Abstract
This chapter reviews the neurobiological effects of stress sensitivity and CIT treatment observed in our nonhuman primate model of Functional Hypothalamic Amenorrhea (FHA). This type of infertility, also known as stress-induced amenorrhea, is exhibited by cynomolgus macaques. In small populations, some individuals are stress sensitive (SS) and others are highly stress resilient (HSR). The SS macaques have suboptimal secretion of estrogen and progesterone during normal menstrual cycles. SS monkeys also have decreased serotonin gene expression and increased CRF expression compared to HSR monkeys. Recently, we found that s-citalopram (CIT) treatment improved ovarian steroid secretion in SS monkeys, but had no effect in HSR monkeys. Examination of the serotonin system revealed that SS monkeys had significantly lower Fev (fifth Ewing variant, rodent Pet1), TPH2 (tryptophan hydroxylase 2), 5HT1A autoreceptor and SERT (serotonin reuptake transporter) expression in the dorsal raphe than SR monkeys. However, CIT did not alter the expression of either Fev, TPH2, SERT or 5HT1A mRNAs. In contrast, SS monkeys tended to a higher density of CRF fiber innervation of the dorsal raphe than HSR monkeys, and CIT significantly decreased the CRF fiber density in SS animals. In addition, CIT increased CRF-R2 gene expression in the dorsal raphe. We speculate that in a 15-week time frame, the therapeutic effect of S-citalopram may be achieved through a mechanism involving extracellular serotonin inhibition of CRF and stimulation of CRF-R2, rather than alteration of serotonin-related gene expression.
Keywords: citalopram, stress, serotonin, corticotropin releasing factor, urocortin, Fev, TPH2, SLC6A4, 5HT1A, CRF-R2, macaques
Exposure to stressful stimuli can lead to a variety of secondary diseases such as anxiety, depression, cardiovascular disease, and immune suppression (McEwen, 2002). Reproductive dysfunction has been recently added to this growing list of stress-related disorders (Xiao et al., 1999, Cameron, 2000). Clinically, one of the most common forms of infertility is stress-induced infertility, known as Functional Hypothalamic Amenorrhea (FHA; (Berga and Loucks, 2006)). FHA is defined as a sustained absence of normal menstrual cycles despite healthy reproductive organs and the capability of showing normal physiologic functioning (Reifenstein, 1946). International estimates indicate a 9% prevalence of infertility of 12 months (Boivin et al., 2007) and approximately 30% of women presenting at infertility clinics in the United States are diagnosed with Functional Hypothalamic Amenorrhea (FHA), which is lately called Stress-Induced Amenorrhea (Reindollar et al., 1986, Marcus et al., 2001, Berga and Loucks, 2006). Psychometric testing has shown that women with FHA report an increased amount of psychosocial stress in their lives compared to control populations, or women with other forms of infertility, despite experiencing comparable numbers of stressful life events (Giles and Berga, 1993, Fioroni et al., 1994, Marcus et al., 2001). Women with FHA tend to be normal weight and carefully watch their food intake, scoring higher on eating disorder inventories than a control population, but not in the range of having eating disorders (Laughlin et al., 1998, Marcus et al., 2001). They also tend to exercise on a regular basis, often to control life stress (Berga and Girton, 1989, Giles and Berga, 1993, Berga et al., 2003). Thus, there is a body of knowledge indicating that FHA, as well as other forms of stress-induced reproductive dysfunction, results from exposure to a combination of common psychosocial and metabolic life stresses (Williams et al., 2007). Clearly, not all women who experience these everyday life stresses develop fertility problems, suggesting that there is a range of sensitivity to developing stress-induced reproductive dysfunction in normal women.
We have developed an experimental nonhuman primate model of FHA in cynomolgus macaques (Macaca fascicularis), which like women, display monthly menstrual cycles with no seasonal variation. Our stress paradigm was modeled on the mild levels of psychosoical stress and the diet and exercise habits reported by women with FHA. (Biller et al., 1990, Berga et al., 1997, Marcus et al., 2001, Williams et al., 2007). Indeed, we found that mild psychosocial stress (relocation) combined with a mild diet and/or a moderate exercise regimen suppresses reproductive function in about a third of cynomolgus macaques, which reverses upon stress removal (Williams et al., 1997, Cameron, 2000, Bethea et al., 2005a, Williams et al., 2007).
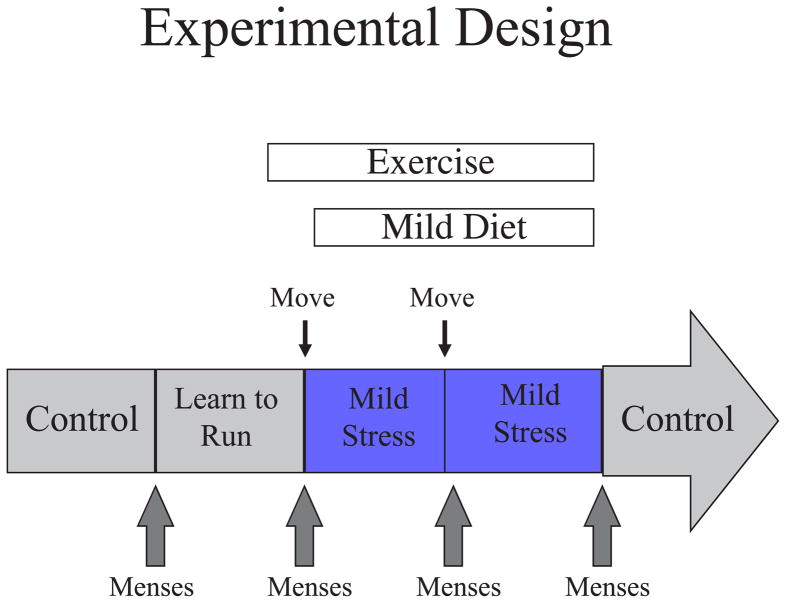
Figure 1 illustrates the paradigm employed. Cynomolgus monkeys are monitored until regular menstrual cycles are present. When the paradigm is started, the animals are observed through 2 control cycles during which the animal first observes another monkey on the treadmill and then is placed on the treadmill and learns to run. At the beginning of the third cycle, the animal is moved to another room with unfamiliar animals (psychosocial stress), placed on 80% of ad libitum monkey chow and allowed to run 5 days per week. After 30 days, the animal is moved again to another room with unfamiliar conspecifics and continues the diet and exercise. Following 30 days in the second room, the animal is returned to its original room and the diet and exercise are ceased.
Figure 1.
Schematic diagram of experimental design.
Using this monkey model, we find that some individuals are sensitive to stress-induced suppression of reproductive hormone secretion, while others are stress-resilient. We designate animals ‘highly stress-resilient’ (HSR) if they maintain normal menstrual cyclicity when exposed to two cycles of combined stress; or medium stress-resilient (MSR) if they are ovulatory in the first stress cycle, but anovulatory in the second stress cycle, or stress-sensitive (SS) if they become anovulatory as soon as stress is initiated. These designations are straightforward, but they apply only to our model of FHA at this time. We have shown that compared to HSR animals, the MSR+SS animals have slightly higher daytime cortisol levels and show a larger increase in cortisol when stressed, which is blocked by antalarmin (Herod et al., 2011a, Herod et al., 2011b).
Two major neural systems that are involved in the mediation of stress are (1) the serotonin system and (2) the corticotropin releasing factor (CRF) system. Underpinning serotonin neurotransmission is the expression of genes that determine synthesis, uptake, degradation and autoreceptors. Fev is an ETS domain transcription factor that determines whether a neuron is serotonergic, and functions specifically in the differentiation and maintenance of serotonin neurons (Hendricks et al., 1999, Hendricks et al., 2003). Tryptophan hydroxylase (TPH2) is the rate limiting enzyme in serotonin synthesis; the serotonin reuptake transporter (SERT) governs the re-uptake of serotonin from the synapse and beyond; monoamine oxidases A and B (MAO-A, MAO-B) catalyze the degradation of serotonin and other biogenic amines; and the 5HT1A autoreceptor decreases serotonin neuronal excitation. Dysfunction of serotonin-related gene products has been documented in patients with depression, anxiety or impulse disorders (Mann et al., 2001, Arango et al., 2002) and in animal models of depression, anxiety and impulsive behavior (Champoux et al., 2000). Individual polymorphisms in these genes have also been linked to affective disorders in humans (Arango et al., 2003, Souery et al., 2003, D’Souza and Craig, 2008). In animal models, a number of these polymorphisms interact with the environment, called gene x environment interactions, which in turn appear to produce alterations in behavior resembling neuropsychiatric disorders (Newman et al., 2005).
In many stressful situations the hypothalamic-pituitary-adrenal (HPA) axis becomes activated with increases in CRF, ACTH and cortisol, and CRF may inhibit GnRH release (Williams et al., 1990, Wang and Millam, 1999, Dobson et al., 2003). The CRF system has been implicated in the etiology of depression (Morimoto et al., 1993, Weiss et al., 1994, Nemeroff, 2004b), and a stress-induced elevation in corticotropin releasing factor (CRF) in the hypothalamic paraventricular nucleus (PVN) is thought to underlie the hyperactivity of the HPA axis in depression (Holsboer, 1999, Keck and Holsboer, 2001, de Kloet et al., 2005). In clinical studies, individuals with depression, anxiety or suicide exhibited more CRF neurons in the hypothalamus than normal individuals (Raadsheer et al., 1994, Bao et al., 2005). Moreover, CRF neurons and fibers are found in numerous limbic structures outside of the hypothalamus, including the dorsal raphe nucleus, and they mediate stress responses (Frim et al., 1990, Delville et al., 1992, Keegan et al., 1994).
Another peptide system involved in the regulation of stress and anxiety is the urocortin system, which bears some homology with CRF (Vaughan et al., 1995). Three urocortin (UCN) peptides have been characterized; UCN1, UCN2 and UCN3; and both CRF and UCN1 fibers innervate the primate dorsal raphe nucleus (Vasconcelos et al., 2003, Linthorst, 2005). UCN1 is involved in the stress adaptation response (Kozicz, 2003) and in stress-induced anxiety (Skelton et al., 2000). Moreover, UCN1 neurons respond to alcohol and may affect alcohol preference and consumption (Ryabinin and Weitemier, 2006).
Hence, the ‘CRF system’ is comprised of several related ligands and receptors. The ligands are CRF and the urocortins 1, 2 and 3 (UCN1, UCN2, UCN3). CRF and urocortins mediate their effects by activating two known G-protein coupled receptors, CRF-R1 and CRF-R2 (Dautzenberg and Hauger, 2002). CRF has a higher affinity for CRF-R1 than CRF-R2. In contrast, UCN1 binds CRF-R2 with higher affinity than it binds CRF-R1 (Vaughan et al., 1995). The distribution of the CRF-R1 and CRF-R2 receptors is distinct and implies diverse physiological functions, as evidenced by the divergent phenotypes of CRF-R1- and CRFR2-null mice (Smith et al., 1998, Timpl et al., 1998, Muller et al., 2003, Keck et al., 2005). It is thought that CRF-R1 mediates anxious behavior and the HPA axis response to stress, while CRF-R2 mediates stress-coping behaviors such as anxiolytic behaviors, dearousal, and cardioprotection (Valdez et al., 2002). Therefore, either inhibition of CRF-R1 and/or stimulation of CRF-R2 could decrease stress sensitivity, anxiety and related depressive behaviors. In addition, CRF and UCN1 also interact with binding proteins, CRFBP and sCRFR2α (Behan et al., 1995b). Each binding protein binds CRF or UCN1 extracellularly and is thought to prevent receptor activation (Behan et al., 1995a, Kemp et al., 1998).
In the past decade, the relationship between CRF signaling and serotonin transmission has attracted significant interest. There is a prominent serotonergic projection to the PVN (Petrov et al., 1992, Hanley and Van de Kar, 2003) where 5HT2A and 5HT2C receptors are robustly expressed in monkeys (Gundlah et al., 1999). There is also a reciprocal CRF projection from the PVN to the midbrain dorsal and median raphe nuclei, the location of serotonin neurons that project to the forebrain (Luiten et al., 1985, Portillo et al., 1998). In a human study, CRF fibers and terminals apposed serotonin cell bodies and primary dendrites in the raphe region (Ruggiero et al., 1999). In rodents, CRF-R1 and CRF-R2 receptors have been observed in the dorsal raphe nucleus. Once released into the raphe, CRF and UCN1 bind to CRF-Receptor 1 (CRF-R1) and CRF-Receptor 2 (CRF-R2) and regulate serotonin neurotransmission (Hammack et al., 2003a, Hammack et al., 2003b, Clark et al., 2007). However, CRF has complex and opposing effects depending on the dose used and the endpoint examined (Pernar et al., 2004).
In a previous group of characterized monkeys, we found that in non-stressed conditions there are differences in the functioning of these key neural systems in stress-sensitive vs. stress-resilient monkeys [reviewed in (Bethea et al., 2008)]. Stress-sensitive animals chronically have lower release of serotonin (5HT) in response to fenfluramine (Bethea et al., 2005a), and have a down regulation of the central serotonergic system as indicated by less serotonin reuptake transporter (SERT) gene expression, less 5HT-1A receptor gene expression and less expression of the genes that degrade serotonin (MAO-A and MAO-B) in the raphe nucleus (Bethea et al., 2005b). Moreover, stress-sensitive animals exhibited significantly elevated CRH gene expression (Centeno et al., 2007) in the PVN of the hypothalamus. As described above, the PVN sends a CRF projection to the midbrain serotonin system, suggesting that stress-sensitive animals would have an increase in CRF fibers in the raphe.
Recently, we characterized a new population of monkeys as stress-sensitive and stress-resilient, and then treated them with escitalopram (s-citalopram) or placebo for 15 weeks in the absence of stress (Cameron et al., 2004). S-citalopram is an antidepressant drug used to treat major depressive disorder, generalized anxiety disorder, social anxiety disorder, or panic disorder. S-citalopram belongs to a class of drugs known as selective serotonin reuptake inhibitors (SSRIs); it is the S-stereoisomer (enantiomer) of the earlier mixed isomer formulation, and it has the highest affinity for the transporter of any of the SSRIs. Since there was an increase in estradiol and progesterone secretion only in the SS group treated with s-citalopram, it suggested that central neural drive to the reproductive axis was improved with s-citalopram treatment (Cameron et al., 2004). For convenience, we will refer to s-citalopram as citalopram or CIT.
How citalopram action was transduced to an increase in ovarian steroid secretion became of interest. The pharmacological actions of citalopram indicated that the serotonin system was a primary cite of action. Therefore, we examined the expression of 4 pivotal genes that regulate the function of the serotonin neural system, Fev, TPH2, SERT and 5HT1A. Because we had observed higher CRF gene and protein expression in the PVN of stress-sensitive animals, we also questioned whether citalopram affected the CRF and UCN1 stress-related peptide projection systems in the midbrain raphe region. Finally, we examined the expression of the CRF-R2 receptor in the dorsal raphe.
Based upon the menstrual response to the combined psychosocial and metabolic stress, the monkeys were labeled highly stress resilient (HSR, n=8), medium stress resilient (MSR, n=4), stress sensitive (SS, n=13). CIT or placebo was administered for 15 weeks after the animals were returned to control conditions. Hence, they received CIT in non-stressed conditions. Serum concentrations of CIT were maintained in a target range of 20-40 ng/ml (Lima et al., 2009). Menstrual cyclicity and reproductive hormones were monitored throughout the citalopram treatment.
The citalopram treatment was physiologically efficacious as indicated by an improvement in ovarian steroid secretion (Cameron et al., 2004). The groups that showed some sensitivity to stress (MSR and SS groups) were combined for hormone measurements only (n=17) and compared to the highly stress resilient group (n=8). In stress sensitive monkeys, citalopram treatment significantly increased peak estradiol levels in the follicular phase of the menstrual cycle from 360±67 to 544±82 pg/ml (p < 0.05) and increased peak progesterone levels in the luteal phase of the menstrual cycle from 6.7±1.4 to 11.3± 1.8 ng/ml (p < 0.05). In contrast, vehicle- and citalopram-treated stress-resilient monkeys had similar peak estradiol and peak progesterone, before and after treatment, which averaged 575±100 pg/ml and 11.5±3 ng/ml, respectively (Cameron et al., 2004).
For neuroanatomical analysis, we only compared the ends of the spectrum, that is HSR and SS animals. A 2 × 2 block design was used with an n of four in each group, that is HSR+Placebo, HSR+Citalopram, SS+Placebo and SS+Citalopram. One of the blocks from the HSR+Citalopram group was apparently poorly perfused and adequate signals were not obtained, which left 3 animals in the HSR+citalopram group and 4 animals in all of the other groups for further analysis.
In situ hybridization (ISH) assays with digoxigenin were applied to determine the expression of Fev and CRF-R2 (Berg-von der Emde et al., 1995, Lima et al., 2009). In situ hybridization (ISH) assays with 35S-UTP were applied to determine the expression of TPH2, SERT and 5HT1A (Lima et al., 2009). Immunocytochemical (ICC) assays were applied to examine the innervation of the raphe by CRF and UCN1 axons as previously described (Weissheimer, 2010). Densitometric and stereological analysis was applied to quantify the ISH and ICC assays as previously described (Lima et al., 2009, Weissheimer, 2010).
Fev
Fev (a homolog of Pet1 in rats) is an ETS domain transcription factor that determines whether a neuron is serotonergic; and it functions specifically in the differentiation and maintenance of serotonin neurons. Fev is absolutely necessary for a neuron to become serotonergic and it is not expressed in any other neurons of the CNS. Indeed, the rodent homolog of Fev marks and is restricted to, the entire rostral-caudal extent of the rat serotonergic hindbrain raphe complex (Hendricks et al., 1999). Conserved Fev binding sites are present in or near the promoter regions of the human and mouse TPH2, 5-HT1A receptor, serotonin transporter, and aromatic L-amino acid decarboxylase genes, whose expression is characteristic of the serotonergic neuron phenotype (Hendricks et al., 1999, Hendricks et al., 2003). We questioned whether stress-sensitivity or citalopram affected Fev gene expression.
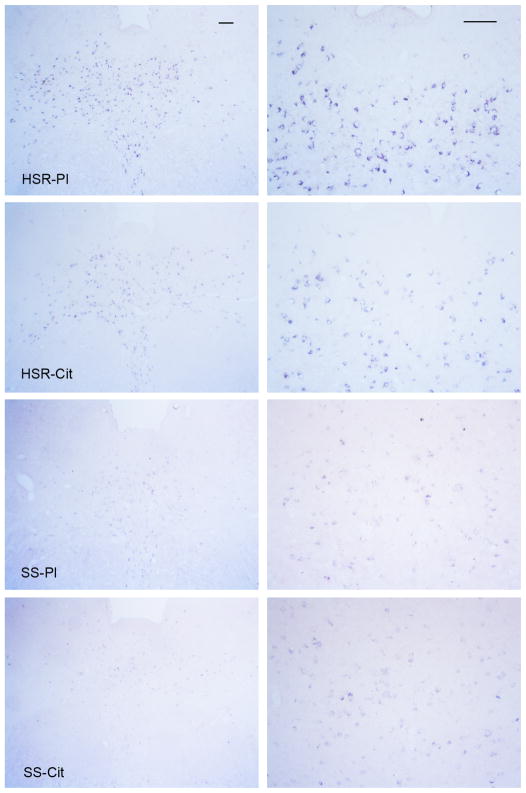
As illustrated in Figure 2, there was robust expression of Fev in the HSR groups compared to the SS groups. Fev expression was restricted to the neurons of the raphe nuclei and the presence of Fev indicates that these neurons are solely serotonergic. Fev expression was significantly lower and the number of detectable Fev-positive neurons was reduced in the stress-sensitive monkeys compared to stress-resilient monkeys, but citalopram had no effect in either group (Figure 3). Thus, stress-sensitive macaques have reduced expression of the gene that governs serotonin-related gene expression and neuron function, but a change in Fev gene expression is not involved in the citalopram-induced increase in ovarian steroid secretion. The reduction in Fev-positive cell number strongly suggests that stress-sensitive animals have fewer serotonin neurons. It may be argued that serotonin neurons with little or no Fev expression were missed, but this seems to be a contradiction in terms. Other reports contend that Fev/Pet1 is a key transcriptional regulator of genes required specifically for the terminal serotonergic phenotype (Hendricks et al., 1999). Extending this line of reasoning (with a very sensitive assay), one could deduce that if a neuron does not have detectable Fev expression, then it is unlikely to be a serotonin neuron. This observation explains why stress-sensitive monkeys have lower expression of all of the serotonin-related genes and why, even with lower but adequate levels of ovarian steroids, they have compromised serotonin function. Very likely, this type of deficit has a developmental component.
Figure 2.
Illustration of Fev expression in the rostral dorsal raphe in representative monkeys from each treatment group at two magnifications. Digoxigenin in situ hybridization was applied. The scale bar equals 100 μm. There is robust expression of Fev in the HSR-placebo (HSR-Pl) and HSR-citalopram (SHR-Cit) groups, but Fev expression is markedly reduced in the SS-placebo (SS-Pl) and SS-citalopram (SS-Cit) groups. From (Lima et al., 2009).
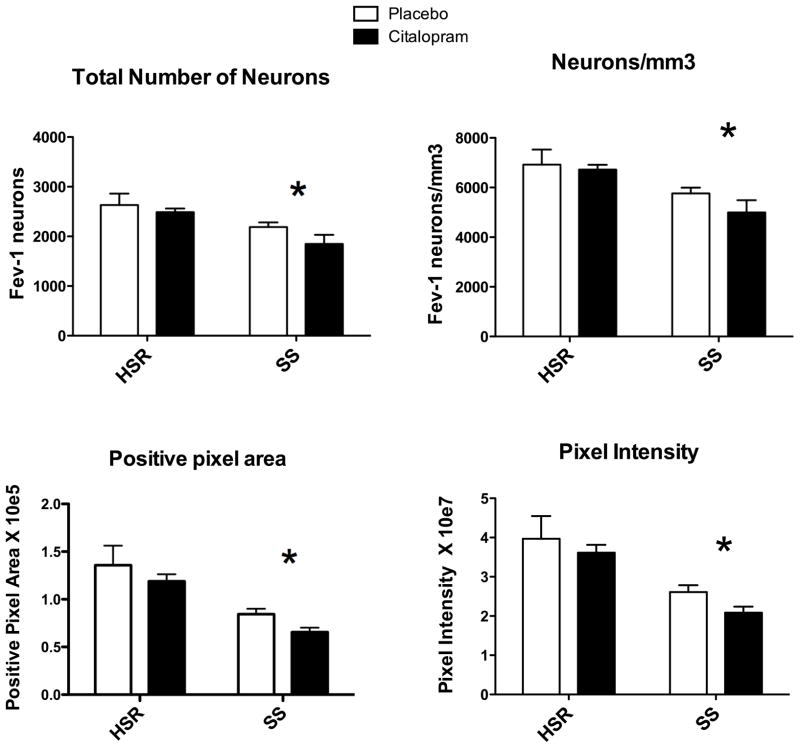
Figure 3.
Overall average of several Fev parameters for each group (n=3–4/group). From (Lima et al., 2009)
Top Left - The number of neurons in each level was summed generating the total number of neurons per animal. The mean of the total number of neurons for each group is illustrated. There was a significant effect of stress sensitivity (*p = 0.008), but no effect of treatment (p= 0.18) and no interaction (p=0.57).
Top Right - The number of Fev positive serotonin neurons per mm3 volume is illustrated. There was a significant effect of stress sensitivity (*p = 0.008), but no effect of treatment (p= 0.31) and no interaction (p=0.54).
Bottom Left - The average positive pixel area is illustrated. There was a significant effect of stress sensitivity (*p = 0.001), but no effect of treatment (p= 0.167) and no interaction (p=0.938).
Bottom Right - The intensity of each positive pixel was obtained and summed for each level. The average of the summed pixel intensity across all 8 levels was obtained for each animal and this was used to obtain the group means of positive pixel intensity as illustrated. There was a significant effect of stress sensitivity (*p = 0.002), but no effect of treatment (p= 0.23) and no interaction (p=0.81).
TPH2
TPH2 is the rate limiting or committal enzyme in the overall synthesis of serotonin in the brain and it converts the precursor, tryptophan to 5-hydroxytryptophan, which is then converted to 5-hydroxytryptamine or serotonin. Hence, the level or activity of this pivotal enzyme plays a role in the concentration of serotonin achieved in cell bodies. It appears that newly synthesized 5-HT is preferentially released. However, the release of 5-HT is dependent on neuronal activity, and it is not always linked to the synthesis of 5-HT (Hery and Ternaux, 1981). We questioned whether stress-sensitivity or citalopram affected TPH2 gene expression with radioactive ISH.
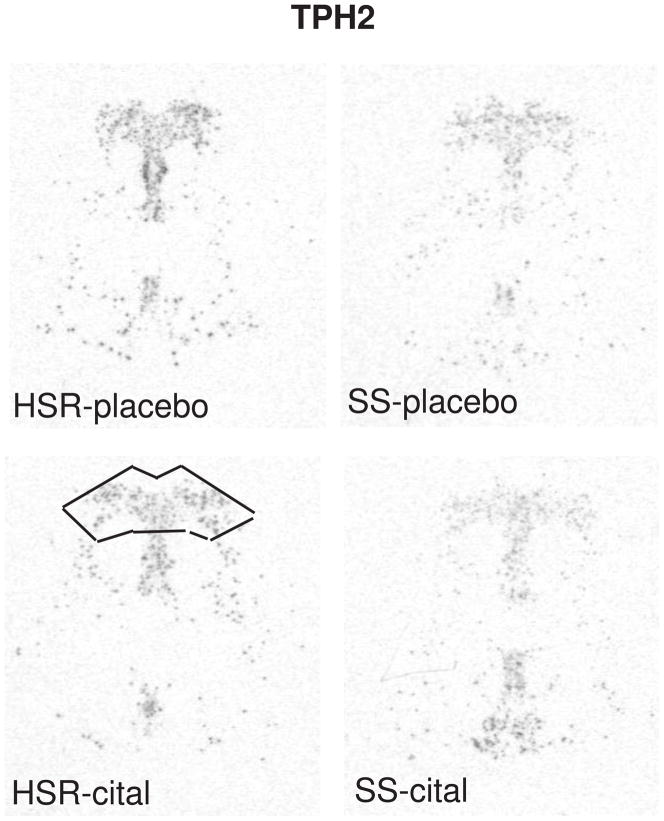
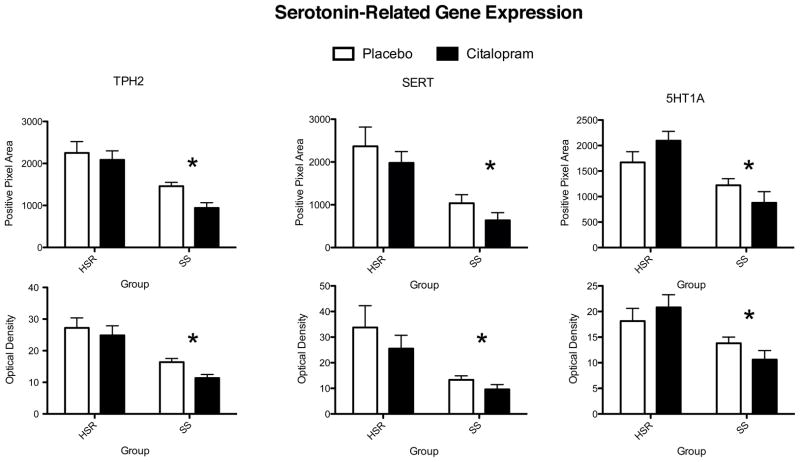
The autoradiographic signal for TPH2 mRNA at a rostral level of the dorsal raphe nucleus is illustrated in Figure 4. TPH2 gene expression was lower in the SS animals compared to the HSR animals, but citalopram treatment for 15 weeks did not alter TPH2 gene expression (Figure 7). This indicates that serotonin synthesis in stress-sensitive monkeys is compromised and that a change in TPH2 gene expression is not involved in the citalopram-induced increase in ovarian steroid secretion. We previously showed that stress-sensitive monkeys secrete less prolactin than stress-resilient monkeys after challenge with fenfluramine, a serotonin releaser and reuptake blocker (Bethea et al., 2005a), indicating that stress-sensitive monkeys have lower available serotonin than stress-resilient monkeys. The lower expression of TPH2 mRNA may provide a molecular basis for the lower availability of serotonin, and together the data indicate that serotonin synthesis is decreased in stress-sensitive individuals. Consistent with these observations, tryptophan depletion and reduction of serotonin causes transient depression only in sensitive individuals (Hasler et al., 2004). The decrease in TPH2 expression is due in part to the reduction in serotonin cell number, but close examination of the autoradiographs also suggests that TPH2 expression is reduced in individual cells.
Figure 4.
In situ hybridization autoradiograms of TPH2 signal in the dorsal raphe nucleus of representative HSR and SS macaques treated with placebo or citalopram for 15 weeks. Scattered neurons of the rostral median raphe are also visible underneath the decussation of the cerebellar peduncles. The box illustrates the area analyzed at this morphological level. The same box was applied to all sections at this level. Each of the 7 levels had a different sized box that was held constant for all of the sections at that morphological level. From (Lima et al., 2009).
Figure 7.
Histograms illustrating the average positive pixels and optical density of TPH2, SERT and 5HT1A signal in the dorsal raphe nucleus of HSR and SS macaques treated with placebo or citalopram for 15 weeks. For all genes, there was a significant effect of stress sensitivity on optical density and on positive pixel area. However, there was no effect of drug treatment on optical density or on positive pixel area and there was no interaction. From (Lima et al., 2009).
| Stress Sensitivity | Drug Treatment | Interaction | ||||
|---|---|---|---|---|---|---|
| OD | Positive pixel area | OD Positive pixel area | OD | Positive pixel area | ||
| TPH2 | *p=0.0002 | *p=0.0003 | p=0.13 | p= 0.10 | p=0.37 | p=0.57 |
| SERT | *p=0.005 | *p=0.001 | p=0.27 | p= 0.22 | p=0.66 | p=0.98 |
| 5HT1A | *p=0.004 | *p=0.001 | p=0.89 | p= 0.83 | p=0.17 | p=0.072 |
Deneris and colleagues have shown in mice that the TPH2 promoter region contains Fev binding sites and that in adults Fev is still a critical transcription factor for the transcription of TPH2 (Hendricks et al., 1999, Hendricks et al., 2003, Liu et al., 2010). Thus, the lower expression of Fev may underlie the lower expression of TPH2 in stress-sensitive monkeys.
TPH enzyme activity is regulated by posttranslational phosphorylation by protein kinase A at serine 58 (Johansen et al., 1995, 1996). Moreover, only phosphorylated TPH can interact with an accessory protein called 14-3-3 and this interaction is absolutely necessary for catalytic activity (Banik et al., 1997). So, there are many other avenues by which serotonin synthesis could be regulated in addition to gene expression. Recently, new knowledge regarding environmental alteration of methylation in the genome has emerged (Fuchikami et al., 2010, Lesch, 2011). We have speculated that the animals become stress-sensitive due to early life trauma, which may lead to changes in methylation and in turn, transcription. Investigation of methylation on the TPH2 gene and the impact of environment are needed.
SERT
The primary means by which serotonergic neurons control extracellular serotonin concentrations is via the serotonin reuptake transporter (SERT), which acts to reduce the concentration of serotonin in the synaptic cleft (Blakely et al., 1994). Removal of serotonin from the extracellular space and terminating serotonin neurotransmission are the major tasks of SERT (Blakely et al., 1994). The SERT proteins are predominantly located presynaptically at serotonin nerve terminals (Hoffman et al., 1998), but SERT protein is also present on serotonin cell bodies and dendrites for uptake of serotonin released somato-dendritically (Blakely et al., 1994, Hoffman et al., 1998). SERT is also found along axons, but whether it is functioning or being transported is uncertain. It has been suggested that axonal SERT is functional and it contributes to extrasynaptic transmission (Tao-Cheng and Zhou, 1999, Lu et al., 2003). Dysfunction of SERT-mediated uptake of serotonin has been implicated in depression and anxiety disorders (Siever et al., 1991). Moreover, SERT is the site of action of widely used antidepressants known as selective serotonin reuptake inhibitors (Barker and Blakely, 1995). Therefore, we questioned whether stress-sensitivity or citalopram affected SERT gene expression.
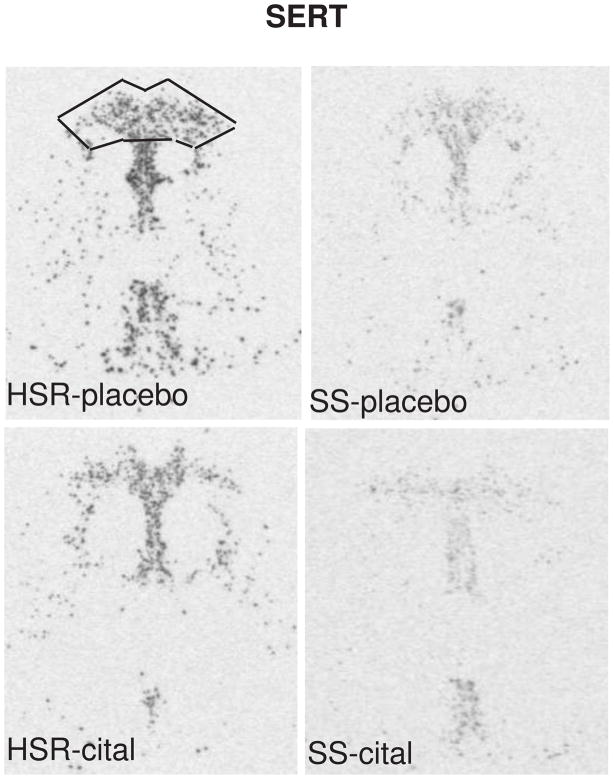
The autoradiographic signal for SERT mRNA at a rostral level of the dorsal raphe nucleus is illustrated in Figure 5. SERT was robustly expressed in the HSR animals treated with placebo or citalopram. There was an apparent decrease in SERT mRNA in the SS animals treated with placebo or citalopram. Quantitatively, SERT gene expression was lower in the SS animals compared to the HSR animals, but citalopram treatment for 15 weeks did not alter SERT gene expression (Figure 7). This indicates that serotonin transporter synthesis in stress-sensitive monkeys may be compromised, but a change in SERT gene expression is not involved in the citalopram-induced increase in ovarian steroid secretion. The lower expression of SERT mRNA expression in SS monkeys compared to HSR monkeys, independently confirms our earlier report with a different cohort of monkeys (Bethea et al., 2005b). If the lower level of mRNA translates to a lower level of SERT protein expression, then stress-sensitive monkeys would have markedly reduced SERT protein and function. The expression of SERT appears to correlate with the level of serotonin produced. Although somewhat paradoxical, in cases where serotonin is compromised, SERT is also reduced. Several approaches have shown that there is a reduced density of SERT in patients with depression and in the postmortem brain tissue of suicide victims (Lawrence et al., 1990, Drevets et al., 1992, Hrdina et al., 1993, Arango et al., 2002). Regulation of the transporter protein in the presynaptic membrane is more dependent on the concentration of serotonin in the synapse than driven by gene expression according to the “use it or lose it” hypothesis of Blakely and colleagues (Blakely and Bauman, 2000, Blakely et al., 2005, Steiner et al., 2008). That is, amine reuptake is a regulated process in synaptic plasticity and not just a determinant of transmitter clearance. Citalopram, thought to increase synaptic serotonin, may have caused an increase in transporter protein. However, this appears to have little impact on gene expression.
Figure 5.
In situ hybridization autoradiograms of SERT signal in the dorsal raphe nucleus of representative HSR and SS macaques treated with placebo or citalopram for 15 weeks. Scattered neurons of the rostral median raphe are also visible underneath the decussation of the cerebellar peduncles. The box illustrates the area analyzed at this morphological level. The same box was applied to all sections at this level. Each of the 7 levels had a different sized box that was held constant for all of the sections at that morphological level. From (Lima et al., 2009).
Much attention has been placed on a polymorphism in the SERT gene, which may lead to a decrease in SERT expression (Bondy et al., 2000). However, cynomolgus macaques do not have the orthologus deletion event. Rather, all cynomolgus macaques have the long allele (Bethea et al., 2005b). Hence, the SERT polymorphism is not responsible for the decrease in SERT mRNA in these stress-sensitive monkeys. Nonetheless, it is attractive to speculate that early life experience may alter the methylation of SERT contributing to stress-sensitivity in our model of FHA. Even in nonhuman primates, mothering skills differ. Recent studies found that the methylation status of the SERT promoter was significantly lower in human mothers with depressed mood symptoms at 26 weeks gestation (Devlin et al., 2010). In addition, childhood trauma may create long-lasting changes in methylation of the promoter region of 5HTT in women (Beach et al., 2010, 2011).
Recent studies with conditional knockout mice indicate that SERT transcription is dependent on Fev expression in adulthood and that the SERT promoter contains Fev binding sites (Liu et al., 2010). Therefore, it is probable that the lower expression of SERT is due to the lower expression of Fev in the stress-sensitive monkeys, but 15 weeks of citalopram did not significantly affect Fev or SERT gene expression.
The human SERT protein has six phosphorylation sites that are acted upon by protein kinase C (PKC) and PKA (Blakely et al., 1998). The phosphorylation of SERT promotes transporter internalization and decreases its membrane expression and serotonin uptake (Ramamoorthy et al., 1998). The regulation of SERT through this mechanism is usage dependent. When serotonin molecules pass through the portal of SERT protein, the phosphorylation and internalization of SERT is inhibited whereas when the passage of serotonin is blocked by SSRIs (and other compounds such as cocaine and methamphetamine), the phosphorylation and internalization of the SERT accelerate (Ramamoorthy and Blakely, 1999). Hence, there could be multiple points by which SERT expression may be disrupted such as gene methylation and transcription, translation, protein phosphorylation, trafficking, and stability.
5HT1A
The most widely studied of the 5HT autoreceptors is the 5HT1A receptor. The 5HT1A autoreceptor subtype is of significant importance because it inhibits serotonin neural firing (Sprouse and Aghajanian, 1986), which leads to a suppression of serotonin synthesis, serotonin turnover and the release of serotonin in diverse projection areas (Bohmaker et al., 1993, Sharp et al., 1993, Singh and Lucki, 1993). A characteristic of compounds with medium to high efficacy for blocking 5HT1A receptors is an antidepressant-like activity when combined with selective serotonin reuptake inhibitors (Singh and Lucki, 1993). Serotonin reuptake inhibitors block serotonin reuptake via the serotonin transporter (SERT). However, the therapeutic efficacy of serotonin reuptake inhibitors is typically delayed for several weeks. Hypothetically, this delay is due at least in part to acute increases in extracellular serotonin concentrations in the raphe, which inhibit neuronal firing via the 5HT1A autoreceptor (Goodwin, 1996). Therefore, blockade of the autoreceptor may prevent the delay in therapeutic efficacy of the serotonin reuptake inhibitors, increasing their effectiveness. Indeed, a 5HT1A antagonist was found to potentiate the antidepressant effects of a serotonin reuptake inhibitor in depressed patients (Artigas et al., 1994). Hence, we questioned whether stress-sensitivity or citalopram affected 5HT1A gene expression.
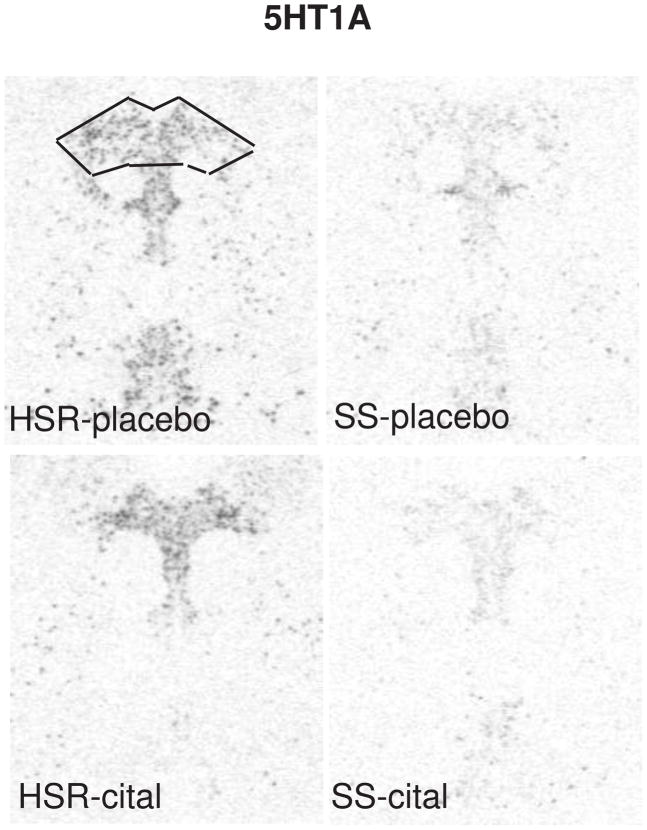
The autoradiographic signal for 5HT1A mRNA at a rostral level of the dorsal raphe nucleus is illustrated in Figure 6. 5HT1A was robustly expressed in the HSR animals treated with placebo or citalopram. There was an apparent decrease in 5HT1A mRNA in the SS animals treated with placebo or citalopram. Quantitively, 5HT1A gene expression was lower in the SS animals compared to the HSR animals, but citalopram treatment for 15 weeks did not alter 5HT1A gene expression (Figure 7). This indicates that 5HT1A autoreceptor synthesis in stress-sensitive monkeys may be compromised, but a change in 5HT1A gene expression is not involved in the citalopram-induced increase in ovarian steroid secretion. In our previous study, 5HT1A mRNA expression tended to be lower in stress-sensitive animals, but it did not reach statistical significance probably due to the small sample size and small effect (Bethea et al., 2005b). Now it is confirmed that there is a real deficit in 5HT1A gene expression in stress-sensitive individuals. Indeed, it indicates that in the previous study there was a type II error, in that we did not detect a real difference. Unfortunately, this is a risk in primate studies that are constrained by costs. In a different model, we showed that 5HT1A binding correlated with 5HT1A gene expression (Lu and Bethea, 2002). Therefore, the stress-sensitive monkeys probably have lower 5HT1A receptor density. These results are consistent with clinical studies that showed decreased 5HT1A binding in multiple brain regions of patients with depression (Drevets et al., 2000) and 5HT1A subsensitivity has been linked to late luteal phase dysphoric disorder (Yatham, 1993). Subordinate and chronically stressed male tree shrews have lower 5HT1A binding across a variety of brain regions including the hippocampus and frontal cortex (Flugge et al., 1998). Chronic stress also reduced post-synaptic 5HT1A binding in the hippocampus of rats (Watanabe et al., 1993), but there was no effect on 5HT1A autoreceptor binding in the raphe nuclei (Pare and Tejani-Butt, 1996). Paradoxically however, antidepressants are thought to decrease 5HT1A binding (Hjorth et al., 2000) and 5HT1A antagonists are beneficial adjuncts to antidepressant treatment (Singh and Lucki, 1993, Artigas et al., 1994, Romero and Artigas, 1997).
Figure 6.
In situ hybridization autoradiograms of 5HT1A signal in the dorsal raphe nucleus of representative HSR and SS macaques treated with placebo or citalopram for 15 weeks. Scattered neurons of the rostral median raphe are also visible underneath the decussation of the cerebellar peduncles. The box illustrates the area analyzed at this morphological level. The same box was applied to all sections at this level. Each of the 7 levels had a different sized box that was held constant for all of the sections at that morphological level. From (Lima et al., 2009).
It is interesting to note that in adult mice, Fev does not appear to regulate the expression of the 5HT1A autoreceptor, although during embryogenesis Fev determines 5HT1A expression in serotonin neurons (Liu et al., 2010). Nonetheless, in Fev conditional knockout mice, there was an unusual disruption of 5HT1A function in that the receptor did not bind the 5HT1A ligand, 8OH-DPAT nor inhibit neuronal firing. These data indicate that adequate expression of the receptor at gene and protein levels may not always lead to adequate function of the receptor. However, significantly reduced expression likely leads to significantly reduced receptor density. We think that the strong positive correlation between 5HT1A expression and the expression of Fev, TPH2 and SERT indicates a similar regulatory mechanism is at work. Altogether, gene expression and previous fenfluramine challenges point to overall reduced serotonin neuronal function in stress-sensitive macaques, but citalopram did not change serotonin-related gene expression.
CRF Fiber Density
The knowledge of CRF innervation of the serotonergic raphe nuclei (Sakanaka et al., 1987, Ruggiero et al., 1999) coupled with the detection of CRF receptors in the raphe (Chalmers et al., 1995, Van Pett et al., 2000) forged the link between the CRF and serotonin systems. Subsequently, the opposing effects of CRF and UCN on serotonin were established in rodents. Administration of CRF directly into the DRN inhibits serotonergic activity, and CRF-R1 antagonists block this effect (Price et al., 1998). CRF also has opposing effects on serotonin activity depending on the concentration and temporal relation to a stressor (Kirby et al., 2000, Forster et al., 2006). The stimulatory effect of UCN and CRF-R2 is supported by increased 5-HT efflux in the basolateral amygdala (a projection region of the DRN) with intra-DRN administration of the CRF-R2 agonist, UCN2. This effect was completely blocked by antisauvagine-30 (ASV-30), a relatively selective CRF-R2 antagonist (Amat et al., 2004).
CRF producing neurons and projections are widespread and play a role in stress-related psychiatric disorders (Clark and Kaiyala, 2003). CRF innervation of the serotonergic dorsal raphe nucleus has been described and CRF receptors are found in the dorsal raphe of rodents (Lowry et al., 2000, Linthorst, 2005, Valentino et al., 2009). Moreover, CRF regulates serotonin release (Summers et al., 2003, Thomas et al., 2003, Clark et al., 2007, Forster et al., 2008). Previous studies demonstrated that serotonin innervates CRF neurons in the hypothalamus (Phelix et al., 1992, Saphier et al., 1994) and newer data indicate that serotonin decreases CRF (Stout et al., 2002, Lowry et al., 2009), perhaps via inhibitory interneurons (Bethea and Centeno, 2008). Therefore, we hypothesized that citalopram increased extracellular serotonin, which decreased CRF production and delivery to extrahypothalamic regions, including the dorsal raphe. This could further facilitate serotonin neurotransmission and further decrease CRF, thereby establishing a feed forward loop.
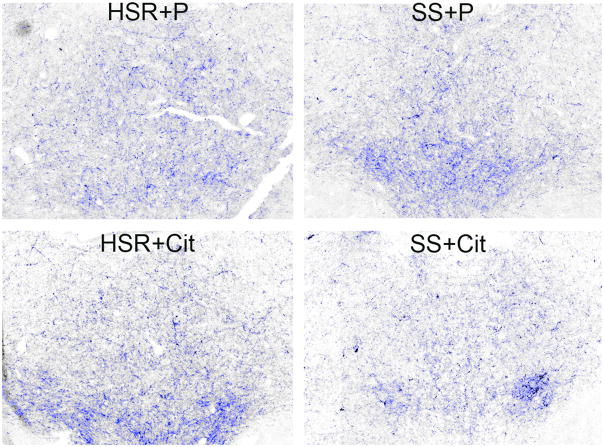
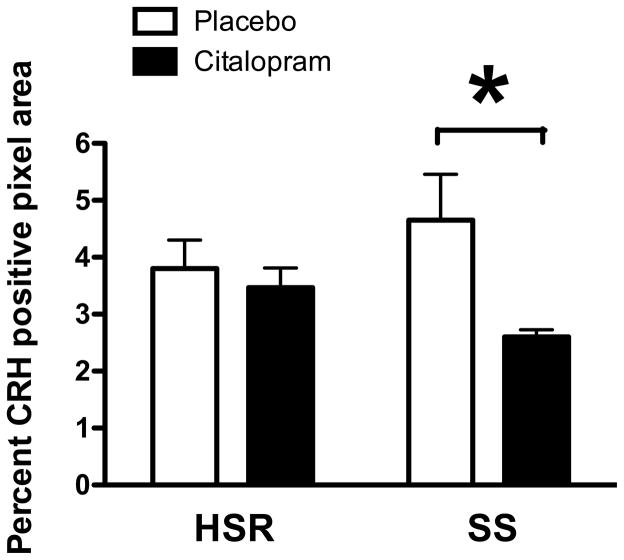
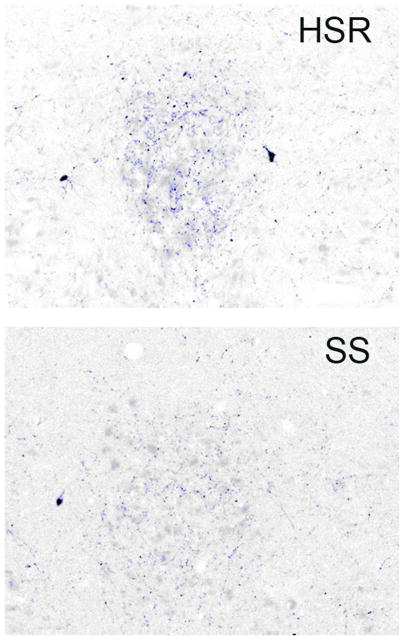
Robust CRF fiber staining was observed in the dorsal raphe nucleus as illustrated in Figure 8. The positively stained fibers were segmented and highlighted in blue. Perceptibly, there appears to be a decrease in CRF fiber density in the SS animal treated with citalopram (CIT) compared to the SS animal treated with placebo (P). Quantitatively, CRF fiber density in the dorsal raphe nucleus tended higher in stress-sensitive individuals, and the administration of citalopram reduced CRF fiber density only in the stress-sensitive group (Figure 9). The CRF innervation of the dorsal raphe is believed to originate in the caudal portion of the PVN and in the amygdala where we previously observed an increase in CRF in stress-sensitive individuals relative to stress-resilient individuals (Centeno et al., 2007). This difference was reflected in the somewhat higher CRF fiber density in the dorsal raphe of the SS animals treated with placebo. CRF inhibits serotonin, so a decrease in CRF innervation of the raphe could increase serotonin neurotransmission (apparently without affecting gene expression). Extrapolating from the current data, it is likely that citalopram reduces CRF gene and protein expression in the PVN and amygdala, which in turn leads to a decrease in CRF fiber density in the raphe. Although a similar effect has been observed in rodents with other antidepressants (Stout et al., 2002, Lowry et al., 2009), the mechanism of action has not been addressed. We speculate that SSRI’s elevate serotonin in the synaptic cleft, which in turn decreases CRF production and neurotransmission. Further studies to look at CRF in the hypothalamus and thalamus from this same cohort of animals are planned.
Figure 8.
Photomicrographs of CRF fiber staining in the dorsal raphe nucleus. Stereology montage (×10) of CRF fiber staining in the dorsal raphe nucleus in a representative animal from each treatment group is as follows: HSR+P - Highly stress resilient treated with placebo; HSR+Cit- highly stress resilient treated with s-citalopram; SS+P - stress sensitive treated with placebo; SS+Cit - stress sensitive treated with s-citalopram. Sections were immunostained for CRF and segmented into positive- and negative stained pixels. Positive pixels are highlighted in blue. Cell bodies or debris were erased prior to pixel quantitation. Visually, there appeared to be a lower density of CRF fibers in the SS+Cit treated animal. From (Weissheimer et al., 2010).
Figure 9.
Average CRF-positive pixels across 4 levels of the dorsal raphe nucleus in all groups expressed as percent of total area examined. From (Weissheimer et al., 2010). There was a significant effect of treatment (p=0.0496), but no effect of stress sensitivity (p=0.6755) and no interaction (p=0.3027). SS+CIT group exhibited significantly lower CRF fiber density compared to SS+placebo. Asterisk represents a significant difference (Bonferroni, p<0.05).
UCN1 Fiber Density
UCN1 decreases feeding behavior, and also participates in the stress response (Spina et al., 1996, Oki and Sasano, 2004, Pan and Kastin, 2008). UCN1 binds to CRF-R2 with greater affinity than CRF suggesting that UCN1 preferentially binds CRF-R2. Both UCN1 knockout mice and CRF-R2 knockout mice exhibit increased anxiety-like behavior (Pan and Kastin, 2008). Thus, it has been generalized that activation of CRF-R1 by CRF increases anxiety-like behavior whereas activation of CRF-R2 by UCN1 decreases anxiety-like behavior. CRF-R1 and R2 receptors are present in the dorsal raphe of macaques (Sanchez et al., 2010). Therefore, we hypothesized that UCN1 fiber density would be lower in SS animals and that CIT would increase UCN1 fiber density.
A small, but fairly consistent UCN1 fiber plexus amenable to measurement was detected in an area between the EW nucleus and the dorsal raphe nucleus near the serotonergic caudal linear nucleus. Sections of the midbrain that were rostral to the dorsal raphe nucleus and encompassing the caudal linear nucleus, containing the UCN1 fiber plexus were used for analysis. UCN1 fiber staining in this area from representative HSR and SS animals is illustrated in Figure 10. UCN1 fiber density was significantly lower in SS than HSR animals. However, citalopram had no effect on UCN1 fiber density although this little plexus may not represent UCN1 fiber density in other regions (data not shown). Several studies have examined the effect of stress on UCN1. Our observation of different expression of UCN1 in SS and HSR animals suggests that the reverse is also true. That is, UCN1 is higher in stress-resilient individuals and it may ameliorate the response to stress. Norepinehprine and the locus ceruleus have also been implicated in stress and CRF-related peptide interactions (Koob, 1999) and cholinergic neurons in the EW nucleus are adjacent to UCN1 neurons, as well (May et al., 2008). Clearly, the afferent innervation of the midbrain UCN1 neurons needs to be defined.
Figure 10.
Photomicrographs of UCN1-positive fibers in the midbrain, rostral to the dorsal raphe nucleus, in a representative highly stress-resilient (HSR) animal and a stress-sensitive (SS) animal. Sections were immunostained for UCN1 and segmented into positive- and negative stained pixels. Positive pixels are highlighted in blue. Cell bodies or debris were erased prior to pixel quantitation. Visually, there were few UCN1 fibers in the SS groups. From (Weissheimer et al., 2010).
CRF-R2
CRF receptors have been detected in the raphe of rodents (Chalmers et al., 1995, Van Pett et al., 2000) and primates (Sanchez et al., 2010). The opposing effects of CRF and UCN on serotonin were established in rodents. As mentioned earlier, there are two types of CRF receptors, CRF-R1 and CRF-R2. Administration of CRF directly into the DRN inhibits serotonergic activity, and CRF-R1 antagonists block this effect (Price et al., 1998). CRF also has opposing effects on serotonin activity depending on the concentration and temporal relation to a stressor (Kirby et al., 2000, Forster et al., 2006). Conversely, the stimulatory effect of UCN acting via CRF-R2 was supported by increased 5-HT efflux in the basolateral amygdala (a projection region of the DRN) with intra-DRN administration of the CRF-R2 agonist, UCN2. This effect was completely blocked by antisauvagine-30 (ASV-30), a relatively selective CRF-R2 antagonist (Amat et al., 2004). Following our observation that citalopram decreased CRF innervation of the dorsal raphe, we questioned whether the CRF receptors in the dorsal raphe were also regulated. We hypothesized that stress-sensitive animals would exhibit lower levels of anxiolytic CRF-R2 compared to stress-resilient animals, and that administration of citalopram would elevate CRF-R2. CRF-R2 expression was examined with digoxigenin ISH.
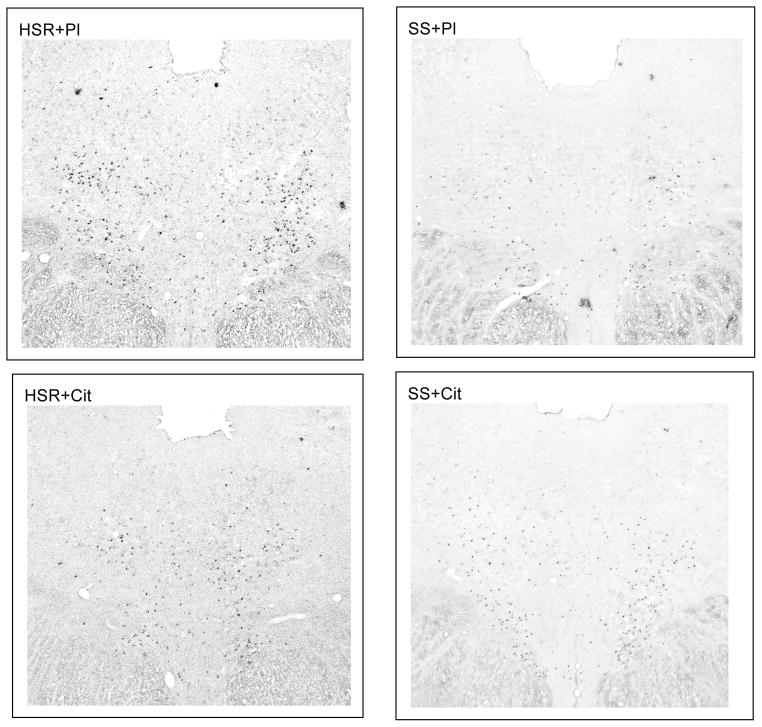
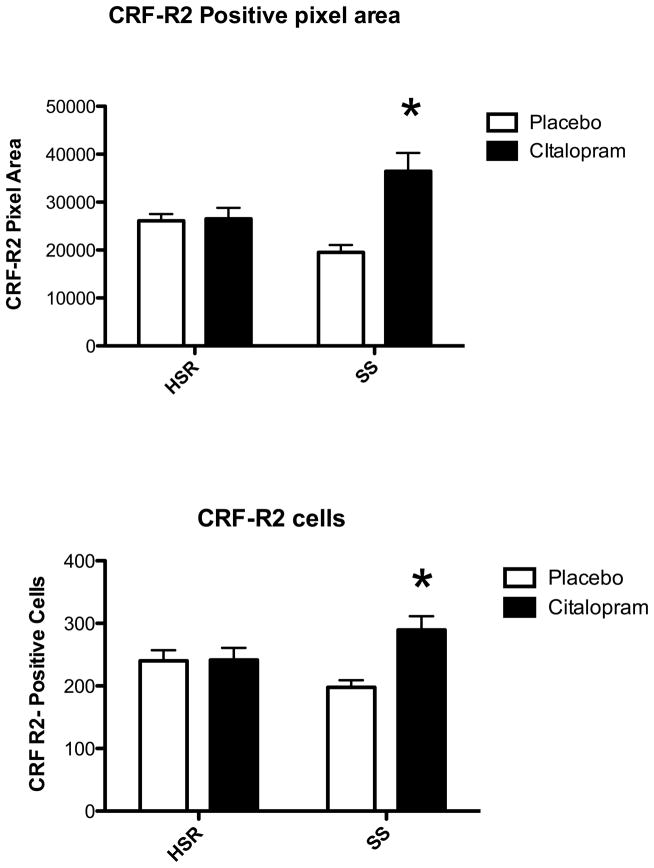
CRF-R2 expression was robust and representative sections from an animal in each group are illustrated in Figure 11. The entire dorsal raphe nucleus was outlined for analysis excluding the dorsal raphe intrafascicularis. We obtained the CRF-R2 positive pixel area and the number of CRF-R2 positive cells. CRF-R2 tended lower in the placebo treated SS animals compared to the placebo treated HSR animals. There was a significant increase in CRF-R2 positive pixel area and in the number of CRF-R2 positive neurons only in stress-sensitive monkeys treated with citalopram (Figure 12).
Figure 11.
Photomicrographs of CRF-R2 digoxigenin ISH in a representative animal from each group at a level that was midway between the most rostral and most caudal sections examined. The stress sensitive animal treated with placebo (SS+Pl) shows fewer strongly positive neurons than the stress resilient animal treated with placebo (HSR+Pl). Treatment of the stress-sensitive animal with citalopram (SS+Cit) appeared to increase the number of strongly positive cells over placebo treatment. However, treatment of the stress-resilient animal with citalopram (HSR+Cit) had little effect compared to HSR+Pl.
Figure 12.
Top. Average CRF-R2-positive pixels across 5 levels of the dorsal raphe in all groups. There was no effect of stress sensitivity (p=0.50), but there was a significant effect of citalopram treatment (p=0.004) and a significant interaction. (p=0.0058). The SS+CIT group exhibited significantly higher CRF-R2 positive pixels compared to the other groups. Asterisk represents a significant difference (Bonferonni, p<0.05). Bottom. Average number of CRF-R2-positive cells across 5 levels of the dorsal raphe in all groups. There was no effect of stress sensitivity (p=0.87), but there was a significant effect of citalopram treatment (p=0.023) and a significant interaction. (p=0.0259). The SS+CIT group exhibited significantly higher CRF-R2 positive pixels compared to the other groups. Asterisk represents a significant difference (Bonferonni, p<0.05).
In rodents, double ISH revealed that CRF-R2 was expressed exclusively in serotonin neurons at midlevels of the dorsal raphe, whereas, at caudal levels, CRF-R2 was expressed in both serotonin and GABAergic neurons (Day et al., 2004). In addition, administration of UCN2 into the DRN increased c-fos expression in labeled 5-HT neurons (Amat et al., 2004). In a different model, we have shown that CRF-R1 and CRF-R2 are expressed and regulated in laser captured serotonin neurons from macaques (Sanchez et al., 2010). Hence, it is likely that the increase in CRF-R2 expression that we observed in stress-sensitive monkeys occurred on serotonin neurons. How citalopram increased gene expression of CRF-R2 but not Fev, TPH2, SERT or 5HT1A in serotonin neurons is unresolved.
Apposing the expression of UCN1 and its receptor, CRF-R2, in this model is complex, but may be approached. We observed significantly higher UCN1 and slightly higher CRF-R2 in SR animals compared to SS animals. This suggests that UCN1 and CRF-R2 may contribute to stress-resilience. Administration of citalopram increased CRF-R2 in SS animals, but not in SR animals and UCN1 was not affected. Together, these actions may balance the interaction of UCN1 with CRF-R2 towards stress-resilience. In addition, CRF was reduced by citalopram and studies are underway to determine the expression of CRF-R1.
Overview and Correlations
The neurobiological basis of individual differences in stress sensitivity and the potential pharmacological remediation is an important issue in obvious fields such as psychiatry and immunology. A large body of clinical research has found that individuals may be stress sensitive or stress resilient, and that stress sensitive individuals have an increased incidence of mood and anxiety disorders. More recently, gynecologists have recognized that individual differences in stress sensitivity play a role in the pathology of infertility (Giles and Berga, 1993, Marcus et al., 2001). We used a nonhuman primate model to study stress sensitivity, which was defined as cessation of ovulation due to a combination of psychosocial (relocation) and metabolic (diet) stress. Genetic, environmental, neurobiological and psychosocial factors contribute to stress resilience and stress sensitivity in humans and animal models. Of the neurobiological factors contributing to stress sensitivity, changes in functional aspects of the serotonin and CRF systems play a leading role.
We previously showed that monkeys who are sensitive to stress-induced amenorrhea have reduced secretion of prolactin after fenfluramine challenge, fewer serotonin neurons and decreased expression of SERT and 5HT1A expression (Bethea et al., 2008). We also showed that monkeys who are sensitive to stress-induced amenorrhea exhibit significantly more CRF mRNA and CRF protein in the caudal region of the PVN, significantly more CRF mRNA in the thalamus and a significantly higher CRF fiber density in the central nucleus of the amygdala relative to stress-resilient animals under nonstressed basal conditions (Centeno et al., 2007). In contrast, rodent strains with different sensitivities to stress exhibit no difference in hypothalamic CRF expression (Gomez et al., 1996).
In our model, stress induces reproductive dysfunction in sensitive individuals, but even in non-stressed conditions, stress-sensitive monkeys have chronically lower ovarian steroid secretion. In a new cohort of animals, 15 weeks of citalopram treatment elevated estrogen and progesterone secretion in stress-sensitive animals. This work has approached the neural systems acted upon by citalopram, which in turn are thought to impinge upon the GnRH system and ultimately determine ovarian steroid secretion. Since citalopram is a selective serotonin reuptake inhibitor, we examined its efect on serotonin-related gene expression (Lima et al., 2009). In addition, due to the involvement of the CRF system in stress and the link between serotonin and CRF, we examined CRF and UCN1 innervation of the serotonin system (Weissheimer, 2010) and CRF-R2 expression in the dorsal raphe.
It is important to note that we found that activation of the HPA axis does not appear to be the primary mechanism by which an individual develops sensitivity to stress-induced reproductive dysfunction (Herod et al., 2011a). SS monkeys (n = 5) did not differ from MSR (n = 5) or HSR (n = 7) monkeys in 1) the diurnal release of ACTH and cortisol, 2) ACTH and cortisol in response to an acute psychological stress, 3) the percent suppression of cortisol in response to dexamethasone negative feedback, 4) the diurnal release of ACTH and cortisol following exposure to mild psychosocial and metabolic stress, 5) the concentration of cortisol in hair, or 6) adrenal weight. However, MSR + SS monkeys (n = 10) did secrete more cortisol than HSR monkeys during the daytime hours (1000-1800) following exposure to a novel social environment and reduced diet. Thus, we concluded that increased activity of the HPA axis is unlikely to be the primary mechanism causing increased sensitivity to stress-induced reproductive dysfunction (Herod et al., 2011a).
First, the expression of 4 genes that determine a significant portion of serotonin neuronal function, i.e., Fev, TPH2, SERT and 5HT1A autoreceptor, was examined in stress- sensitive and stress-resilient monkeys treated with citalopram or placebo for 15 weeks in the absence of stress. We found that the expression of each of these genes was significantly reduced in the stress-sensitive animals, but that citalopram treatment did not alter their expression. Blood levels of citalopram were maintained in a therapeutic range and the citalopram treatment was effective in increasing ovarian steroid secretion in the stress- sensitive animals prior to euthanasia, suggesting that the treatment was efficacious (Cameron et al., 2004). This data agrees with a previous report on the absence of expression consequences with SSRI treatment in rodents (Spurlock et al., 1994). Nonetheless, it could have been argued that the rodents were not depressed or stressed, so the SSRI may not elicit an overt effect. Our citalopram treatment of stress-sensitive macaques refutes that argument. Clearly the stress-sensitive macaques have a deficit in serotonin function, which citalopram may have corrected, but the mechanism did not involve a change in serotonin related gene expression.
The lower expression of TPH2, SERT and 5HT1A is likely a consequence of the lower expression of Fev. The TPH2, SERT and 5HT1A promoters contain a Fev binding site and Fev is thought to be the terminal differentiation gene controlling the expression of serotonin specific genes such as TPH2, SERT and the 5HT1A receptor. Therefore, Fev determines whether a neuron is serotonergic or not. Thus, the lower expression of TPH2, SERT and 5HT1A may reflect the lower serotonin cell number as well as lower Fev expression in individual cells in the stress-sensitive animals.
It is noteworthy that in Pet1 (Fev) knock out mice, the serotonin neurons fail to differentiate and remaining ones show deficient expression of serotonin-related genes. Moreover, these mice show heightened anxiety-like and aggressive behavior as adults (Hendricks et al., 2003). The stress-sensitive monkeys, who have deficient Fev expression, and hence fewer serotonin neurons, as well as reduced expression of the other serotonin–related genes, also show heightened anxiety-like behavior in a temperament test. In parallel studies, we used a series of tests for anxious behavior that were adapted for monkey behavioral testing (Cameron, 2003, Bethea et al., 2004) to assess temperamental characteristics in stress-sensitive vs. stress-resilient monkeys. We found that stress-sensitive monkeys show increased behavioral agitation when placed in a novel situation (Herod et al., 2006), a characteristic associated with low central serotonin release in a number of psychiatric disorders. Behaviors such as excessive worry or fear (Schulkin et al., 1998, Kalin and Shelton, 2003, Myers and Davis, 2007), inhibited approach to novelty/hypoactivity (Biederman et al., 1990, Hirshfeld et al., 1992, Fox, 2004), and increased agitation/hyperactivity in response to novelty (Kelly et al., 1997, Strekalova et al., 2005, Shroff et al., 2006) have all been observed in both clinical tests and animal models of anxiety. The pathophysiologic mechanisms underlying psychomotor agitation include dysregulation of dopaminergic, serotonergic, noradrenergic and GABAergic systems (Lindenmayer, 2000), several of which show differences between stress-sensitive and stress-resilient animals (Bethea et al., 2008).
Citalopram is a selective serotonin reuptake inhibitor (SSRI) that alleviates many of the symptoms of serotonin deficiency, initially by increasing extracellular serotonin (Smith et al., 2000). SSRIs are also thought to induce neuronal plasticity (Manji et al., 2003) although whether this is a direct effect of the SSRI, or an effect of the elevated extracellular serotonin, has not been fully resolved. Nonetheless, SSRI’s have a delayed efficacy, which has lead to questions of whether changes in gene expression may be involved. In the hippocampus of male tree shrews, chronic stress causes changes in gene expression that are reversed by administration of antidepressants (Alfonso et al., 2004) and microarray analysis found a significant number of gene changes in the dorsal raphe nucleus of naïve rats treated with fluoxetine (Conti et al., 2007). However, an early study in which naive rats were treated with a variety of antidepressants for four weeks reported no significant changes in TPH1, SERT or 5HT1A receptor mRNAs (Spurlock et al., 1994). Later it was reported that four or eight weeks of fluoxetine (7.5 mg/kg/day) increased TPH2 mRNA in the dorsal raphe nucleus, which correlated with antidepressant action in the forced swim test, but two weeks of treatment had no effect (Shishkina et al., 2007). The same treatment decreased SERT mRNA at all time points. Nonetheless, we observed no effect of 15 weeks of citalopram on Fev, TPH2, SERT or 5HT1A mRNAs in the dorsal raphe nucleus of stress-resilient or stress-sensitive monkeys. Thus, our results are more consistent with the early study of showing lack of antidepressant action on serotonin-related gene expression in rats (Spurlock et al., 1994).
The lack of an effect of citalopram on serotonin-related gene expression suggests that changes in expression of Fev, TPH2, SERT or 5HT1A mRNAs in the dorsal raphe were not central to the therapeutic action of citalopram. It is well accepted that SSRIs increase extracellular serotonin (Hjorth et al., 2000) and either serotonin and/or antidepressants probably increase pivotal growth factors that stimulate neuronal remodeling (Alfonso et al., 2005, Turner et al., 2006). Citalopram did increase CRF-R2 gene expression in serotonin neurons, but the mechanism is unclear. Perhaps the CRF receptors are regulated by the amount of CRF delivered to serotonin neurons, and citalopram decreased CRF by elevating synaptic serotonin. In this scenario, a decrease in CRF may increase CRF-R2. Likewise, an increase in extracellular serotonin may increase CRF-R2, but again, how?
In our model of hypothalamic amenorrhea, citalopram increased serum estrogen and progesterone concentrations in the stress-sensitive, but not the stress-resilient monkeys. It may be questioned whether the citalopram-induced increase in estrogen and progesterone could exert feedback regulation on serotonin-related gene expression. In our model of surgical menopause with hormone replacement therapy (HRT), the steroid hormone concentrations are adjusted from nearly absent (Ovx control) to levels observed in the early to mid-luteal phase (HRT groups), whereas in the stress model, the steroid hormones change from adequate (with placebo) to better (with citalopram) in the SS group. The citalopram-induced adjustment does not appear to exert the same kind of dynamic effect on TPH2 expression as the HRT adjustment.
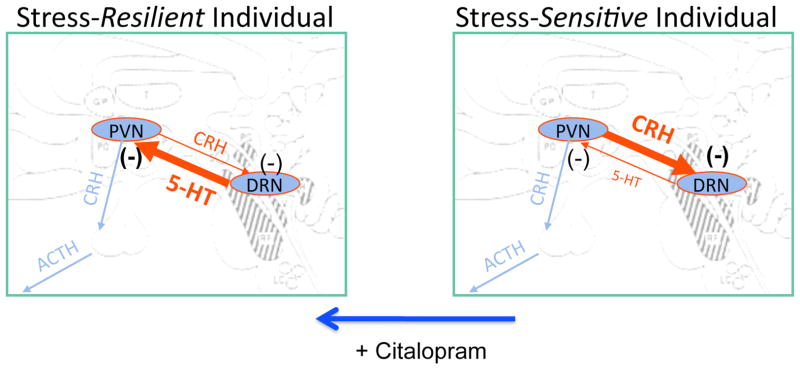
As illustrated in Figure 19, there is a higher density of CRF fibers and a lower density of UCN1 fibers in the midbrain raphe region in stress-sensitive macaques compared to stress-resilient macaques. It is reasonable to hypothesize that in SS animals, the higher CRF and lower UCN1 innervations lead to a decrease in serotonin neurotransmission, which in turn contributes to their stress sensitivity. In addition, with 15 weeks of s-citalopram treatment, there was a decrease in CRF fiber density in the dorsal raphe of stress sensitive animals. The decrease in CRF delivered to the dorsal raphe could allow serotonin to further increase, producing a feed forward circuit in which CRF was further decreased. Together, these actions could reduce anxiety and sensitivity to stress. Citalopram reduced CRF fiber density in the dorsal raphe only in stress-sensitive animals, which correlates closely with the observed increase in ovarian steroid secretion in response to citalopram only in stress-sensitive animals. Thus, citalopram appears to readjust CRF and CRF-R2 receptors toward a stress-resilient state. The CRF cell bodies, which innervate the dorsal raphe, are located in the PVN and amygdala; and in a reciprocal fashion, serotonin innervates CRH neurons in the PVN and amygdala (Bassett and Foote, 1992, Laflamme et al., 1999 Lowry, 2000#3320, Ruggiero et al., 1999, Kirby et al., 2000, Asan et al., 2005, Clark et al., 2007). It is attractive to speculate that citalopram may act to increase serotonin release in the PVN and amygdala of stress-sensitive animals, which in turn would decrease CRF production. The citalopram-induced upregulation of CRF-R2 on serotonin neurons could be a direct effect of citalopram, extracellular serotonin or secondary to the decrease in CRF delivered to the raphe.
It is also noteworthy that stress-sensitive animals exhibited reduced serotonin gene expression, elevated CRF in the PVN and higher CRF fiber density in the dorsal raphe nucleus in the absence of external stress (Centeno et al., 2007, Bethea et al., 2008, Weissheimer, 2010). There are numerous studies in humans indicating that childhood trauma leads to vulnerability to stress and affective disorders in adulthood (Nemeroff, 2004a), Since the cynomolgus monkeys are caught and imported, we speculate that the vulnerability of some individuals may be due to “childhood trauma” in the wild such as variable foraging demands on the mother or abuse (Coplan et al., 2010).
In conclusion, stress-sensitive monkeys have lower expression of the Fev gene in individual neurons, and fewer detectable serotonin neurons than stress-resilient monkeys. This deficit has downstream consequences including lower expression of TPH2, SERT and 5HT1A genes, as well as elevated expression of hypothalamic GAD67 and CRH and finally, decreased transport of LHRH to the median eminence (Bethea et al., 2008). These shifts in CNS systems lead to a decrease in ovarian production of estrogen and progesterone although ovulation was adequate without exogenous stress. Preliminary data indicate that the stress-sensitive animals exhibit higher levels of behavioral agitation/anxiety in temperament tests. Administration of citalopram increases estrogen and progesterone production, but it does not alter gene expression of Fev, TPH2, SERT or 5HT1A in the dorsal raphe nucleus. Rather, it decreases CRH innervation in the raphe. In addition, there was an increase in anxiolytic CRF-R2 receptors on serotonin neurons. Any decrease in CRF to the raphe would increase serotonin neurotransmission. Any increase in serotonin function would elevate mood, increase stress resilience and decrease anxiety (Graeff, 2002, Hammack et al., 2002, Hanley and Van de Kar, 2003, Hendricks et al., 2003, Lowry et al., 2009). From the opposite perspective, stress increases CRF production, which under chronic conditions could decrease serotonin neurotransmission resulting in vulnerability to stress, depression and anxiety (Stout et al., 2002, Valentino et al., 2009). This suggests that the mechanism of action of citalopram involves post-translational events in the serotonin system, which may in turn alter the CRH system leading to further elevation of serotonin neurotransmission in nonhuman primates.
Figure 13.
Schematic representation of our hypothesis regarding the neurobiology of stress sensitivity and the effect of citalopram. Based upon the current data, we speculate that even in the absence of stress, stress-sensitive animals have an overactive CRF system and an underactive serotonin system, compared to stress-resilient animals. Administration of citalopram for 15 weeks reduced CRF innervation of the DRN and increased CRF-R2 expression on serotonin neurons. Therefore, we speculate that citalopram normalized the CRF system and either directly or indirectly, upregulated CRF-R2 expression. Citalopram did not affect serotonin-related gene expression, but based upon its mechanism of action, we speculate that synaptic serotonin was increased, which in turn reduced CRF production.
Highlights.
Monkeys were characterized as stress sensitive or stress resilient by their ovulatory response during metabolic and psychosocial stress.
Stress sensitive monkeys showed an improvement in ovarian function after 15 weeks of citalopram treatment
Stress sensitive monkeys have reduced gene expression of Fev, TPH2, SERT and 5HT1A compared to stress resilient monkeys
Citalopram treatment did not improve Fev, TPH2, SERT or 5HT1A gene expression in stress sensitive monkeys
Stress sensitive monkeys have greater CRF fiber density and less CRF-R2 expression in the raphe compared to stress resilient monkeys.
Citalopram treatment decreased CRF fiber density and increased CRF-R2 expresssion in the dorsal raphe of stress sensitive monkeys
Acknowledgments
Supported by the Eunice Kennedy Shriver NICHD through cooperative agreement U54 HD 18185 as part of the specialized Cooperative Centers Program in Reproduction and Infertility Research, NIH grant MH62677 to CLB, NIH grant HD 62618 to JLC, and NIH RR000163 for the operation of ONPRC
We are very grateful for the dedicated work of J.A. Bytheway, S. Guay, D. Kerr, and N. Rockcastle for the performance of the physiological studies treating monkeys with citalopram. We are also indebted to the collaboration with D.A. Axelson and J.M. Perel in the Dept. of Psychiatry at the University of Pittsburgh who measured blood citalopram levels. We thank S.R. Ojeda (Division of Neuroscience, ONPRC) for his help with the non-isotopic in situ hybridization procedure. Technical assistance from the Primate Core laboratory of the Center for Research in Reproductive Physiology at the University of Pittsburgh was also greatly appreciated. We also thank Yibing Ja, M.S., ONPRC Cell and Molecular Biology Core, for cloning and sequencing of the Fev amplicon and Dr. Anda Cornea, ONPRC Imaging Core for expert assistance with stereological analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alfonso J, Frasch AC, Flugge G. Chronic stress, depression and antidepressants: effects on gene transcription in the hippocampus. Rev Neurosci. 2005;16:43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- Alfonso J, Pollevick GD, Van Der Hart MG, Flugge G, Fuchs E, Frasch AC. Identification of genes regulated by chronic psychosocial stress and antidepressant treatment in the hippocampus. Eur J Neurosci. 2004;19:659–666. doi: 10.1111/j.1460-9568.2004.03178.x. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Arango V, Huang YY, Underwood MD, Mann JJ. Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res. 2003;37:375–386. doi: 10.1016/s0022-3956(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Artigas F, Perez V, Alvarez E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Banik U, Wang G-A, Wagner PD, Kaufman S. Interaction of phosphorylated tryptophan hydroxylase with 14-3-3 proteins. J Biol Chem. 1997;272:26219–26225. doi: 10.1074/jbc.272.42.26219. [DOI] [PubMed] [Google Scholar]
- Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- Barker EL, Blakely RD. Norepinephrine and serotonin transporters. Molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, Ltd; 1995. pp. 321–333. [Google Scholar]
- Bassett JL, Foote SL. Distribution of corticotropin-releasing factor-like immunoreactivity in squirrel monkey (Saimiri sciureus) amygdala. J Comp Neurol. 1992;323:91–102. doi: 10.1002/cne.903230108. [DOI] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: an examination of the Iowa Adoptee sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:710–713. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at 5HTT mediates the impact of child sex abuse on women’s antisocial behavior: an examination of the Iowa adoptee sample. Psychosom Med. 2011;73:83–87. doi: 10.1097/PSY.0b013e3181fdd074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995a;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Behan DP, Maciejewski D, Chalmers D, De Souza EB. Corticotropin releasing factor binding protein (CRF-BP) is expressed in neuronal and astrocytic cells. Brain Res. 1995b;698:259–264. doi: 10.1016/0006-8993(95)01014-m. [DOI] [PubMed] [Google Scholar]
- Berg-von der Emde K, Dees WL, Hiney JK, Hill DF, Dissen GA, Costa ME, Moholt-Siebert M, Ojeda SR. Neurotrophins and the neuroendocrine brain: different neurotrophins sustain anatomically and functionally segregated subsets of hypothalamic dopaminergic neurons. J Neurosci. 1995;15:4223–4237. doi: 10.1523/JNEUROSCI.15-06-04223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril. 1997;67:1024–1030. doi: 10.1016/s0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- Berga SL, Girton LG. The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatric Clinics of North America. 1989;12:105–116. [PubMed] [Google Scholar]
- Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann N Y Acad Sci. 2006;1092:114–129. doi: 10.1196/annals.1365.010. [DOI] [PubMed] [Google Scholar]
- Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–981. doi: 10.1016/s0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML. Ovarian steroid treatment decreases corticotropin-releasing hormone (CRH) mRNA and protein in the hypothalamic paraventricular nucleus of ovariectomized monkeys. Neuropsychopharmacology. 2008;33:546–556. doi: 10.1038/sj.npp.1301442. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005a;83:148–155. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behav Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005b;132:151–166. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Rosenbaum JF, Hirshfeld DR, Faraone SV, Bolduc EA, Gersten M, Meminger SR, Kagan J, Snidman N, Reznick JS. Psychiatric correlates of behavioral inhibition in young children of parents with and without psychiatric disorders. Arch Gen Psychiatry. 1990;47:21–26. doi: 10.1001/archpsyc.1990.01810130023004. [DOI] [PubMed] [Google Scholar]
- Biller BMK, Federoff HJ, Koenig JI, Klibanski A. Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 1990;70:311–317. doi: 10.1210/jcem-70-2-311. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J Exper Biology. 1994;196:236–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 2005;20:225–231. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry. 1998;44:169–178. doi: 10.1016/s0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Bohmaker K, Eison AS, Yocca FD, Mellar E. Comparative effects of chronic 8-OH-DPAT, gepirone and ipsapirone treatment on the sensitivity of somatodendritic 5HT1A autoreceptors. Neuropharmacology. 1993;32:527–534. doi: 10.1016/0028-3908(93)90048-8. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- Bondy B, Erfurth A, De Jonge S, Kruger M, Meyer H. Possible association of the short allele of the serotonin transporter promoter gene polymorphism (5-HTTLPR) with violent suicide. Mol Psychiatry. 2000;5:193–195. doi: 10.1038/sj.mp.4000678. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Reproductive dysfunction in primates, behaviorally induced. In: Fink G, editor. Encyclopedia of Stress. New York: Academic Press; 2000. pp. 366–372. [Google Scholar]
- Cameron JL. Stress and reproduction. In: Henry HL, Norman AW, editors. The Encyclopedia of Hormones. Elsevier Science; 2003. pp. 433–438. [Google Scholar]
- Cameron JL, Bytheway JA, Guay S, Bethea CL, Kerr DI, Rockcastle N, Perel JM, Axelson DA. Treatment with a serotonin reuptake inhibitor increases reproductive hormone secretion in stress sensitive monkeys. 34th Annual Meeting of the Society for Neuroscience; San Diego, CA. 2004. [Google Scholar]
- Centeno ML, Sanchez RL, Reddy AP, Cameron JL, Bethea CL. Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology. 2007;86:277–288. doi: 10.1159/000109877. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Lesch KP, Heils A, Nielsen DA, Higley JD, Suomi SJ. Serotonin transporter gene polymorphism and neurobehavioral development in rhesus monkey neonates. American Journal Primatology. 2000;51 (Suppl 1):50–51. [Google Scholar]
- Clark MS, Kaiyala KJ. Role of corticotropin-releasing factor family peptides and receptors in stress-related psychiatric disorders. Semin Clin Neuropsychiatry. 2003;8:119–136. doi: 10.1053/scnp.2003.50011. [DOI] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Hoplight BJ, Neumaier JF. Chronic low dose ovine corticotropin releasing factor or urocortin II into the rostral dorsal raphe alters exploratory behavior and serotonergic gene expression in specific subregions of the dorsal raphe. Neuroscience. 2007;146:1888–1905. doi: 10.1016/j.neuroscience.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, Bilbe G, Hoyer D, Bartfai T. Region-specific transcriptional changes following the three antidepressant treatments electroconvulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Abdallah CG, Kaufman J, Gelernter J, Smith EL, Perera TD, Dwork AJ, Kaffman A, Gorman JM, Rosenblum LA, Owens MJ, Nemeroff CB. Early-life stress, corticotropin-releasing factor, and serotonin transporter gene: A pilot study. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza UM, Craig IW. Functional genetic polymorphisms in serotonin and dopamine gene systems and their significance in behavioural disorders. Prog Brain Res. 2008;172:73–98. doi: 10.1016/S0079-6123(08)00904-7. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Delville Y, Stires C, Ferris CF. Distribution of corticotropin-releasing hormone immunoreactivity in golden hamster brain. Brain Res Bull. 1992;29:681–684. doi: 10.1016/0361-9230(92)90138-n. [DOI] [PubMed] [Google Scholar]
- Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson H, Ghuman S, Prabhakar S, Smith R. A conceptual model of the influence of stress on female reproduction. Reproduction. 2003;125:151–163. doi: 10.1530/rep.0.1250151. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nucl Med Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioroni L, Fava M, Genazzani AD, Facchinetti F, Genazzani AR. Life events impact in patients with secondary amenorrhoea. J Psychosom Res. 1994;38:617–622. doi: 10.1016/0022-3999(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Flugge G, Kramer M, Rensing S, Fuchs E. 5HT1A-receptors and behaviour under chronic stress: selective counteraction by testosterone. Eur J Neurosci. 1998;10:2685–2693. [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA. Temperament and early experience form social behavior. Ann N Y Acad Sci. 2004;1038:171–178. doi: 10.1196/annals.1315.025. [DOI] [PubMed] [Google Scholar]
- Frim DM, Robinson BG, Pasieka KB, Majzoub JA. Differential regulation of corticotropin-releasing hormone mRNA in rat brain. Am J Physiol. 1990;258:E686–692. doi: 10.1152/ajpendo.1990.258.4.E686. [DOI] [PubMed] [Google Scholar]
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig. 2010;7:251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril. 1993;60:486–492. [PubMed] [Google Scholar]
- Gomez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- Goodwin GM. How do antidepressants affect serotonin receptors? The role of serotonin receptors in the therapeutic and side effect profile of the SSRIs. J Clinical Psychiatry. 1996;57:9–13. [PubMed] [Google Scholar]
- Graeff FG. On serotonin and experimental anxiety. Psychopharmacology (Berl) 2002;163:467–476. doi: 10.1007/s00213-002-1112-4. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Mol Brain Res. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003a;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003b;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley NR, Van de Kar LD. Serotonin and the neuroendocrine regulation of the hypothalamic--pituitary-adrenal axis in health and disease. Vitam Horm. 2003;66:189–255. doi: 10.1016/s0083-6729(03)01006-9. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Herod SM, Bytheway J, Dolney R, Cameron JL. Female monkeys who are sensitive to stress-induced reproductive dysfunction show increases in certain forms of anxious behavior. Annual Meeting of the Society for Neuroscience; Atlanta, GA. 2006. [Google Scholar]
- Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab. 2011a;300:E28–36. doi: 10.1152/ajpendo.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011b;300:E19–27. doi: 10.1152/ajpendo.00224.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hery F, Ternaux JP. Regulation of release processes in central serotoninergic neurons. J Physiol (Paris) 1981;77:287–301. [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. J Psychopharmacol. 2000;14:177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- Johansen PA, Jennings I, Cotton RG, Kuhn DM. Tryptophan hydroxylase is phosphorylated by protein kinase A. J Neurochem. 1995;65:882–888. doi: 10.1046/j.1471-4159.1995.65020882.x. [DOI] [PubMed] [Google Scholar]
- Johansen PA, Jennings I, Cotton RG, Kuhn DM. Phosphorylation and activation of tryptophan hydroxylase by exogenous protein kinase A. J Neurochem. 1996;66:817–823. doi: 10.1046/j.1471-4159.1996.66020817.x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Keck ME, Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. 2001;22:835–844. doi: 10.1016/s0196-9781(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Keck ME, Ohl F, Holsboer F, Muller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29:867–889. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Keegan CE, Herman JP, Karolyi IJ, O’Shea KS, Camper SA, Seasholtz AF. Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology. 1994;134:2547–2555. doi: 10.1210/endo.134.6.8194481. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Kemp CF, Woods RJ, Lowry PJ. The corticotrophin-releasing factor-binding protein: an act of several parts. Peptides. 1998;19:1119–1128. doi: 10.1016/s0196-9781(98)00057-6. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Kozicz T. Neurons colocalizing urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neuroscience. 2003;116:315–320. doi: 10.1016/s0306-4522(02)00772-8. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Feuvrier E, Richard D, Rivest S. Involvement of serotonergic pathways in mediating the neuronal activity and genetic transcription of neuroendocrine corticotropin-releasing factor in the brain of systemically endotoxin-challenged rats. Neuroscience. 1999;88:223–240. doi: 10.1016/s0306-4522(98)00369-8. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83:25–32. doi: 10.1210/jcem.83.1.4502. [DOI] [PubMed] [Google Scholar]
- Lawrence KM, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT uptake sites, labelled with [3H]paroxetine, in antidepressant-free depressed suicides. Brain Res. 1990;526:17–22. doi: 10.1016/0006-8993(90)90244-6. [DOI] [PubMed] [Google Scholar]
- Lesch KP. When the Serotonin Transporter Gene Meets Adversity: The Contribution of Animal Models to Understanding Epigenetic Mechanisms in Affective Disorders and Resilience. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2010_109. [DOI] [PubMed] [Google Scholar]
- Lima FB, Centeno ML, Costa ME, Reddy AP, Cameron JL, Bethea CL. Stress sensitive female macaques have decreased fifth Ewing variant (Fev) and serotonin-related gene expression that is not reversed by citalopram. Neuroscience. 2009;164:676–691. doi: 10.1016/j.neuroscience.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer JP. The pathophysiology of agitation. J Clin Psychiatry. 2000;61(Suppl 14):5–10. [PubMed] [Google Scholar]
- Linthorst AC. Interactions between corticotropin-releasing hormone and serotonin: implications for the aetiology and treatment of anxiety disorders. Handb Exp Pharmacol. 2005:181–204. doi: 10.1007/3-540-28082-0_7. [DOI] [PubMed] [Google Scholar]
- Liu C, Maejima T, Wyler SC, Casadesus G, Herlitze S, Deneris ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–1198. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Plant A, Windle RJ, Shanks N, Wood SA, Ingram CD, Renner KJ, Lightman SL, Summers CH. Fluoxetine inhibits corticotropin-releasing factor (CRF)-induced behavioural responses in rats. Stress. 2009;12:225–239. doi: 10.1080/10253890802309861. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution and function in female macaques. Molecular Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, Gray AN, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Brent DA, Arango V. The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacology. 2001;24:467–477. doi: 10.1016/S0893-133X(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76:310–316. doi: 10.1016/s0015-0282(01)01921-5. [DOI] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin-containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008;507:1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. The End of Stress As We Know It. Washington, DC: Joseph Henry Press; 2002. [Google Scholar]
- Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N. The central role of corticotropin-releasing factor (CRF-41) in psychological stress in rats. J Physiol. 1993;460:221–229. doi: 10.1113/jphysiol.1993.sp019468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004a;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Nemeroff CC. Early-life adversity, CRF dysregulation, and vulnerability to mood and anxiety disorders. Psychopharmacol Bull. 2004b;38:14–20. [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Oki Y, Sasano H. Localization and physiological roles of urocortin. Peptides. 2004;25:1745–1749. doi: 10.1016/j.peptides.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2008;84:148–156. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare WP, Tejani-Butt SM. Effect of stress on the behavior and 5-HT system in Sprague-Dawley and Wistar Kyoto rat strains. Integr Physiol Behav Sci. 1996;31:112–121. doi: 10.1007/BF02699783. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J Comp Neurol. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Serotonin-CRF interaction in the bed nucleus of the stria terminalis: a light microscopic double-label immunocytochemical analysis. Brain Res Bull. 1992;28:943–948. doi: 10.1016/0361-9230(92)90217-l. [DOI] [PubMed] [Google Scholar]
- Portillo F, Carrasco M, Vallo JJ. Separate populations of neurons within the paraventricular hypothalamic nucleus of the rat project to vagal and thoracic autonomic preganglionic levels and express c-Fos protein induced by lithium chloride. J Chem Neuroanat. 1998;14:95–102. doi: 10.1016/s0891-0618(97)10022-9. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18(6):492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. Journal of Biological Chemistry. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- Reifenstein ECJ. Psychogenic or “hypothalamic” amenorrhea. Med Clin North Am. 1946;30:1103–1114. doi: 10.1016/s0025-7125(16)35908-9. [DOI] [PubMed] [Google Scholar]
- Reindollar RH, Novak M, Tho SP, McDonough PG. Adult-onset amenorrhea: a study of 262 patients. Am J Obstet Gynecol. 1986;155:531–543. doi: 10.1016/0002-9378(86)90274-7. [DOI] [PubMed] [Google Scholar]
- Romero L, Artigas F. Preferential potentiation of the effects of serotonin uptake inhibitors by 5HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. J Neurochem. 1997;68:2593–2603. doi: 10.1046/j.1471-4159.1997.68062593.x. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V. Corticotropic-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience. 1999;91:1343–1354. doi: 10.1016/s0306-4522(98)00703-9. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Bethea CL. Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience. 2010;171:893–909. doi: 10.1016/j.neuroscience.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphier D, Welch JE, Farrar GE, Nguyen NQ, Aguado F, Thaller TR, Knight DS. Interactions between serotonin, thyrotropin-releasing hormone, and substance P in the CNS regulation of adrenocortical secretion. Psychoneuroendocrinology. 1994;19:779–797. doi: 10.1016/0306-4530(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, Mcewan BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids:implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Sharp T, McQuade R, Bramwell S, Hjorth S. Effect of acute and repeated administration of 5HT1A receptor agonists on 5HT release in rat brain in vivo. Naunyn-Schmiedebergs Arch Pharmacol. 1993;348:339–346. doi: 10.1007/BF00171331. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Shroff H, Reba L, Thornton LM, Tozzi F, Klump KL, Berrettini WH, Brandt H, Crawford S, Crow S, Fichter MM, Goldman D, Halmi KA, Johnson C, Kaplan AS, Keel P, LaVia M, Mitchell J, Rotondo A, Strober M, Treasure J, Woodside DB, Kaye WH, Bulik CM. Features associated with excessive exercise in women with eating disorders. Int J Eat Disord. 2006;39:454–461. doi: 10.1002/eat.20247. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Kahn RS, Lawlor BA, Trestman RL, Lawrence TL, Coccaro EF. II. Critical issues in defining the role of serotonin in psychiatric disorders. Pharmacological Reviews. 1991;43:509–525. [PubMed] [Google Scholar]
- Singh A, Lucki I. Antidepressant-like activity of compounds with varying efficacy at 5HT1A receptors. Neuropharmacology. 1993;32:331–340. doi: 10.1016/0028-3908(93)90153-t. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Owens MJ, Nemeroff CB. The neurobiology of urocortin. Regul Pept. 2000;93:85–92. doi: 10.1016/s0167-0115(00)00180-4. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Smith TD, Kuczenski R, George-Griedman K, Malley JD, Foote SL. In vivo microdialysis assessment of extracellular serotonin and dopamine levels in awake monkeys during sustained fluoxetine administration. Synapse. 2000;38:460–470. doi: 10.1002/1098-2396(20001215)38:4<460::AID-SYN11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Souery D, Oswald P, Linkowski P, Mendlewicz J. Molecular genetics in the analysis of suicide. Ann Med. 2003;35:191–196. doi: 10.1080/07853890310008242. [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. (−)-Propanolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5HT1A selective agonists. Eur J Pharmacol. 1986;128:295–298. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- Spurlock G, Buckland P, O’Donovan M, McGuffin P. Lack of effect of antidepressant drugs on the levels of mRNAs encoding serotonergic receptors, synthetic enzymes and 5HT transporter. Neuropharmacology. 1994;33:433–440. doi: 10.1016/0028-3908(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout SC, Owens MJ, Nemeroff CB. Regulation of corticotropin-releasing factor neuronal systems and hypothalamic-pituitary-adrenal axis activity by stress and chronic antidepressant treatment. J Pharmacol Exper Therap. 2002;300:1085–1092. doi: 10.1124/jpet.300.3.1085. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Dolgov O, Bartsch D. Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol. 2005;16:171–180. doi: 10.1097/00008877-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Summers CH, Kampshoff JL, Ronan PJ, Lowry CA, Prestbo AA, Korzan WJ, Renner KJ. Monoaminergic activity in subregions of raphe nuclei elicited by prior stress and the neuropeptide corticotropin-releasing factor. J Neuroendocrinol. 2003;15:1122–1133. doi: 10.1111/j.1365-2826.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Zhou FC. Differential polarization of serotonin transporters in axons versus soma-dendrites: an immunogold electron microscopy study. Neuroscience. 1999;94:821–830. doi: 10.1016/s0306-4522(99)00373-5. [DOI] [PubMed] [Google Scholar]
- Thomas E, Pernar L, Lucki I, Valentino RJ. Corticotropin-releasing factor in the dorsal raphe nucleus regulates activity of lateral septal neurons. Brain Res. 2003;960:201–208. doi: 10.1016/s0006-8993(02)03882-9. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiatry. 2006;59:1128–1135. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vasconcelos LA, Donaldson C, Sita LV, Casatti CA, Lotfi CF, Wang L, Cadinouche MZ, Frigo L, Elias CF, Lovejoy DA, Bittencourt JC. Urocortin in the central nervous system of a primate (Cebus apella): sequencing, immunohistochemical, and hybridization histochemical characterization. J Comp Neurol. 2003;463:157–175. doi: 10.1002/cne.10742. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Wang R, Millam JR. Corticotropin-releasing hormone-immunopositive nerve elements in apposition to chicken gonadotropin-releasing hormone I-containing perikarya in Japanese quail (Coturnix coturnix japonica) brain. Cell Tissue Res. 1999;297:223–228. doi: 10.1007/s004410051350. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Sakai RR, McEwen BS, Mendelson S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, Nemeroff CB. Depression and anxiety: role of the locus coeruleus and corticotropin releasing factor. Brain Research Bulletin. 1994;35:561–572. doi: 10.1016/0361-9230(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Weissheimer KV, Herod SM, Cameron JL, Bethea CL. Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorhea. Neuroendocrinology. 2010;92:224–234. doi: 10.1159/000319257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Nishihara M, Thalabard JC, Grosser PM, Hotchkiss J, Knobil E. Corticotropin-releasing factor and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Electrophysiological studies. Neuroendocrinology. 1990;52:133–137. doi: 10.1159/000125563. [DOI] [PubMed] [Google Scholar]
- Williams NI, Berga SL, Cameron JL. Mild metabolic stress potentiates the suppressive effect of psychological stress on reproductive function in female cynomolgus monkeys. Endocrine Society. 1997:Abstracts PI-367. [Google Scholar]
- Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–276. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M. Stress and the menstrual cycle: short- and long-term response to a five-day endotoxin challenge during the luteal phase in the rhesus monkey. J Clin Endocrinol Metab. 1999;84:623–626. doi: 10.1210/jcem.84.2.5448. [DOI] [PubMed] [Google Scholar]
- Yatham LN. Is 5HT1A receptor subsensitivity a trait marker for late luteal phase dysphoric disorder? A pilot study. Can J Psychiatry. 1993;38:662–664. doi: 10.1177/070674379303801007. [DOI] [PubMed] [Google Scholar]