Abstract
Background:
Olfactory dysfunction is an established nonmotor feature of idiopathic Parkinson disease (PD), which may precede disease onset. Olfaction is likely disturbed in patients with PD with leucine-rich repeat kinase (LRRK2) G2019S mutations, although the degree of impairment is debated. It is also unclear whether mutation carriers who have not yet manifested with PD have olfactory disturbances.
Methods:
Thirty-one subjects with LRRK2 G2019S mutation–related PD (PD-manifesting carriers [PD-MC]), 30 subjects with PD without mutations (PD noncarriers [PD-NC]), 28 mutation carrier family members (nonmanifesting carriers [NMC]), and 46 controls completed the University of Pennsylvania Smell Identification Test (UPSIT). Generalized estimating equations were applied to determine whether olfactory score was associated with PD and LRRK2 mutation status.
Results:
As expected, having PD was associated with impaired olfaction regardless of LRRK2 mutation status. More importantly, however, impaired olfaction was increased overall in LRRK2 carriers both with and without PD, though the impairment was only present in a subset of NMCs. Compared to controls, the mean score was lower among NMC (difference = −3.518, p = 0.006), MC (difference = −7.677, p < 0.0001), and idiopathic PD (PD-NC) (difference = −13.810, p < 0.0001). Olfaction was better among MC (PD-MC) than non-LRRK2 PD (PD-NC) (difference = 6.13, p = 0.0012). Group differences from the continuous analysis were maintained in dichotomous analysis stratifying at 15th percentile for age and gender.
Conclusion:
Olfaction is impaired in LRRK2 G2019S–mutation related PD, although less overall than other PD. Further, olfaction is impaired in a subset of LRRK2 NMC, suggesting that olfaction may be a marker for development of PD in this group, and that longitudinal studies are warranted.
Parkinson disease (PD) due to mutations in the LRRK2 gene appears to more closely mimic idiopathic PD than any other genetic etiology.1 Yet there are still gaps and uncertainty in our knowledge of LRRK2 clinical expression. For example, there is controversy regarding the clinical course of LRRK2 PD and whether progression is similar or less rapid compared with idiopathic PD.2–4 The range and severity of nonmotor features associated with LRRK2 mutations is also not well-defined. Several studies suggest that olfactory disturbance is a feature of LRRK2 PD3,5–8 but the degree of the impairment is debated. Finally, it is uncertain whether carriers who have not yet developed PD have abnormal olfaction,6,7 and whether this may be an endophenotype or trait of carrying the mutated LRRK2 gene.
Hyposmia is a common nonmotor feature of PD, present in 70%–100% of subjects with PD9,10 and may discriminate PD from atypical forms such as vascular parkinsonism and corticobasal degeneration.11–13 Loss of neurons and Lewy body deposition are also noted in the anterior olfactory bulb in PD.14 Olfaction has been reported as normal in some genetic etiologies of parkinsonism, such as that due to parkin mutations,15 but is abnormal in PINK1 and glucocerebrosidase-related PD.16–18 However, the association with olfaction in LRRK2 PD is less clear: olfaction has been reported as normal,19 not as significantly affected, or as consistently impaired as in idiopathic PD.3,5–8 Olfactory loss may precede clinical PD by at least 4 years,20 and thus may be a marker of developing PD. Reports of olfaction in LRRK2 mutation carriers without PD, a group that is at increased risk of developing PD, are both limited and conflicting.6,7 In order to systematically examine whether olfaction is affected in LRRK2 PD compared with idiopathic PD, and determine whether olfaction is impaired in mutation carriers who have not yet manifested PD, we studied LRRK2 G2019S mutation carriers with PD-MC (manifesting carriers [MC]), unaffected family members with LRRK2 mutations (nonmanifesting carriers [NMC]), subjects with PD without LRRK2 mutations (PD noncarriers, PD-NC), and healthy controls without a family history of PD (controls).
METHODS
Sixty-one subjects with PD (31 with LRRK2 G2019S mutations [PD-MC] and 30 without [PD-NC]), 28 mutation carrier family members (NMC), and 46 controls were recruited from parent studies of Genetics and PD at Beth Israel Medical Center and the Einstein Aging Study (EAS) at Albert Einstein College of Medicine. At Beth Israel, all study subjects were systematically examined by movement disorders specialists. A diagnostic checklist was completed, and only those subjects rated as having met stringent diagnostic criteria for PD21 were included. One family member who was determined to have PD was not diagnosed prior to the examination, and is included in the PD-MC group. Family members with G2019S mutations as well as spouse and laboratory controls without a family history of PD were included. At the EAS, formal neurologic evaluation including completion of the Unified Parkinson's Disease Rating Scale (UPDRS)22 was performed by a physician; for this study, a subset of elderly controls without parkinsonian features and with a Clinical Dementia Rating Scale score of less than 1 were included. Any potential subject who had a known respiratory tract infection or active allergies was also excluded from the study.
The encapsulated odor University of Pennsylvania Smell Identification Test (UPSIT) was self-administered by using standard 40-odor identification23 either at the time of the visit or at the subject's home, and returned by mail. Subjects were instructed to choose a response from the 4 choices listed. Tests which included incomplete responses were excluded from the analysis.
DNA was available from blood or buccal swab drawn at the parent study. LRRK2 genotyping was performed as previously described.24 All subjects were blinded to their mutation status except for 2 mutation carriers with PD and one nonmanifesting mutation carrier who had undergone clinical genetic testing.
Demographic characteristics and clinical scores among groups were summarized with descriptive statistics. Raw UPSIT scores were calculated as the number of correct identifications, ranging from 0 to 40, with 40 representing perfect olfaction. Analysis was performed first on the raw UPSIT scores as the primary outcome. Scores were also categorized using normative data for age and gender as previously reported by Doty,25 with a dichotomous cut at the 15th percentile.26 Generalized estimating equations (GEE) were applied to account for the correlations among measurements of subjects from the same family and to compare continuous UPSIT scores among the different groups, adjusting for age and gender, with a logistic link for the dichotomized UPSIT score. Analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC).
Standard protocol approvals, registrations, and patient consents.
The study procedures were approved by the respective internal review boards at Beth Israel Medical Center and Albert Einstein College of Medicine, and all subjects gave informed consent.
RESULTS
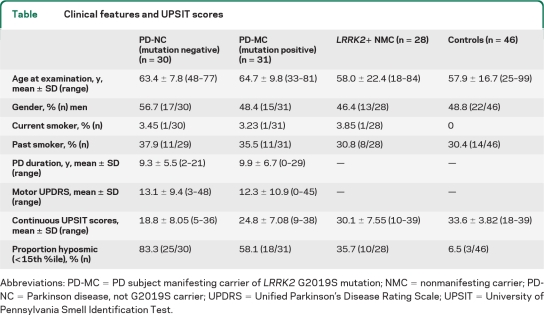
Demographic and clinical features are shown in the table. The non-LRRK2 PD (PD-NC) and MC (PD-MC) groups did not differ by age, duration of disease, UPDRS score at time of visit, or current or prior smoking. The NMC did not differ compared with controls in regards to age, gender, or current and prior smoking. However, compared with the controls, those with PD were older (p = 0.024). The MC and the NMC were not different in age, gender, or smoking status.
Table.
Clinical features and UPSIT scores
Abbreviations: PD-MC=PD subject manifesting carrier of LRRK2 G2019S mutation; NMC=nonmanifesting carrier; PD-NC=Parkinson disease, not G2019S carrier; UPDRS=Unified Parkinson’s Disease Rating Scale; UPSIT=University of Pennsylvania Smell Identification Test.
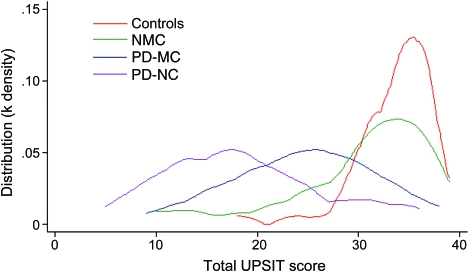
Analysis of continuous UPSIT scores is shown in the table and in the figure. As anticipated, older age was associated with lower UPSIT scores (worse olfaction, p < 0.0001). Prior smoking and gender were not associated with worse olfaction. In the GEE model adjusting for age, gender, and taking family relationship into account, both PD and LRRK2 PD as well as carrying the LRRK2 mutation without manifesting symptoms were associated with worse olfaction: compared to controls, the mean score was lower among NMC (mean estimate of difference = −3.518, 95% confidence interval [CI] −6.004, −1.03, p = 0.006), PD-MC (difference = −7.677, 95% CI −10.507, −4.846, p < 0.0001) and PD-NC (difference = −13.810, 95% CI −16.824, −10.795, p < 0.0001) as well as all PD (PD-NC and PD-MC combined) (difference = −8.984, 95% CI −11.512, −6.457, p < 0.0001).
Figure. Kernel density plot demonstrating distribution of University of Pennsylvania Smell Identification Test (UPSIT) scores in manifesting carriers, Parkinson disease (PD), nonmanifesting carriers, and controls.
Olfaction was better among PD-MC than non-LRRK2 PD (PD-NC) (difference = 6.13, 95% CI 2.422, 9.845, p = 0.0012).
While harboring the G2019S mutation was associated with lower scores among both NMC and PD-MC, the PD-MC group had a worse mean score than NMC (difference = −4.159, 95% CI −7.943, −0.376, p = 0.0312). The expected interaction between harboring the LRRK2 mutation and having PD (p < 0.0001) was observed.
In the analysis of categorical UPSIT scores (based on age and gender normative data at the 15th percentile; table), group differences which were noted for the raw UPSIT score were maintained in the dichotomous analysis: LRRK2 PD subjects (PD-MC) were more likely to be hyposmic than both NMC (OR = 3.05, p = 0.032) and controls (OR = 25.69, p < 0.0001), but less likely than non-LRRK2 PD subjects (PD-NC) (OR = 0.27, p = 0.034); LRRK2 NMC were more likely to be hyposmic than controls (OR = 8.44, p = 0.004).
DISCUSSION
Olfactory dysfunction is established as a common nonmotor feature of PD9,28,28 and there is a correlation between olfactory dysfunction and [99mTc] TRODAT-1 SPECT dopamine transporter density.29 Screening of first-degree relatives who have not developed motor features of PD, but have abnormal dopamine metabolism on PET, suggests that impairment in olfaction precedes clinical PD and is associated with dopaminergic cell loss.30–32 In this, the largest series of olfaction in LRRK2-related PD studied to date, our data support that olfactory disturbances, while less severe than idiopathic PD, are also a prominent feature of LRRK2 G2019S–related PD.33 Further, our data suggest that olfactory dysfunction is a feature of carrying the LRRK2 G2019S mutation that may occur without manifesting motor features. Because olfactory disturbances are not as severe in NMC overall compared to MC, and because this was a cross-sectional study, it is unclear whether all NMC with olfactory disturbances will evolve to develop PD or whether they represent an intermediate endophenotype that is not an immediate precursor to development of PD.
The pathophysiologic basis of LRRK2 PD is still not well understood: most autopsy reports demonstrate α-synuclein deposition with Lewy bodies as well as nigral degeneration.6,34 However, a range of pathology is noted, with few cases demonstrating nigral degeneration in isolation35 and others showing tau inclusions.36 In idiopathic PD, Lewy bodies and Lewy neurites are noted in the olfactory bulb; further, olfactory pathology is thought to occur as an early or initial event according to the staging schema for PD progression proposed by Braak et al.37 In the limited LRRK2 cases reported, α-synuclein accumulation in the rhinencephalon was shown in 4 cases of G2019S mutation PD6 and Lewy body deposition in the olfactory bulb was demonstrated in one LRRK2 Y1699C mutation case.19 These findings suggest that the effects of mutant LRRK2 include olfactory pathology, at least in a subset of carriers. Hence abnormal olfaction noted in our unaffected mutation carriers could represent the first stage in progression to PD. However, the temporal characteristics of LRRK2-related pathology are not established and may not follow Braak's staging schema. Evaluation of other nonmotor features, including transcranial sonography, and functional imaging and longitudinal follow-up will help determine whether olfactory involvement is necessarily part of inexorable progression of PD pathology or may represent a more restricted gene effect.
Similar to PINK1(17 and glucocerebrosidase-associated PD,16,18 but unlike PD due to parkin mutations,15 most8 but not all19 LRRK2 mutation studies support the idea that olfaction is impaired in this genetic form.8 While the degree of olfactory pathology has not been formally quantified in PD and LRRK2 PD, our clinical data support the notion that LRRK2 pathology may not be as extensive. A meta-analysis of LRRK2 mutation subjects with olfactory testing demonstrated that only 51% of LRRK2 G2019S mutation patients had significant olfactory loss.3 By virtue of the study design, however, LRRK2 cases could not be readily compared with controls or other PD cases. Whereas this suggests better olfaction than in idiopathic PD, it also highlights the methodologic concerns about how to rate olfactory abnormalities, and whether these should be considered relative to population norms, or whether each research group reporting olfactory scores needs to develop a large control sample.
Several reports have analyzed G2019S family members, with heterogenous results.6–8 Studies to compare olfaction between NMCs and family members who are noncarriers are currently underway and should help determine whether olfactory disturbance segregates with LRRK2 or whether it represents an intrafamilial abnormality, suggesting other possibly modifying PD genes.
A potential limitation of our study is that while we have sampled one of the largest groups of NMCs, including elderly NMCs, we did not have a large control sample for every decade and gender. Hence, we chose to report both continuous data compared with our laboratory controls as well as categorical normative data obtained through studies of 3,928 (1,819 men and 2,109 women) US controls.25
By demonstrating a difference between nonmanifesting carriers and controls, we suggest that in a subset of LRRK2 mutation carriers, UPSIT may identify nonmotor features of preclinical PD. However, we did not have enough NMCs to define subgroups; only 35% of NMCs fell below the 15th percentile for age and gender and thus some carriers may not have olfactory disturbances or may have only minimal olfactory changes. Longitudinal studies are necessary to determine the temporal relationship between olfaction and the development of motor signs.28,32 It is hoped that better understanding of motor and nonmotor features of LRRK2 PD will shed light on the pathophysiology of this genetic PD subtype.
ACKNOWLEDGMENT
The authors thank Jeannie Soto-Valencia, Monica Sethi, and Paul Soto (Beth Israel Medical Center) for their assistance in data collection and recruitment; the subjects, families, and EAS study subjects for their participation; and Charlotte Magnotta for recruitment (Einstein Aging Study).
- CI
- confidence interval
- EAS
- Einstein Aging Study
- MC
- manifesting carrier
- NC
- noncarriers
- NMC
- nonmanifesting carrier
- PD
- Parkinson disease
- UPDRS
- Unified Parkinson's Disease Rating Scale
- UPSIT
- University of Pennsylvania Smell Identification Test
AUTHOR CONTRIBUTIONS
Dr. Saunders-Pullman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. K. Stanley: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision. Dr. Wang: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. Dr. San Luciano: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, statistical analysis. Dr. Shanker: drafting/revising the manuscript, acquisition of data, study supervision. Dr. Hunt: drafting/revising the manuscript, contribution of vital reagents/tools/patients. Dr. Severt: drafting/revising the manuscript, acquisition of data. D. Raymond: drafting/revising the manuscript, acquisition of data, study supervision. Dr. Ozelius: drafting/revising the manuscript, acquisition of data, study supervision. Dr. Lipton: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis, study supervision, obtaining funding. Dr. Bressman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding.
DISCLOSURE
Dr. Saunders-Pullman serves on the Scientific Advisory Board of the Dystonia Medical Research Foundation. She has received research support from the NIH/NINDS, the Michael J Fox Foundation for Parkinson's Research, the Thomas Hartman Foundation for Parkinson's Research, the Bachmann-Strauss Dystonia Parkinson's Foundation, and the Marcled Foundation. She has received an honorarium from GE Healthcare. K. Stanley reports no disclosures. Dr. Wang receives research support from Bristol-Myers Squibb. Dr. San Luciano has received research support from the American Academy of Neurology Foundation. Dr. Shanker serves as a consultant for Teva Pharmaceutical Industries Ltd. and ECRI. Dr. Hunt reports no disclosures. Dr. Severt has received speaker honoraria from Teva Pharmaceutical Industries Ltd., Novartis, Ipsen, and Allergan, Inc.; and receives research support from Teva Pharmaceutical Industries Ltd., Ceregene, and Chelsea Therapeutics. D. Raymond reports no disclosures. Dr. Ozelius serves on scientific advisory boards for the Dystonia Medical Research Foundation, the Bachmann-Strauss Dystonia & Parkinson Foundation, the Benign Essential Blepharospasm Research Foundation, and the National Spasmodic Dysphonia Association; is listed as an author on patents re: Torsin, Torsin genes and methods of use, and Nucleic acids, methods and kits for the diagnosis of DYT6 primary torsion dystonia; receives research support from the NIH, the Dystonia Medical Research Foundation, and the Bachmann Strauss Dystonia Parkinson Foundation; and receives royalties from Athena Diagnostics, Inc. for a patent re: Torsin, Torsin genes and methods of use. Dr. Lipton serves on scientific advisory boards for Allergan, Inc., Merck Serono,, Neuralieve, Inc., and Pfizer Inc.; has received funding for travel from the American Headache Society, Cognimed, Diamond Headache Clinic Research, Market Force Communications, Merck Serono, Migraine Research Foundation, Scienta, and Talley Management; serves as Associate Editor of Cephalalgia and on the editorial boards of Neurology® and Headache; receives royalties from publishing Headache in Clinical Practice (Isis Medical Media, 2002), Headache in Primary Care (Isis Medical Media, 1999), Wolff's Headache (Oxford University Press, 2001, 2008), Managing Migraine: A Physician's Guide (BC Decker, 2008), and Managing Migraine: A Patient's Guide (BC Decker, 2008); has received speaker honoraria from the National Headache Foundation, the University of Oklahoma, the American Academy of Neurology, the Annenberg Foundation, Merck Serono, GlaxoSmithKline, and Coherex Medical; serves as a consultant for Allergan, Inc., Autonomic Technologies, MAP Pharmaceuticals, Inc., Neuralieve, Inc., and Novartis; receives/has received research support from AstraZeneca, Ortho McNeil, GlaxoSmithKline, Merck Serono, ProEthic Pharmaceutical, Inc., Advanced Bionics, the NIH/NIA, St. Jude Medical, the Migraine Research Foundation, and the National Headache Foundation; and holds stock options in Minster Pharmaceuticals plc. Dr. Bressman serves on scientific advisory boards for the Bachmann Strauss Dystonia and Parkinson's Foundation, the Michael J Fox Foundation for Parkinson's Research, and the Dystonia Medical Research Foundation; holds a patent re: THAP1 gene testing; and receives research support from the NIH and the Michael J Fox Foundation for Parkinson's Research.
REFERENCES
- 1. Klein C, Schlossmacher MG. The genetics of Parkinson's disease: implications for neurological care. Nat Clin Pract Neurol 2006;2:136–146 [DOI] [PubMed] [Google Scholar]
- 2. Clark LN, Wang Y, Karlins E, et al. Frequency of LRRK2 mutations in early- and late-onset Parkinson's disease. Neurology 2006;67:1786–1791 [DOI] [PubMed] [Google Scholar]
- 3. Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol 2008;7:583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Latourelle JC, Sun M, Lew MF, et al. The Gly1920Ser mutation in LRRK2 is not fully penetrant in familial Parkinson's disease: the GenePD study. BMC Med 2008;6:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferreira JJ, Guedes LC, Rosa MM, et al. High prevalence of LRRK2 mutations in familial and sporadic Parkinson's disease in Portugal. Mov Disord 2007;22:1194–201 [DOI] [PubMed] [Google Scholar]
- 6. Silveira-Moriyama L, Guedes LC, Kingsbury A, et al. Hyposmia in G2019S LRRK2-related parkinsonism: clinical and pathologic data. Neurology 2008;71:1021–1026 [DOI] [PubMed] [Google Scholar]
- 7. Lohman E, Leclere L, De Anna F, et al. French Parkinson's Disease Genetic Study Group: a clinical, neuropsychological and olfactory evaluation of a large family with LRRK2 mutations. Parkinsonism Relat Disord 2009;15:273–276 [DOI] [PubMed] [Google Scholar]
- 8. Silveira-Moriyama L, Munhoz RP, Carvalho HJ, et al. Olfactory heterogeneity in LRRK2 related parkinsonism. Mov Disord Epub 2010 Sep 3 [DOI] [PubMed] [Google Scholar]
- 9. Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease state, or disease duration. Neurology 1988;38:1237–1244 [DOI] [PubMed] [Google Scholar]
- 10. Katzenschlager R, Lees AJ. Olfaction and Parkinson's syndromes: its role in differential diagnosis. Curr Opin Neurol 2004;14:417–23 [DOI] [PubMed] [Google Scholar]
- 11. Doty RL, Golbe LI, McKeown DA, Stern MB, Lehrach CM, Crawford D. Olfactory testing differentiates between progressive supranuclear palsy and idiopathic Parkinson's disease. Neurology 1993;43:962–965 [DOI] [PubMed] [Google Scholar]
- 12. Wenning GK, Shephard B, Hawkes C, Petruckevitch A, Lees AJ, Quinn N. Olfactory function in atypical parkinsonian syndromes. Acta Neurol Scand 1995;91:247–250 [DOI] [PubMed] [Google Scholar]
- 13. Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cooksont N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein ϵ4. Neuropathol Appl Neurobiol 2003;29:503–510 [DOI] [PubMed] [Google Scholar]
- 14. Daniel SE, Hawkes CH. Preliminary diagnosis of Parkinson's disease by olfactory bulb pathology. Lancet 1992;340:186. [DOI] [PubMed] [Google Scholar]
- 15. Khan NL, Katzenschlager R, Watt H, et al. Olfaction differentiates parkin disease from early-onset parkinsonism and Parkinson's disease. Neurology 2004;62:1224–1226 [DOI] [PubMed] [Google Scholar]
- 16. Goker-Alpan O, Lopez G, Vithayathil J, Davis T, Hallet M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol 2008;65:1353–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferraris A, Ialongo T, Passali Gl, et al. Olfactory dysfunction in parkinsonism caused by PINK1 mutations. Mov Disord 2009;24:2350–2357 [DOI] [PubMed] [Google Scholar]
- 18. Saunders-Pullman R, Hagenah J, Dhawan V, et al. Gaucher disease ascertained through a Parkinson's center: imaging and clinical characteristics. Mov Disord Epub 2010 May 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khan NL, Jain S, Lynch JM, et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain 2005;128:2786–2796 [DOI] [PubMed] [Google Scholar]
- 20. Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk of future Parkinson's disease. Ann Neurol 2008;63:167–173 [DOI] [PubMed] [Google Scholar]
- 21. Pankratz N, Nichols WC, Uniacke SK, et al. Genome screen to identify susceptibility genes for Parkinson disease in a sample without parkin mutations. Am J Hum Genet 2002;71:124–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fahn S, Elton RL, members of the UPDRS Development Committee Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Caine DB, eds. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163 [Google Scholar]
- 23. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502 [DOI] [PubMed] [Google Scholar]
- 24. Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med 2006;354:424–425 [DOI] [PubMed] [Google Scholar]
- 25. Doty RL. The Smell Identification Test Administration Manual, 3rd ed. Haddon Heights, NJ: Sensonics; 1995 [Google Scholar]
- 26. Jennings D, Stern M, Siderowf A. The PARS study: evaluating pre-clinical parkinsonian features in a healthy hyposmic cohort. Neurology 2010;74(suppl 2):A76 Abstract [Google Scholar]
- 27. Hawkes CH. Olfaction in neurodegenerative disorder. Mov Disord 2003;18:354–372 [DOI] [PubMed] [Google Scholar]
- 28. Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters EC, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 2004;56:173–181 [DOI] [PubMed] [Google Scholar]
- 29. Siderowf A, Newberg A, Chou KL, et al. 99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology 2005;64:1716–1720 [DOI] [PubMed] [Google Scholar]
- 30. Sommer U, Hummel T, Cormann K, et al. Detection of presymptomatic parkinson's disease: combining smell tests, transcranial sonography, and SPECT. Mov Disord 2004;19:1196–1202 [DOI] [PubMed] [Google Scholar]
- 31. Muller A, Mungersdorf M, Reichmann H, Strehle G, Hummel T. Olfactory function in parkinsonian syndromes. J Clin Neurosci 2002;9:521–524 [DOI] [PubMed] [Google Scholar]
- 32. Haehner A, Hummel T, Hummel C, et al. Olfactory loss may be a first sign of idiopathic Parkinson's disease. Mov Disord 2007;2:839–842 [DOI] [PubMed] [Google Scholar]
- 33. Stanley K, Shanker V, Raymond D, et al. Olfactory dysfunction associated with LRRK2 G2019S mutations. Neurology 2010;74(suppl 2):A253 Abstract [Google Scholar]
- 34. Ross OA, Toft M, Whittle AJ, et al. LRRK2 and Lewy body disease. Ann Neurol 2006;59:388–393 [DOI] [PubMed] [Google Scholar]
- 35. Gaig C, Marti MJ, Ezquerra M, Rey MJ, Cardozo A, Tolosa E. G2019S LRRK2 mutations causing Parkinson's disease without Lewy bodies. J Neurol Neurosurg Psychiatry 2007;78:626–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gilks WP, Abou-Sleiman PM, Ghandi S, et al. A common LRRK2 mutation in Parkinson's disease. Lancet 2005;365:415–416 [DOI] [PubMed] [Google Scholar]
- 37. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]