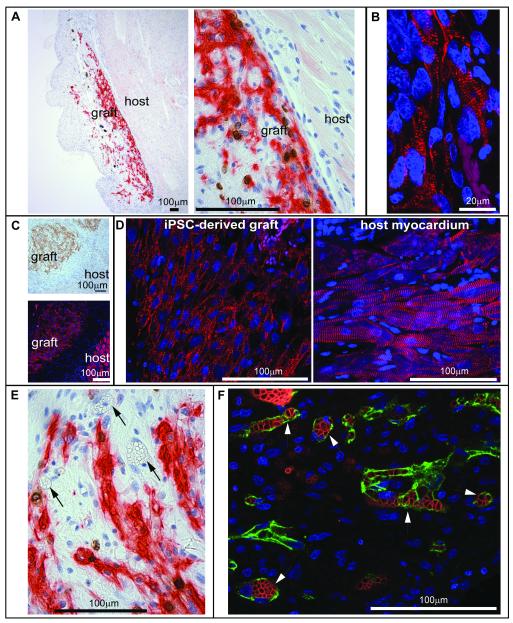
Finally, we investigated the viability of human bioengineered cardiac tissue to engraft in the hearts of athymic rats. Constructs derived from hCM-only and human tri-culture constructs containing cardiomyocytes, endothelial cells, and MSCs were conditioned by static stress and engrafted onto the epicardial surfaces of uninjured athymic rat hearts (n=5 for hCM-only, n=4 for Tri-cell, n=1 for iPSC-derived hCM-only). Hearts were harvested one week later and studied histologically. When a subset of 3 hearts were probed for the presence of human cells by in situ hybridization for human-specific centromeric repeats, the human cardiac constructs were readily identified on the epicardial surface in each heart (data not shown). Furthermore, grafts with GFP-expressing MSCs were visible by fluorescence on the surface of the heart in animals receiving tri-culture constructs (Online Figure IV, D). The implanted constructs expressed the human cardiac marker βMHC in all 10 engrafted animals (Figure 8A, C, E) as well as the cardiac transcription factor Nkx2.5 (data not shown). These human cardiomyocytes were often in close proximity to the host myocardium (Figure 8A), and there was no foreign body reaction to the implanted construct. Engrafted human ESC-derived and iPSC-derived cardiac constructs both showed sarcomeric banding, indicative of intracellular contractile organization, as well as similar cell to cell alignment, indicating intercellular organization (Figure 8B, D, respectively). Furthermore, a microvascular network was present throughout the constructs, and these blood vessels were filled with host erythrocytes (Figure 7E, F). A subset of the host-perfused vessels stained for human-specific CD31 (Figure 7F) within the human tri-culture construct grafts (4 out of 4 grafts) but not in the hCM grafts (rare vessels in 1 out of 5 grafts, likely from “contaminating” endothelial cells), indicating that the endothelial network which had self-organized in vitro within the prevascularized construct could form bona fide blood vessels in vivo.
Figure 8. Cardiac engraftment.
Human cardiac tissue constructs were pulsed for 1 day with BrdU and sutured onto myocardium of athymic rats. After 1 week in vivo, the hearts were excised, sectioned, and immunostained. A) βMHC and BrdU double immunostain of hESC-derived, hCM only cardiac tissue construct. Left, graft overview, right, close apposition at graft-host interface in the myocardium. B) α-actinin immunofluorescence showed sarcomeric organization of engrafted human cardiomyocytes from the hESC-derived, hCM only cardiac tissue construct. C) IPSC-derived cardiac tissue construct engrafted onto the myocardium. Top, βMHC, bottom, α-actinin, showing the graft-host interface. D) Left, close-up of the engrafted iPSC-derived cardiac construct, right, close-up of the native myocardium (red, α-actinin). E) Within the βMHC positive area of engrafted hESC-derived constructs, patent blood vessels were filled with erythrocytes (arrowheads), indicating host perfusion of the graft. F) In engrafted hESC-derived Tri-cell human cardiac tissue constructs, human CD31 immunostaining (green) was used to mark human vessels and Ter119 (red) to mark erythrocytes. Endothelial structures of human origin containing red blood cells are indicated with arrows.
Discussion
During development, the heart responds to biomechanical cues as well as signals from resident cells and matrix to determine overall cardiac size and architecture.6, 7 Cellular and mechanical factors are also important determinants of the modification that occurs when the post-natal heart has to maintain performance while adapting to physiological change such as growth, pregnancy, exercise conditioning, or injury.6, 41, 42 Although animal models have been critical in our understanding of the factors that modulate cardiac tissue development and homeostasis, as discussed above, these have limitations due to species-intrinsic differences in cardiac size, heart rate and so on. To our knowledge this is the first study to examine the effects of mechanical stress and vascularization on cardiomyocyte architecture, proliferation, and maturation in human cardiac tissue. Our major findings are: 1) both static and cyclic stress conditioning promote human cardiomyocyte alignment and hypertrophy within a collagen 3-D matrix, 2) mechanical stress and endothelial cell co-culture both induce human cardiomyocyte proliferation, and 3) stromal cells of various types, including human MSCs, increase vascular network formation within bioengineered human cardiac tissue. Furthermore, these optimized human cardiac tissue constructs can 4) be generated from human iPSC-derived cells and 5) can generate active forces responsive to changes in construct length, analogous to Starling curves generated in the intact heart.
Multiple studies have shown that interactions between cardiomyocytes and endothelial cells are necessary for normal rodent and avian myocardial development.7 For instance, endothelial-specific knockout experiments using Tie2-cre mice43 have been used to identify a number of signaling molecules within the endothelium, including Tie2 itself44, neurofibromin,45 and EphrinB2,46 which modulate myocardial development and trabecular architecture. In fact, co-culture of human endothelial cells and signaling though EphB4 receptor tyrosine kinase has been determined to be critical for high yield generation of cardiomyocytes from EphB4-null mouse ESCs,47 although it is not yet clear if this effect is due to a direct influence on differentiation or on cardiomyocyte survival or proliferation. Similarly, production of neuregulin by endocardial cells is essential, as well as myocyte-specific expression of its cognate receptor ErbB4, for formation of ventricular trabeculae.48-50 It is likely, but not yet shown, that many of these endothelial-cardiomyocyte interactions directly translate to the human myocardium.
Some progress has been made towards creating in vitro models of vascularized skeletal muscle51 and even human cardiovascular tissue with ESC-derived cardiomyocytes. For example, Caspi et al. seeded hESC-derived cardiomyocytes and vascular cells onto poly-l-lactic acid scaffolds, and they also observed that endothelial cells enhance cardiomyocyte proliferation.21 Because synthetic scaffolds may not allow normal cellular remodeling and are known to elicit foreign body reactions that may limit graft-host integration, our group has been exploring scaffold-free29, 30 or natural matrices for tissue engineering such as collagen type I and type III, the major components of the primate myocardial matrix.27 We found that scaffold-free human myocardial constructs, comprised only of cardiomyocytes and the matrix they secrete, survive poorly after transplantation, but the addition of endothelial and stromal cells markedly enhances vascularization and survival.30 Furthermore, when implanted onto the heart, they showed no foreign body reaction at the graft-host interface.
While scaffold-free approaches show promise, the cardiomyocytes in these constructs are developmentally immature, and their assembly in rotary culture via cell-cell adhesion makes it difficult to control tissue architecture. The desire to control cardiomyocyte hypertrophy and fiber alignment prior to engraftment led us to test collagen hydrogels as vehicles for tissue engineering. Previous studies reported that cyclic stress induces cardiomyocyte alignment in rat neonatal cardiomyocytes within a collagen gel.25, 26 No reports, to our knowledge, have previously demonstrated stress-induced development of alignment in human cardiomyocytes or of alignment of myocardial matrix with stress conditioning. Because isolated type I and III fibrillar collagens can polymerize into triple helical fibrils in vitro, it was long thought that the process of fiber polymerization in tissue occurred via self-assembly.52, 53 However, it has more recently been established that cell surface integrins play a role in facilitating the assembly of type I and type III collagen fibers in MEF cultures, and that this assembly is independent of collagen synthesis.54 The data presented here suggest that cell-directed organization of collagen matrix occurs in cardiomyocyte cultures as well, and that stress increases the ability of cells to organize these fibers and align them with vectors of external force.
It has been postulated that mechanical stress modulates architecture of the developing,55, 56 mature57, 58 and injured myocardium.58, 59 In vitro, the establishment of cardiomyocyte cellular organization due to stress has been studied thus far in rat neonatal cardiomyocytes. Optimal sarcomere length in rNCs is re-established during sudden longitudinal static stress and involves PKCε phosphorylation,60 whereas detection of transverse static stress is accomplished through FAK and ERK1/2 phosphorylation.61 Sensing directionality of mechanical stress has been hypothesized to be a mechanism by which cardiomyocytes may add sarcomeres in series in response to diastolic stretch or in parallel due to increased systolic stress, leading to re-establishment of homeostasis and preservation of cardiac performance under changing physiological conditions.62 Here we demonstrated that human cardiomyocytes within unstressed constructs show no more alignment than cells in 2-dimensional cell culture. Conversely, static and cyclic stress conditioning both promoted cell and matrix alignment much closer to that measured in native cardiac muscle (Figures 1, 3, 4). And, while cyclic stress conditioning promoted more cardiomyocyte hypertrophy within the construct, we were surprised that it conferred no additional benefit to cardiomyocyte alignment over static stress conditioning. It is possible that further increased exogenous cyclic stress—either greater amplitude, faster rate, or longer culture conditions than used here—may further improve alignment. However, our pilot studies showed that conditions of 10% elongation caused mechanical failure in some constructs, most often at the points of attachment to the nylon tabs, and conditions of 5% cyclic stress up to 7 days did not show an increase in alignment over 7 day static stress (data not shown). Another possible explanation for comparable alignment in static and cyclic stress conditions is the spontaneous contractions observed in both situations. The cardiomyocytes may induce their own cyclic stress conditioning under “static” external loading conditions.
One of the only other reports on human myocardial tissue engineering demonstrated an increase in both endothelial structure formation and proliferation due to co-culture with stromal cells.21 This study found a similar significant increase in vascular structure formation, however, we observed that the presence of stromal cells actually decreases endothelial cell proliferation within the human constructs (Online Figure IV, C). Several differences exist between the two studies, including the scaffold material (poly l-lactic acid versus collagen), the metric used for measurement of proliferation (Ki67 versus BrdU incorporation) and the stromal cell type, i.e. we investigated both MEFs and human MSCs. The latter are clinically more relevant due to their human origin, smooth muscle differentiation capabilities,63 and putative cardiac function benefit upon injection following injury.64-67 We found similar results with both stromal cell types. Furthermore, the time point of the proliferation measurement in the study by Caspi et al. is not clearly delineated, although other analyses within that study have endpoints from 1 hr to 7 days. In our study, we measured proliferation after 5 days in culture with a 1 day pulse of BrdU. At 5 days, significant vascular structure development was evident. Endothelial cells within the cardiac construct may be involved in a high degree of proliferation followed by apoptosis as part of normal vascular pruning, and the presence of stromal cells may play a role stabilizing the nascent endothelial cell structures.
In summary, we developed a collagen-based, bioengineered human cardiac tissue construct in a self-organizing co-culture with endothelial and stromal cells and demonstrated the development of cardiomyocyte alignment, proliferation, and hypertrophy due to mechanical stress and co-culture. Furthermore, we determined that these constructs engraft into the myocardium with cardiac and endothelial contributions of human origin, and ascertained that engrafted constructs are perfused through connections to host vasculature within a week. These cardiac constructs may provide additional engraftment benefit over cell injection therapies for infarct repair due to positioning of a cardiac repair construct over and across an infarct scar rather than within it. This differential placement may increase therapeutic performance due to 1) separation of the graft from the inflammatory infarct environment and 2) positioning which may promote electrical coupling with intact myocardium on both sides of the infarct.68 These studies validate mechanical conditioning and vascular and stromal co-culture as practical and constructive methods of affecting human cardiomyocyte organization, replication, and maturation in bioengineered human cardiac tissue.
Supplementary Material
Novelty and Significance.
What is known?
Cardiomyocytes can be cultured in monolayer or 3-dimensional matrices of synthetic or natural origin; interactions with endothelial cells are necessary for normal myocardial development.
Human cardiomyocytes can be generated from human embryonic stem cells or reprogrammed “induced pluripotent stem cells” derived from differentiated tissues.
Rat neonatal cardiomyocytes cultured in 3-dimensional collagen matrix respond to mechanical strain with organization and hypertrophy.
What new information does this article contribute?
In a 3-dimensional bioengineered cardiac tissue generated with a type I collagen scaffold, human cardiomyocyte proliferation and vascular structure formation is promoted in vitro by co-culture with vascular and stromal cell types.
Mechanical stress conditioning promotes human cardiomyocyte proliferation, intercellular organization, matrix/scaffold organization, and cellular hypertrophy.
This bioengineered human cardiac muscle is spontaneously contractile, responds appropriately to stretch with increased force of contraction, and can engraft onto the heart, where it is perfused through anastomosis of engineered human vascular networks to the host coronary circulation.
The regulation of heart growth by mechanical stresses and vascularization is poorly understood, yet both these factors are necessary for the heart to reach its proper size, shape and architecture. We therefore investigated the effects of human vascular cells and mechanical stress conditioning of human myocardium derived in vitro using tissue engineering approaches. We found that vascular cells increased proliferation of cardiomyocytes in 3-dimensional bioengineered tissue generated using a type I collagen scaffold. Mechanical stress conditioning induced intercellular organization, matrix organization, cardiomyocyte proliferation and hypertrophy. Furthermore, we discerned that addition of a third, stromal cell type allowed more complex vascular structures to form within the human bioengineered cardiac tissue. Moreover, this engineered human tissue was able to contract spontaneously and synchronously and respond with increased force of contraction when stretched, demonstrating Force/Length Relationships analogous to Starling Curves generated in the intact heart. Finally, we determined that these bioengineered cardiac tissue constructs can be engrafted onto the heart in vivo and are quickly perfused by host coronary circulation through connection to the pre-existing human vascular network. This work has implications for both models of human cardiac development as well as human therapeutics using vascularized human cardiac tissue.
Acknowledgements
We thank Douglas White for assistance with alignment quantification, Sarah Dupras and Jennifer Deem for their surgical expertise, Sarah Fernandes for human centromeric probe staining, and Benjamin Van Biber and Michael Laflamme for their assistance with hESC differentiation. We thank Garvey Imaging Lab at the UW for providing instrumentation for confocal microscopy and Dr. Ron Seifert for his guidance in the use of that equipment. We also thank the Electron Microscopy Core Facility at the Pathology Department of the UW and Stephanie Lara of that facility for assistance in preparing our samples.
Sources of Funding This work was supported by the following National Institutes of Health Grants: R01HL084642, R01HL64387, P01HL094374 and P01GM81619 (to CEM), T32HL007312-Experimental Pathology of Cardiovascular Disease training grant, the UW’s MSTP grant (to NLT), the UW’s Mouse Metabolic Phenotyping Center U24 DK076126, as well as funding from the Children’s Cardiomyopathy Foundation and the Mend a Heart Foundation.
Non-standard Abbreviations and Acronyms
- 2-D
Two dimensional
- 3-D
Three dimensional
- βMHC
β-myosin heavy chain
- BrdU
5-bromo-2-deoxyuridine
- CD31
Cluster of differentiation molecule 31 (also Platelet endothelial cell adhesion molecule 1)
- cTnT
Cardiac troponin T
- EphB4
Ephrin type-B receptor 4
- Endos
Endothelial cells
- ESC
Embryonic stem cell
- GFP
Green fluorescent protein
- hCM
Human cardiomyocyte
- hESC
Human embryonic stem cell
- HPRT
hypoxanthine phosphoribosyltransferase 1
- HUVEC
Human umbilical vein endothelial cell
- iPSC
Induced pluripotent stem cell
- MEF
Mouse embryonic fibroblast
- MSC
Marrow stromal cell
- rNC
Rat neonatal cardiomyocyte
- SEM
Standard error of the mean
Footnotes
Disclosures Dr. Murry’s laboratory receives a research grant from Surmodics Corporation.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montgomery MO, Jiao Y, Phillips SJ, Singh G, Xu J, Balsara R, Litvin J. Alterations in sheep fetal right ventricular tissue with induced hemodynamic pressure overload. Basic Res Cardiol. 1998;93:192–200. doi: 10.1007/s003950050086. [DOI] [PubMed] [Google Scholar]
- 2.Tobita K, Keller BB. Right and left ventricular wall deformation patterns in normal and left heart hypoplasia chick embryos. Am J Physiol Heart Circ Physiol. 2000;279:H959–969. doi: 10.1152/ajpheart.2000.279.3.H959. [DOI] [PubMed] [Google Scholar]
- 3.Voronov DA, Alford PW, Xu G, Taber LA. The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev Biol. 2004;272:339–350. doi: 10.1016/j.ydbio.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Kira Y, Nakaoka T, Hashimoto E, Okabe F, Asano S, Sekine I. Effect of long-term cyclic mechanical load on protein synthesis and morphological changes in cultured myocardial cells from neonatal rat. Cardiovasc Drugs Ther. 1994;8:251–262. doi: 10.1007/BF00877334. [DOI] [PubMed] [Google Scholar]
- 5.Kuruvilla L, Kartha CC. Molecular mechanisms in endothelial regulation of cardiac function. Mol Cell Biochem. 2003;253:113–123. doi: 10.1023/a:1026061507004. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Macdonald ST, Farthing CR. Molecular mechanisms controlling the coupled development of myocardium and coronary vasculature. Clin Sci (Lond) 2006;111:35–46. doi: 10.1042/CS20060003. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 13.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu WZ, Santana LF, Laflamme MA. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS One. 2009;4:e5407. doi: 10.1371/journal.pone.0005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 17.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itzhaki I, Schiller J, Beyar R, Satin J, Gepstein L. Calcium handling in embryonic stem cell-derived cardiac myocytes: of mice and men. Ann N Y Acad Sci. 2006;1080:207–215. doi: 10.1196/annals.1380.017. [DOI] [PubMed] [Google Scholar]
- 19.Radisic M, Park H, Martens TP, Salazar-Lazaro JE, Geng W, Wang Y, Langer R, Freed LE, Vunjak-Novakovic G. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J Biomed Mater Res A. 2008;86:713–724. doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blan NR, Birla RK. Design and fabrication of heart muscle using scaffold-based tissue engineering. J Biomed Mater Res A. 2008;86:195–208. doi: 10.1002/jbm.a.31642. [DOI] [PubMed] [Google Scholar]
- 21.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 22.Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80:305–312. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 23.Birla RK, Huang YC, Dennis RG. Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng. 2007;13:2239–2248. doi: 10.1089/ten.2006.0359. [DOI] [PubMed] [Google Scholar]
- 24.Shimko VF, Claycomb WC. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A. 2008;14:49–58. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 26.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000;14:669–679. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 27.Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62:757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 28.Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, Torok-Storb B, Ratner BD, Pabon L, Murry CE. VEGF Induces Differentiation of Functional Endothelium From Human Embryonic Stem Cells. Implications for Tissue Engineering. Arterioscler Thromb Vasc Biol. 2009;30:80–89. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15:1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 33.Xu C, Police S, Hassanipour M, Gold JD. Cardiac bodies: a novel culture method for enrichment of cardiomyocytes derived from human embryonic stem cells. Stem Cells Dev. 2006;15:631–639. doi: 10.1089/scd.2006.15.631. [DOI] [PubMed] [Google Scholar]
- 34.Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999;100:193–202. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- 35.Stevens KR, Rolle MW, Minami E, Ueno S, Nourse MB, Virag JI, Reinecke H, Murry CE. Chemical dimerization of fibroblast growth factor receptor-1 induces myoblast proliferation, increases intracardiac graft size, and reduces ventricular dilation in infarcted hearts. Hum Gene Ther. 2007;18:401–412. doi: 10.1089/hum.2006.161. [DOI] [PubMed] [Google Scholar]
- 36.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 37.Borges LF, Gutierrez PS, Marana HR, Taboga SR. Picrosirius-polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron. 2007;38:580–583. doi: 10.1016/j.micron.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. 2005;13:198–204. doi: 10.1111/j.1067-1927.2005.130211.x. [DOI] [PubMed] [Google Scholar]
- 39.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilfiker-Kleiner D, Limbourg A, Drexler H. STAT3-mediated activation of myocardial capillary growth. Trends Cardiovasc Med. 2005;15:152–157. doi: 10.1016/j.tcm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 41.O’Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, Simpson PC. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest. 2003;111:1783–1791. doi: 10.1172/JCI16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shyu KG. Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clin Sci (Lond) 2009;116:377–389. doi: 10.1042/CS20080163. [DOI] [PubMed] [Google Scholar]
- 43.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 44.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 45.Gitler AD, Zhu Y, Ismat FA, Lu MM, Yamauchi Y, Parada LF, Epstein JA. Nf1 has an essential role in endothelial cells. Nat Genet. 2003;33:75–79. doi: 10.1038/ng1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- 47.Chen K, Bai H, Arzigian M, Gao YX, Bao J, Wu WS, Shen WF, Wu L, Wang ZZ. Endothelial cells regulate cardiomyocyte development from embryonic stem cells. J Cell Biochem. 2010;111:29–39. doi: 10.1002/jcb.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 49.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 50.Lai D, Liu X, Forrai A, Wolstein O, Michalicek J, Ahmed I, Garratt AN, Birchmeier C, Zhou M, Hartley L, Robb L, Feneley MP, Fatkin D, Harvey RP. Neuregulin 1 sustains the gene regulatory network in both trabecular and nontrabecular myocardium. Circ Res. 2010;107:715–727. doi: 10.1161/CIRCRESAHA.110.218693. [DOI] [PubMed] [Google Scholar]
- 51.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 52.Kadler KE, Hojima Y, Prockop DJ. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987;262:15696–15701. [PubMed] [Google Scholar]
- 53.Holmes DF, Graham HK, Trotter JA, Kadler KE. STEM/TEM studies of collagen fibril assembly. Micron. 2001;32:273–285. doi: 10.1016/s0968-4328(00)00040-8. [DOI] [PubMed] [Google Scholar]
- 54.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 55.Lin IE, Taber LA. Mechanical effects of looping in the embryonic chick heart. J Biomech. 1994;27:311–321. doi: 10.1016/0021-9290(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 56.Taber LA. Mechanical aspects of cardiac development. Prog Biophys Mol Biol. 1998;69:237–255. doi: 10.1016/s0079-6107(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 57.Arts T, Prinzen FW, Snoeckx LH, Rijcken JM, Reneman RS. Adaptation of cardiac structure by mechanical feedback in the environment of the cell: a model study. Biophys J. 1994;66:953–961. doi: 10.1016/S0006-3495(94)80876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arts T, Delhaas T, Bovendeerd P, Verbeek X, Prinzen FW. Adaptation to mechanical load determines shape and properties of heart and circulation: the CircAdapt model. Am J Physiol Heart Circ Physiol. 2005;288:H1943–1954. doi: 10.1152/ajpheart.00444.2004. [DOI] [PubMed] [Google Scholar]
- 59.Olivetti G, Capasso JM, Sonnenblick EH, Anversa P. Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circ Res. 1990;67:23–34. doi: 10.1161/01.res.67.1.23. [DOI] [PubMed] [Google Scholar]
- 60.Mansour H, de Tombe PP, Samarel AM, Russell B. Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cepsilon and focal adhesion kinase. Circ Res. 2004;94:642–649. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- 61.Senyo SE, Koshman YE, Russell B. Stimulus interval, rate and direction differentially regulate phosphorylation for mechanotransduction in neonatal cardiac myocytes. FEBS Lett. 2007;581:4241–4247. doi: 10.1016/j.febslet.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russell B, Curtis MW, Koshman YE, Samarel AM. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J Mol Cell Cardiol. 2010;48:817–823. doi: 10.1016/j.yjmcc.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–477. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 64.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 65.Chacko SM, Khan M, Kuppusamy ML, Pandian RP, Varadharaj S, Selvendiran K, Bratasz A, Rivera BK, Kuppusamy P. Myocardial oxygenation and functional recovery in infarct rat hearts transplanted with mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2009;296:H1263–1273. doi: 10.1152/ajpheart.01311.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 67.Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, Voridis EM, Papamichail M. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65:321–329. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- 68.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.