Abstract
Background
The combination of genistein 27 mg, cholecalciferol 200 IU, citrated zinc bisglycinate (4 mg elemental zinc) 20 mg per capsule in Fosteum®, a prescription medical food regulated by the FDA and indicated for the dietary management of osteopenia and osteoporosis, was tested for drug interactions and to determine the pharmacokinetic profile for genistein, the principal bone-modulating ingredient in the product.
Methods
In vitro human liver microsome cytochrome P450 (CYP450) assays were used to test the product for potential drug interactions with the isoforms 1A2, 2C8, 2C9, 2C19, 2D6, and 3A4. Due to specific 2C8 and 2C9 inhibition, a steady-state pharmacokinetic study was performed to assess serum genistein concentrations by high-pressure liquid chromatography-coupled mass spectroscopy in healthy fasting (n = 10) and fed (n = 10) postmenopausal women.
Results
The product showed minimal inhibition of 1A2, 2C19, 2D6, and 3A4, exhibiting IC50 > 10 μM, but 2C8 and 2C9 yielded IC50 of 2.5 μM and 2.8 μM, respectively, concentrations which are theroretically achievable when dosing the product twice daily. After seven days of administration in a steady-state pharmacokinetic study, significant differences were found for unconjugated genistein (including free and protein-bound), regarding time to peak concentration (1.88 ± 1.36 hours), maximum concentration reached (0.052 ± 0.055 μM), elimination half-life (2.3 ± 1.6 hours), and area under the concentration-time curve (53.75 ± 17.59 ng · hour/mL) compared with results for total genistein (including glucuronidated and sulfonated conjugates) time to peak concentration (2.22 ± 1.09 hours), maximum concentration reached (2.95 ± 1.64 μM), elimination half-life (10.4 ± 4.1 hours), and area under the concentration-time curve (10424 ± 6290 ng · hour/mL) in fasting subjects. Coadministration of food tended to extend the time and extent of absorption as well as slow elimination of genistein, but not in a statistically significant manner.
Conclusion
Because the serum genistein concentrations achieved during pharmacokinetic testing at therapeutic doses were well below those required for enzyme inhibition in the in vitro liver microsome assays, these results indicate a low potential for drug interactions.
Keywords: genistein, metabolism, pharmacokinetics, drug interactions, medical food
Introduction
Renewed interest in botanically derived therapies has resulted in the recent marketing of products containing high concentrations of isoflavones for bone loss, both as food supplements and as prescription medical foods. Many epidemiological studies support an inverse relationship between isoflavone intake and bone loss and fracture rate. A large prospective study of 24,403 postmenopausal Chinese women, for example, related soy protein and isoflavone consumption to subsequent fracture incidence over a 4.5-year period.1 After age, body mass index, and lifestyle risk factors were controlled, a significant linear negative association was found for both soy protein and isoflavone consumption (≥21 mg daily) and fracture risk.
Genistein is an isoflavone found in small quantities in certain legumes throughout the plant kingdom (Figure 1). Unfermented soybeans are a particularly rich source of genistin, the glucoside precursor of genistein, although the concentration varies with the strain, location, and environmental conditions of cultivation of the plant. Another widely utilized source of genistin is Sophora japonica L.2 Asian populations, for whom fermented soy food and other isoflavone containing plants are dietary staples, are estimated to consume 25–100 mg of isoflavone daily.3 The majority of isoflavone consumption is in the form of aglycone rather than as glucosides. In contrast, intake of isoflavones in the US is estimated at only 0.15–3 mg per day, with much of it being in glucoside forms.4,5 Therefore, non-Asian populations may not reap the benefits of high intake of isoflavone, in particular, genistein aglycone.
Figure 1.
Genistein aglycone.
Mixed isoflavone studies demonstrate positive effects on bone markers and lipid profiles,6 vasomotor symptoms,7 and mood8 in humans, as well as memory in an experimental animal model.9 In ovariectomized osteoporotic rats, Bitto et al showed that genistein restored better quality bone than alendronate, raloxifene, and estradiol as measured by bone mineral density, metabolic bone markers, fracture resistance, and bone histology.10 Additional studies showed that genistein prevented and restored bone in animal models of secondary osteoporosis induced by steroids.11,12 In well-controlled clinical trials, purified genistein (54 mg/day) was shown to improve bone markers and increase bone mineral density over three years at a rate comparable with other standard therapies for osteoporosis.13–16 Other studies have demonstrated the ability of genistein to successfully manage vasomotor symptoms in postmenopausal women.17,18 Genistein, in experimental animal models, has anxiolytic and antidepressant effects.19–21 Genistein has an excellent cardiovascular safety profile in well controlled clinical trials.22 Finally, genistein has a positive cancer risk profile in humans.15,23,24
Despite the widespread consumption of soya, soy products, and their major isoflavones, little has been published regarding the metabolic fate of these molecules. Major metabolites are known to be glucuronides and sulfonates of isoflavones,25 but are poorly characterized, often because no reference standards are available26 and their influence on drug metabolic pathways is unknown. The extent to which genistein and its metabolites bind serum proteins in the body is not known, but is thought to be through an ionic interaction. Due to the introduction of purified and high-dose therapeutic genistein products onto the market, knowledge of the metabolism and pharmacokinetic profile of genistein is imperative if unanticipated interactions with other drugs are to be avoided.
A specially formulated medical food which contains genistein 27 mg, cholecalciferol 200 IU, and citrated zinc bisglycinate (4 mg elemental zinc) 20 mg per capsule (Fosteum®) is taken twice daily under physician supervision for the clinical dietary management of osteopenia and osteoporosis.27 In this study, the interaction of genistein from the formulation was assessed by cytochrome P450 (CYP450) enzyme inhibition assays in human liver microsomes. In addition, a steady-state pharmacokinetic study was performed in healthy fasting and fed postmenopausal female subjects to determine if serum genistein levels become sufficiently high to make drug interactions a possibility. Results for both in vitro drug metabolic studies for the product and the pharmacokinetic profile in postmenopausal women are presented for genistein.
Methods
All chemicals, except where noted, were purchased from Sigma-Aldrich, St Louis, MO. To screen for the potential of genistein drug interactions, the formulation was initially diluted from a 10 mM dimethylsulfoxide stock standardized for genistein and incubated in duplicate at final concentrations of 10 μM and 25 μM (genistein) with pooled probe substrates for CYP450 enzyme isoforms 1A2 (0.25 mg/mL), 2C8 (0.05 mg/mL), 2C9 (0.025 mg/mL), 2C19 (0.5 mg/mL), and 2D6 (0.1 mg/mL) in a 200 μL well volume in 96-well plates (Nunc A/S, Roskilde, Denmark) containing human liver microsomes (from Xenotech LLC, Lenexa, KS) (MDS Pharma Services, King of Prussia, PA).28 Substrate concentrations for each CYP450 isoform were set close to the Km (Table 1).
Table 1.
Standard probe substrates and metabolites (modified Greenford–Ware cocktail) for initial cytochrome P450 enzyme inhibition screens from the literature. Inhibitory activity using human liver microsomes found at 10 μM and 25 μM concentrations of genistein in Fosteum®. IC50 values found for genistein in Fosteum for inhibition of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and CYP2D6
| CYP450 isoform | Probe substrate
|
Probe metabolite | Liver microsome inhibition genistein (10 μM) | Liver microsome inhibition genistein (25 μM) | IC50† (μM) | ||
|---|---|---|---|---|---|---|---|
| Name | Assay concentration (μM) | * Km (μM) | |||||
| CYP1A2 | Phenacetin | 18.8 | 12.5 | Paracetamol | 20% | 46% | >50 |
| CYP2C8 | Paclitaxel | 1 | 5 | 6α-hydroxypaclitaxel | 53% | 80% | 2.5 |
| CYP2C9 | Tolbutamide | 120 | 166 | 4-hydroxytolbutamide | 59% | 87% | 2.8 |
| CYP2C19 | S-mephenytoin | 100 | 40 | 4-hydroxymephenytoin | 22% | 56% | 19 |
| CYP2D6 | Bufuralol | 10 | 5 to 10 | 1-hydroxybufuralol | 13% | 34% | >50 |
| CYP3A4 | Midazolam | 5 | 10 | 1-hydroxymidazolam | −1% | 19% | Not tested |
Notes: Km = the substrate concentration at which the velocity of an enzyme-catalyzed reaction is half maximal;
IC50 = half maximal inhibitory concentration.
Positive control inhibitors and the concentration at which each inhibited CYP450 isoforms to 100% were: furafylline (10 μM) for 1A2, montelukast (10 μM) for 2C8, sulfaphenazole (10 μM) for 2C9, benzylnirvanol (2 μM) for 2C19,29 quinidine (10 μM) for 2D6, and ketoconazole (1 μM) for 3A4. Each inhibitor was diluted from 10 mM dimethylsulfoxide stocks and assayed alongside the product formulation containing genistein diluted from a 2 mM stock dissolved in dimethylsulfoxide. After incubation for 30 minutes in a humidified incubator at 37°C, the reactions were terminated by adding 100 μL of acetonitrile. The assay utilized phosphate buffer (75 mM, pH 7.4) and the NADPH regenerating system (MgCl2, 3.3 mM; glucose-6-phosphate, 3.3 mM; glucose-6-phosphate dehydrogenase, 1 U/mL; NADP+, 1.3 mM) which was added at the beginning of each reaction incubation.
Specific inhibition of CYP450 enzymes was measured in duplicate standardized to the 10 μM of genistein contained in the formulation. The decrease in production of the specific probe metabolites (Table 1) was analyzed using liquid chromatography- mass spectroscopy/mass spectroscopy (LC-MS/MS, Applied Biosystems, Life Technologies Corp, Carlsbad, CA) along with selected reaction monitoring transitions. 30 The signal for each probe metabolite was integrated, and the metabolite to internal standard area ratio was generated. Percent inhibition was calculated by comparing the area ratios of the probe metabolite in the presence and absence of the test article. If genistein in the formulation inhibited an isoform to >50% at 10 μM, an analysis was then performed to establish an IC50 for each enzyme. CYP2C8 was also examined in IC50 experiments due to its homology with the 2C9 isoform31 to assess its potential for drug interaction.
The formulation was diluted from a 2 mM stock standardized for genistein in dimethylsulfoxide and incubated in triplicate at eight final concentrations from 0.016 to 50 μM in half-log steps to determine an IC50 for the CYP450 isoforms A2, 2C8, 2C9, 2C19, and 2D6 using the same assay system described above. The signals for each probe metabolite were integrated and probe metabolite to internal standard peak area ratios were generated. Percent control was calculated by comparing the area ratios of the probe metabolite in the presence and absence of genistein using the following formula:
where raw = peak area ratios of test article, min = peak area ratios of estimated lower limits of detection and max = peak area ratios of solvent control. IC50 s were fitted to the percent control data using XLFit (version 2.0 build 39, ID-BS Ltd). Although in vitro in nature and not tissue-specific, this analysis helps determine whether a human pharmacokinetic study is needed to assess the serum concentration and potential drug interactions with a medical food.
The pharmacokinetic study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board (Quorum IRB, Seattle, WA). Written informed consent was obtained from all subjects. Subjects were free to withdraw from the study at any time, for any reason. Twenty women (aged 50–66, mean 57), at least one year postmenopausal and in good general health were enrolled into the trial. To avoid dietary contamination of the study results, subjects were excluded if they had used supplements containing phytoestrogen/isoflavone for 60 days or consumed products containing soy or isoflavone for at least two weeks prior to the screening visit. Due to preliminary results suggesting the possibility of CYP2C8 or 2C9 inhibition by genistein, use of 2C8/2C9 substrates, ie, warfarin, amitryptyline, fluoxetine, diclofenac, ibuprofen, naproxen, celecoxib, phenytoin, losartan, irbesartan, glipizide, glyburide, and tolbutamide were proscribed for 60 days prior to screening and throughout the study period. No subject discontinued participation in the trial.
This was an open-label, multidose, steady-state pharmacokinetic study. Originally 26 subjects were screened with a physical examination and fasting hematology, chemistry safety laboratory panels, and by interview 1–2 weeks prior to the baseline visit. At the baseline visit, 20 subjects were randomized to take one capsule of the medical food formulation twice daily either with food (breakfast and supper) or on an empty stomach (one hour prior to breakfast and supper). The first dose was taken on the evening of the baseline visit. All subjects were instructed to report eight days later to begin pharmacokinetic sampling.
On the morning of the eighth day, subjects reported to the clinical research site, and trough serum samples were obtained, following which subjects took the study product either on an empty stomach (fasting) or with a light breakfast (fed). Subjects under the fasting condition consumed the breakfast after the one-hour sample draw. Blood samples (about 8 mL per sample) were obtained by venipuncture into vacutainer tubes and allowed to clot at room temperature. Serum samples were taken at hours 1, 2, 4, 6, 8, 10, 12, 24, 36, 48, 72, and 96 after dosing. Samples were then centrifuged for 15 minutes at 1500 rpm and the serum was aliquoted into cryovials and stored at −70°C. Subjects left the clinical site following the 12-hour sampling interval and returned for the 24-, 36-, 48-, 72- and 96-hour blood draws. The study was constructed to record any and all adverse events. All samples remained frozen until analysis by liquid chromatography and mass spectroscopy.
Isolation and high-performance liquid chromatography analysis was done according to Thomas et al, with modifications. 32 To determine unconjugated genistein (defined as free plus protein-bound), aliquots of 0.05 mL for each time point, as well as blank plasma obtained prior to consumption of the formulation and a deuterated genistein (genistein-d4) internal standard (0.1 mL of 50 ng/mL in 20% methanol in distilled water, Cambridge Isotopes Laboratories, Andover, MA) were added together to individual polypropylene culture tubes for extraction of unconjugated genistein. Then 0.2 mL of 0.25 M sodium acetate, pH 5.0, was added to each sample. The determination of total genistein (defined as the sum of unconjugated, glucuronidated, and sulfonated genistein) was performed by the addition of 0.1 mL of 900 U/mL β-glucuronidase and sulfatase in sodium acetate buffer followed by a 1.5-hour incubation at 40°C. Both unconjugated and total genistein samples were vortexed for one minute, then 2.5 mL of 1:1:1 hexane to methyl tert-butyl ether to methylene chloride extraction solvent was added to each tube. The samples were then vortexed gently for 15 minutes followed by a 10-minute centrifugation at 3000 rpm to separate the aqueous and organic layers. The aqueous layer of each sample and controls were then frozen at −80ºC, and the organic layer poured into 10 mL conical glass screw-cap tubes where they were dried with nitrogen gas at 40°C.
The dried extracts were reconstituted with 0.2 mL of 1:1 mobile phase buffer A (0.05% formic acid and 5 mM ammonium formate in distilled water) to mobile phase buffer B (0.05% formic acid and 5 mM ammonium formate in an 80:10:10 ratio, acetonitrile to methanol to distilled water). Samples were vigorously vortexed for five minutes and then centrifuged for two minutes at 1500 rpm to remove any insoluble material. The supernatants were removed and transferred to 0.25 mL polypropylene injection vials with caps for each chromatography run.
A standard curve using >98.5% pure genistein ( Primus Pharmaceuticals Inc, Scottsdale, AZ) in a 1:1 mixture of mobile phase buffer A to mobile phase buffer B was established by injecting 20 μL samples of 0.25 ng/mL to 10,000 ng/mL in two-fold increases. A 20 μL volume was first injected onto the loading column (MetaChem SafetyGuard ODS 10 × 4.6 mm, Agilent Technologies, Santa Clara, CA) and then onto the analytical C18 50 × 4.6 mm 5 μm column (Waters Atlantis, Waters Corp, Milford, MA). The LC program for the loading column was: injection of the sample followed by a one-minute isocratic wash with 20% buffer B, then a linear gradient in 0.5 minutes to 80% buffer B, followed by an isocratic wash with 80% buffer B and, finally, a wash step of 100% buffer B. The LC program after the sample traversed the loading column and then traveled through a switch valve (VICI Valco Instruments Co Inc, Houston, TX) onto the analytical column was: a one-minute isocratic wash with 50% buffer B, a linear gradient from 50%–80% buffer B in 0.5 minutes, followed by an isocratic wash with 80% buffer B. Genistein standards show a retention time of 2.78 minutes on the analytical column.
LC/MS/MS was used to determine the plasma concentration of genistein so that a comparison could be done with the IC50 values established for CYP2C8 and 2C9. The analysis of extracted samples was performed using a Sciex API4000 QTrap MS/MS (AB SCIEX Research and Development, Concord, ON, Canada) equipped with an ESP(+) interface, and was used with an ion source temperature of 450°C. For MS/MS measurements, a collision energy of 30 eV was used for the (M + H)+ transitions used to monitor unlabeled and genistein-d4 (m/z 271.0→215.1 and 275.1→219.1, respectively) with a dwell time of 0.15 seconds and a sampling cone-skimmer potential of 50 V. Isotope dilution for analysis of genistein and characterization of the genistein-d4 was done according to Holder et al for comparison with the samples obtained from both fasting and fed subjects.33
Total genistein concentrations were obtained at each time point in duplicate for each subject and pharmacokinetic analysis performed. The primary variables of interest were Cmax (the maximum observed concentration of total genistein), Tmax (the elapsed time at which Cmax was observed), T1/2 (the elapsed time at which genistein concentration was half of Cmax), and the imputed area under the curve (AUC) estimating the total body exposure to genistein over time. The AUC was computed by interpolating the concentrations of total genistein in the intervals between recordings using trapezoid calculations. Imputation was performed by cubic spline estimation. Each of these variables was computed for each participant, and mean values and standard deviations were computed for the sample. Any value exhibiting a >3 standard deviations (n = 3) from the mean was removed from the analysis. A Student’s t-test was conducted for each measure to see if the observed difference in means was significant. Descriptive statistics were presented for each of the primary outcome variables.
Results
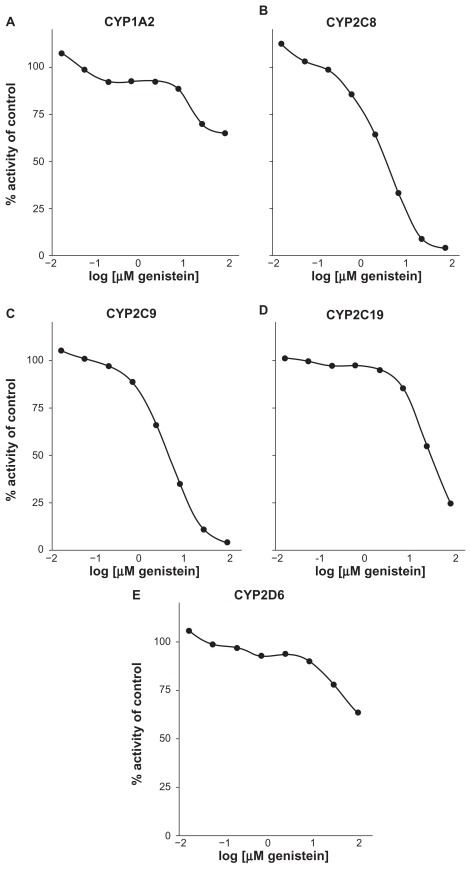
Data from a preliminary in vitro screen of CYP450 inhibitory activity using human liver microsomes at 10 μM and 25 μM concentrations of genistein from the formulation are shown in Table 1. Genistein from the medical food formulation inhibited CYP2C8 and CYP2C9 metabolism of 6α-hydroxypaclitaxel and 4-hydroxytolbutamide by 53% and 59%, respectively, at a 10 μM concentration, which suggests a significant inhibitory effect on both isozymes. Although below the cutoff threshold, genistein also displayed moderate (>20%) inhibition of CYP1A2 and CYP2C19, thus replicating the inhibition of CYP1A2 reported by Hu et al.34 Genistein showed a mild 13% inhibition of CYP2D6 at 10 μM. Based on these data, an additional concentration-ranging experiment was conducted to determine IC50 values for CYP1A2, CYP2C9, CYP2C8, CYP2C19, and CYP2D6.
Figures 2A–E shows the inhibition titration curves for CYP1A2, CYP2C8, CYP2C9, CYP2C19, and CYP2D6 to establish IC50 values. Table 1 also shows a summary of these IC50 titration values found in human liver microsomes. Genistein did not inhibit CYP1A2, CYP2C19, or CYP2D6 at concentrations that would suggest a drug interaction potential by this analysis (IC50s > 50, 19, and >50 μM, respectively, Table 1). The CYP3A4 isoform was not tested by IC50 analysis due to a low initial inhibition in vitro. These data raise the possibility that the genistein-containing formulation might inhibit the metabolism of CYP2C8 (IC50 = 2.5 μM) and CYP2C9 (IC50 = 2.8 μM) to a clinically significant degree. A human pharmacokinetic study was performed to evaluate this potential further.
Figure 2.
Genistein aglycone inhibition titration curves for CYP1A2 (A), CYP2C8 (B), CYP2C9 (C), CYP2C19 (D), and CYP2D6 (E).
To validate the LC methodology, serum was extracted and run under standard conditions. The added internal standard, ie, genistein-d4, was found among background peaks in serum not exposed to the formulation. Unconjugated genistein from subjects produced a distinct peak which aligned with the control genistein-d4 run in serum and with the deuterated standard run without exposure to serum (data not shown). The calibration peak of >98.5% for pure genistein used for determination of genistein concentration in each sample also corresponded directly to those concentrations determined by spiking serum with genistein-d4. Mass spectroscopy analysis of the serum containing unconjugated genistein showed the concentration to be low (30–500-fold less) compared with the total genistein which contained glucuronidated and sulfonated forms in either fasting or fed subjects. Descriptive statistics summarizing the 96-hour pharmacokinetic data are presented for both unconjugated (Table 2A) and total genistein (Table 2B).
Table 2.
Free compared with total genistein aglycone from the medical food formulation under fasting and fed prandial states in postmenopausal women
| A. Unconjugated genistein
| |||
|---|---|---|---|
| Subjects | n | Unconjugated genistein | Mean ± SD |
| Fasting | 8* | Cmax (ng/mL, μM) | 14.1 ± 14.8, 0.052 ± 0.055 |
| Tmax (hours) | 1.88 ± 1.36 | ||
| T1/2 (hours) | 2.3 ± 1.6 | ||
| AUC (ng · hr/mL) | 53.75 ± 17.59 | ||
| Age (yrs) | 56.8 ± 3.9 | ||
| Fed | 10 | Cmax (ng/mL, μM) | 3.01 ± 1.50, 0.011 ± 0.006 |
| Tmax (hours) | 2.10 ± 1.10 | ||
| T1/2 (hours) | 3.4 ± 2.1 | ||
| AUC (ng · hr/mL) | 11.3 ± 7.62 | ||
| Age (yrs) | 56.5 ± 5.7 | ||
|
B. Total genistein | |||
| Subjects | n | Total genistein | Mean ± SD |
| Fasting | 9* | Cmax (ng/mL, μM) | 798.0 ± 441.9, 2.95 ± 1.64 |
| Tmax (hours) | 2.22 ± 1.09 | ||
| T1/2 (hours) | 10.4 ± 4.1 | ||
| AUC (ng · hr/mL) | 10424 ± 6290 | ||
| Age (yrs) | 56.8 ± 3.9 | ||
| Fed | 10 | Cmax (ng/mL, μM) | 701.6 ± 310.7, 2.60 ± 1.15 |
| Tmax (hours) | 3.10 ± 1.73 | ||
| T1/2 (hours) | 11.8 ± 3.7 | ||
| AUC (ng · hr/mL) | 9775 ± 6157 | ||
| Age (yrs) | 56.5 ± 5.7 | ||
Note: Two subjects were removed from the unconjugated genistein analysis of the fasting PK data group and one subject from total genistein analysis of the fed group due to >3 standard deviation differences in Tmax values.
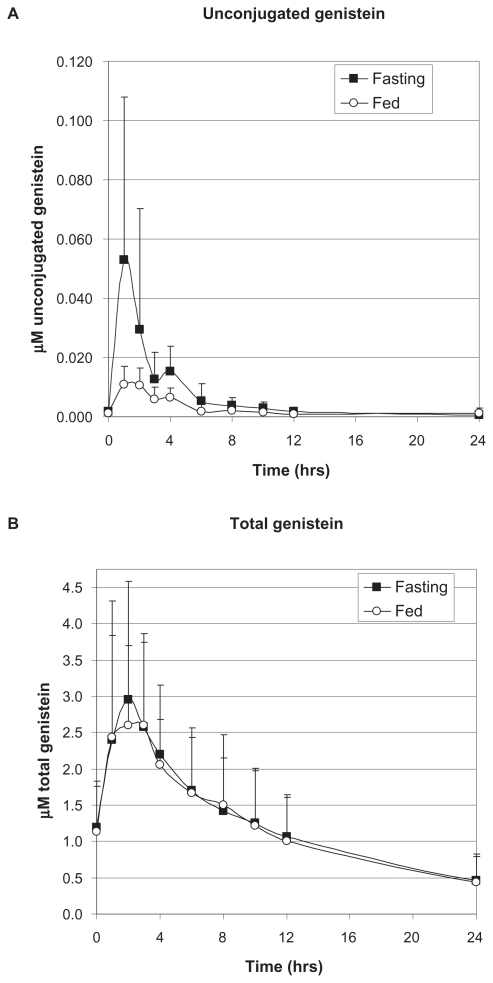
For unconjugated genistein among all fasting subjects, an average Cmax of 14.1 ± 14.8 ng/mL (0.0528 ± 0.077 μM) was reached at an average of 1.88 hours following the final dose of genistein, whereas the average Cmax for fed subjects, 3.01 ± 1.50 ng/mL (0.011 ± 0.006 μM), was reached at an average of 2.1 hours, suggesting that the food matrix decreased and delayed the absorption of genistein (Table 2A). There was no way to determine unconjugated genistein levels from serum after about 24 hours, no matter the fed state, due to limits of detection using the LC/MS method. Although the average mean Cmax for unconjugated genistein was lower among individuals who took the medical food formulation with food compared with fasting subjects, this difference was not statistically significant (P = 0.21). This may be a spurious result, however, due to inaccuracies in detection at low levels of genistein. Two subjects were removed from the unconjugated genistein analysis of the fasting pharmacokinetic data group and one subject from total genistein analysis of the fed group due to more than three standard deviation differences in Tmax values. In fasting subjects, total genistein reached an average Cmax of 798.0 ± 441.9 ng/mL (2.95 ± 1.64 μM) at an average of 2.22 hours following the final dose of genistein, whereas the average Cmax for fed subjects was 701.6 ± 310.7 ng/mL (2.60 ± 1.15 μM), reached at an average of 3.1 hours after the final dose, again suggesting that the food matrix delayed the time course of absorption (Table 2B). The difference in Cmax of total genistein between fasting and fed subjects was only about 14%. This difference was not statistically significant (P = 0.148). The T1/2 of unconjugated genistein for the fasting group was approximately 2.3 hours compared with 3.4 hours for the fed group, suggesting a delay in absorption due to the food matrix (Table 2A). These half-lives were much shorter compared with those found for total genistein, ie, 10.4 hours and 11.8 hours, respectively, for fasting and fed groups. This may be due to second-pass metabolism of the conjugated forms of genistein (Table 2B). Determination of an accurate AUC for unconjugated genistein was difficult due to low detection levels after 24 hours, so approximate AUCs are shown in Table 2A. Area under the curve of total genistein for the fasting group was 10423.7 ± 6290.0 ng · hr/mL and 9775.1 ± 6157.4 ng · hr/mL for the fed group, showing no significant difference (P = 0.79, Table 2B). The pharmacokinetic profile for both fasting and fed subjects is shown in Figure 3AB.
Figure 3.
Pharmacokinetic profile of unconjugated A) and total B) genistein from the medical food in fasting and fed prandial states.
The overall serum clearance of unconjugated genistein was very similar in both fasting and fed subjects, although the fasting group had a more rapid and greater uptake followed by a biphasic excretion (Figure 3A). Total genistein serum clearance, which includes both unconjugated genistein and its metabolites, shows initial equal uptake, but the fed group levels off before exhibiting a similar excretion profile (Figure 3B). This may also represent interference from the food matrix. No adverse events were reported during the study in either the fasting or fed group.
Discussion
The health benefits of isoflavones are directly related to their bioavailability. Bioavailability, in general, is dependent upon an individual’s state of health, bacterial flora in the gut, gender, age, the food matrix in which the compounds are consumed, the mix of isoflavones in the products, and host genetics.35 Genistein is freely absorbed from the intestine, and a large fraction is converted to the 7β-O-glucuronide as it crosses the brush border and ultimately enters the portal vein,36 a process that is influenced by intestinal bacteria.37,38 Recent ex vivo data using isolated human gastrointestinal tract tissue suggest that genistein may also undergo sulfonation in the small intestine, although the extent to which these sulfonates are absorbed following dietary intake is unknown.39 The exact percentages of glucuronidated and sulfonated metabolites after crossing the lumen are also unknown, although it is clear that only a small percentage of the parent molecule remains as unconjugated genistein once it reaches the liver. In the liver, genistein undergoes additional biotransformation via CYP450-mediated hydroxylation,34 followed by glucuronidation and sulfonation by UDP-glucuronosyltransferase and sulfotransferases, respectively.36 Genistein 7β-O-glucuronide can be recovered from bile after infusion of genistein into the small bowel of rats.40 As much as 70% of the genistein recovered from bile is in the form of glucuronidated conjugates, with smaller amounts reappearing in the distal duodenum and jejunum.36 The vast majority of circulating genistein in serum has been found to be in the form of glucuronidated and sulfonated conjugates, which represent excretion forms of the molecule.37 Little is known about the bioactivity of conjugated isoflavones. This represents an area of research that should be explored in the future.
In vitro screening for drug metabolism is an initial and common pharmaceutical industry practice, and required to assure safety of new prescription therapeutics.41 The use of one sample concentration (10 μM) at the apparent Km of the substrate provides a preliminary assessment of the Ki, as described by the Cheng-Prusoff equation.42 At the apparent Km of the substrate, the IC50 value is equal to or double the value of the Ki, for a noncompetitive or competitive inhibitor, respectively. Because most clinically important drug inhibitors have Kis < 10 μM, it is recommended that a sample showing inhibition of ≥50% be further characterized by determination of an IC50 or a Ki.
Genistein has not been shown to activate CYP450 metabolizing enzymes,43 although various inhibitory interactions with human CYP450 enzymes have been noted. Genistein has been found to inhibit minor CYP450 enzymes, such as CYP2A6 (34), CYP1A1*2, –1E and –2A1,44 and CYP1A1.45 Two concentrations, 10 μM and 25 μM, standardized to genistein in the medical food formulation were used in this current analysis. A 25 μM genistein concentration was also included in the analysis because previously published data showed moderate CYP450 inhibition for the 1A2 and 2C19 isoforms.34 The genistein in the formulation was tested for inhibition of six major drug metabolizing CYP450 enzymes as per industry standard drug interaction protocols, rather than focusing on the minor isozymes.46 No interactions of genistein with CYP2D6 were known, but this isozyme was also tested due to the large number of medications with which it interacts.
Genistein did not inhibit CYP2D6 to the extent that it would present a drug interaction potential. Hu et al found that genistein inhibited the CYP1A2 isozyme in vitro, with an IC50 of 16 μM.34 In our study, genistein inhibited CYP1A2 moderately (20%) in a comparable human liver microsome system at 10 μM, but, in contrast with the results of Hu et al, exhibited an IC50 > 50 μM (Table 1). Our results are more similar to those reported by Roberts-Kirchoff et al, who also found moderate inhibition of CYP1A2.45 Unlike data reported on inhibition of CYP3A4,45,47 genistein showed no inhibition at a 10 μM concentration (Table 1). Differences in CYP3A4 results may be due to the different probe substrates used in the analyses. Genistein showed only moderate inhibition of CYP2C19 (22%), but did not present the possibility for a drug interaction with an IC50 value of 19 μM (Table 1). Tolleson et al showed that CYP2C9 catalyzed genistein conversion.48 In our study, genistein inhibited CYP2C8 and CYP2C9 at a sufficient level (53% and 59%, respectively) to potentially cause drug interaction (Table 1). CYP2C8 and 2C9, with extensive gene sequence homology,31 showed similar inhibition, with genistein having IC50 s of 2.5 μM and 2.8 μM, respectively. CYP2C8 and 2C9 detoxify major drug classes, such as nonsteroidal anti-inflammatory drugs, antiepileptic and antidiabetic agents, statins, angiotensin II receptor antagonists, diuretics, and warfarin. Given the possibility that serum genistein levels at 54 mg per day could reach similar concentrations as the IC50 s for these two CYP450 isozymes, a pharmacokinetic study in postmenopausal women was initiated to determine the serum concentration achievable with the recommended therapeutic dose.
Results from isoflavone pharmacokinetic studies have been shown to vary with gender, concomitant food intake, and individual idiosyncrasy.49–51 Several single bolus mixed aglycone isoflavone studies have been performed from a food or supplement matrix. Setchell et al found that administering a single bolus of genistein at 50 mg (n = 6) gave a Tmax for total genistein of 5.2 hours, a Cmax of 341 ± 74 ng/mL (1.26 ± 0.27 μM), T1/2 of 6.78 ± 0.84 hours, and AUC of 4540 ± 1410 ng · h/mL, as determined by gas chromatography and mass spectroscopy analysis.52 Unconjugated genistein was very low and reached a peak at about 12.6 ng/mL ( approximately 0.047 μM). Similar results were obtained by Bloedon et al.53 The Tmax, Cmax, and AUC values from our study were probably greater than those found in previous studies due to the steady-state dosing regimen used in our protocol. Administration of approximately 35 mg of mixed genistein/daidzein over two and four weeks (n = 8) produced steady-state concentrations of 2 μM and 1.7 μM, respectively.54 Unconjugated genistein was not determined in this steady-state administration protocol. The total steady-state concentration is somewhat lower than what was observed in our study, but the difference may be due to the administration of a mixture of isoflavones rather than pure genistein and the lower daily dose. Similar to what was observed in the present study, large standard deviations for Tmax and Cmax were found, suggesting the variability of bacterial flora in the gut or differences in enzyme content and/or in luminal cells in individual subjects, which can modify genistein.
Ullman et al reported in a 14-day steady-state pharmacokinetic study using 60 mg of synthetic genistein (>98.5%, n = 10) a total genistein Cmax of 929.2 ± 327.1 ng/mL (approximately 3.4 μM).55 The AUC was 13544.8 ± 7222.3 ng · hr/mL. Tmax and T1/2 were 6.0 ± 4.0 and 9.7 ± 6.9 hours. The observed Tmax, Cmax, and AUC for the Ullman study,55 using synthetic genistein of similar purity, was similar to our findings, but T1/2 values were more than double what we observed for total genistein. Differences in isoflavone absorption and secretion have been noted between men and women.51 There was a higher proportion of males (n = 8) to females (n = 2) in the Ullman et al study.55 This is in contrast with our study, which was performed in postmenopausal women. A head-to-head study in equivalent populations is needed for proper comparison of naturally derived genistein and synthetic genistein to answer this question. Another study, in which 30 mg of synthetic genistein (98.5%) was given daily for seven days to 12 subjects, showed a total genistein Cmax of approximately 500 ng/mL, with a steady-state AUC of about 5940 ng · hr/mL, a Tmax of 5.3 hours, and a T1/2 of about 8.2 hours for the conjugated and about four hours for unconjugated genistein.56 More than likely, the Cmax and AUC are reduced compared with the Ullman et al study of the same product,55 based on half the dose (30 mg versus 60 mg) being administered.56 Differences in Tmax and T1/2 may be different due to the study containing all females56 compared with the Ullman et al study.55
Although the maximal amount of circulating total genistein found in our pharmacokinetic study is close to the IC50 values for CYP2C8 and 2C9 determined by in vitro studies, over 95% of circulating genistein exists in conjugated forms which are excreted.37 The extent to which genistein metabolites inhibit various CYP450 isozymes is currently unknown, and purified standards are not currently available for this analysis. Little is known about the tissue distribution of unconjugated and conjugated forms to assess tissue-specific interactions.
Using a mouse uterine cytosolic estrogen competitive binding analysis, Zhang et al found that genistein had a 48-fold higher affinity for estrogen receptors than its glucuronides.57 Normally, tissue studies are performed if the serum concentration is high enough to cause a drug interaction to determine tissue-specific accumulation or effects. However, in this case, the unconjugated level of genistein did not reach a level to necessitate tissue studies. Further work is needed on the conjugated forms of genistein to determine if they truly have the potential for drug interactions. Finally, major drug interactions in populations which consume high levels of aglycone isoflavones from fermented foods do not exist.
Deficiencies in this study include the inability to guarantee absolutely the absence of all isoflavone-containing oral intake and the lack of suitable analyses for genistein conjugates, the availability of which would have allowed for a slightly more comprehensive evaluation of total potential genistein bioavailability.
Conclusion
Although this medical food formulation shows a potential for drug interactions in vitro, a steady-state pharmacokinetic study demonstrates that the serum concentration of genistein attained by daily dosing of 54 mg in postmenopausal women does not reach a level which poses a hazard. The intake of food with the formulation, although not statistically significant, does seem to affect the uptake of genistein minimally. Therefore, care must be taken not to consume large amounts of nutrients which compete for intestinal receptors binding genistein, in order to assure proper uptake and bioavailability.
Acknowledgments
The authors wish to thank Dr Alan Kivitz of Altoona, Duncansville, PA, for excellence in conducting the clinical pharmacokinetic study; MDS Pharma Services, Bothell, WA, for CYP450 testing; and PPD Development LP, Chicago, IL, for competently performing the CYP450 analyses.
Footnotes
Disclosure
This study was funded by Primus Pharmaceuticals Inc, Scottsdale, AZ, which manufactures and markets Fosteum®. BPB, RML, and LP are employees of Primus Pharmaceuticals Inc. FS and AB have performed clinical and animal experimental work with genistein which has been partially funded by Primus Pharmaceuticals Inc.
References
- 1.Zhang X, Shu XO, Li H, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005;165(16):1890–1895. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 2.Tian Z, Wan M, Wang Z, Wang B. The preparation of genistein and LC-MS/MS on-line analysis. Drug Dev Res. 2004;61(1):6–12. [Google Scholar]
- 3.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55(1):1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 4.Horn-Ross PL, John EM, Lee M, et al. Phytoestrogen consumption and breast cancer risk in a multiethnic population: The Bay Area Breast Cancer Study. Am J Epidemiol. 2001;154(5):434–441. doi: 10.1093/aje/154.5.434. [DOI] [PubMed] [Google Scholar]
- 5.De Kleijn MJ, van der Schouw YT, Wilson PW, et al. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: The Framingham study(1–4) J Nutr. 2001;131(6):1826–1832. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- 6.Uesugi T, Fukui Y, Yamori Y. Beneficial effects of soybean isoflavone supplementation on bone metabolism and serum lipids in postmenopausal Japanese women: A four-week study. J Am Coll Nutr. 2002;21(2):97–102. doi: 10.1080/07315724.2002.10719200. [DOI] [PubMed] [Google Scholar]
- 7.Russo R, Corosu R. The clinical use of a preparation based on phytooestrogens in the treatment of menopausal disorders. Acta Biomed. 2003;74(3):137–143. [PubMed] [Google Scholar]
- 8.Casini ML, Marelli G, Papaleo E, Ferrari A, D’Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: A randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. 2006;85(4):972–978. doi: 10.1016/j.fertnstert.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Lund TD, Edwin D, Lephart ED. Manipulation of prenatal hormones and dietary phytoestrogens during adulthood alter the sexually dimorphic expression of visual spatial memory. BMC Neurosci. 2001;2:21. doi: 10.1186/1471-2202-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bitto A, Burnett BP, Polito F, et al. Effects of genistein aglycone in osteoporotic, ovariectomized rats: A comparison with alendronate, raloxifene and oestradiol. Br J Pharmacol. 2008;155(6):896–905. doi: 10.1038/bjp.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitto A, Burnett BP, Polito F, et al. Genistein aglycone reverses glucocorticoid- induced osteoporosis and increases bone breaking strength in rats: A comparative study with alendronate. Br J Pharmacol. 2009;156(8):1287–1295. doi: 10.1111/j.1476-5381.2008.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitto A, Polito F, Burnett B, et al. Protective effect of genistein aglycone on the development of osteonecrosis of the femoral head and secondary osteoporosis induced by methylprednisolone in rats. J Endocrinol. 2009;201(3):321–328. doi: 10.1677/JOE-08-0552. [DOI] [PubMed] [Google Scholar]
- 13.Morabito N, Crisafulli A, Vergara C, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17(10):1904–1912. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- 14.Marini H, Minutoli L, Polito F, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann Intern Med. 2007;146(12):839–847. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- 15.Marini H, Bitto A, Altavilla D, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J Clin Endocrinol Metab. 2008;93(12):4787–4796. doi: 10.1210/jc.2008-1087. [DOI] [PubMed] [Google Scholar]
- 16.Atteritano M, Mazzaferro S, Frisina A, et al. Genistein effects on quantitative ultrasound parameters and bone mineral density in osteopenic postmenopausal women. Osteoporos Int. 2009;20(11):1947–1954. doi: 10.1007/s00198-009-0883-4. [DOI] [PubMed] [Google Scholar]
- 17.D’Anna R, Cannata ML, Atteritano M, et al. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: A 1-year randomized, double-blind, placebo-controlled study. Menopause. 2007;14(4):648–655. doi: 10.1097/01.gme.0000248708.60698.98. [DOI] [PubMed] [Google Scholar]
- 18.Evans M, Elliott JG, Sharma P, Berman R, Guthrie N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: A multi-center, randomized, placebo-controlled study. Maturitas. 2011;68:189–196. doi: 10.1016/j.maturitas.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Sapronov NS, Kasakova SB. Effects of synthetic and plant-derived selective modulators of estrogen receptors on depression-like behavior of female rats. Bull Exp Biol Med. 2008;146(1):73–76. doi: 10.1007/s10517-008-0210-7. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Landa JF, Hernández-Figueroa JD, Hernández-Calderón Bdel C, Saavedra M. Anxiolytic-like effect of phytoestrogen genistein in rats with long-term absence of ovarian hormones in the black and white model. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):367–372. doi: 10.1016/j.pnpbp.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Kageyama A, Sakakibara H, Zhou W, et al. Genistein regulated serotonergic activity in the hippocampus of ovariectomized rats under forced swimming stress. Biosci Biotechnol Biochem. 2010;74(10):2005–2010. doi: 10.1271/bbb.100238. [DOI] [PubMed] [Google Scholar]
- 22.Altavilla D, Polito F, Marini H, et al. Effects of genistein aglycone on cardiovascular risk: The effects of the phytoestrogen genistein on the cardiovascular apparatus. Agrofood Industry Hi-Tech. 2008;19(5):16–17. [Google Scholar]
- 23.Atteritano M, Pernice F, Mazzaferro S, et al. Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebo-controlled study. Eur J Pharmacol. 2008;589(1–3):22–26. doi: 10.1016/j.ejphar.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 24.Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: A review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67(7):398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 25.Coldham NG, Howells LC, Santi A, et al. Biotransformation of genistein in the rat: elucidation of metabolite structure by product ion mass fragmentology. J Steroid Biochem Mol Biol. 1999;70(4–6):169–184. doi: 10.1016/s0960-0760(99)00104-1. [DOI] [PubMed] [Google Scholar]
- 26.Heinonen SM, Hoikkala A, Wähälä K, Adlercreutz H. Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects. Identification of new metabolites having an intact isoflavonoid skeleton. J Steroid Biochem Mol Biol. 2003;87(4–5):285–299. doi: 10.1016/j.jsbmb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Nieves JW. Nutritional therapies (including Fosteum) Curr Osteoporos Rep. 2009;7(1):5–11. doi: 10.1007/s11914-009-0002-7. [DOI] [PubMed] [Google Scholar]
- 28.Dierks EA, Stams KR, Lim HK, Cornelius G, Zhang H, Ball SE. A method for the simultaneous evaluation of the activities of seven major human drug-metabolizing cytochrome P450s using an in vitro cocktail of probe substrates and fast gradient liquid chromatography tandem mass spectrometry. Drug Metab Dispos. 2001;29(1):23–29. [PubMed] [Google Scholar]
- 29.Walsky RL, Obach RS. Verification of the selectivity of (+)N-3-benzylnirvanol as a CYP2C19 inhibitor. Drug Metab Dispos. 2003;31(3):343. doi: 10.1124/dmd.31.3.343. [DOI] [PubMed] [Google Scholar]
- 30.Ayrton J, Plumb R, Leavens WJ, Mallett D, Dickins M, Dear GJ. Application of a generic fast gradient liquid chromatography tandem mass spectrometry method for the analysis of cytochrome P450 probe substrates. Rapid Commun Mass Spectrom. 1998;12(5):217–224. doi: 10.1002/(SICI)1097-0231(19980314)12:5<217::AID-RCM146>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 31.Hersh EV, Moore PA. Drug interactions in dentistry: The importance of knowing your CYPs. J Am Dent Assoc. 2004;135(3):298–311. doi: 10.14219/jada.archive.2004.0178. [DOI] [PubMed] [Google Scholar]
- 32.Thomas BF, Zeisel SH, Busby MG, et al. Quantitative analysis of the principle soy isoflavones genistein, daidzein and glycitein, and their primary conjugated metabolites in human plasma and urine using reversed-phase high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 2001;760(2):191–205. doi: 10.1016/s0378-4347(01)00269-9. [DOI] [PubMed] [Google Scholar]
- 33.Holder CL, Churchwell MI, Doerge DR. Quantification of soy isoflavones, genistein and daidzein, and conjugates in rat blood using LC/ ES-MS. J Agric Food Chem. 1999;47(9):3764–3770. doi: 10.1021/jf9902651. [DOI] [PubMed] [Google Scholar]
- 34.Hu M, Krausz K, Chen J, et al. Identification of CYP1A2 as the main isoform for the phase I hydroxylated metabolism of genistein and a prodrug converting enzyme of methylated isoflavones. Drug Metab Dispos. 2003;31(7):924–931. doi: 10.1124/dmd.31.7.924. [DOI] [PubMed] [Google Scholar]
- 35.Larkin T, Price WE, Astheimer L. The key importance of soy isoflavone bioavailability to understanding health benefits. Crit Rev Food Sci Nutr. 2008;48(6):538–552. doi: 10.1080/10408390701542716. [DOI] [PubMed] [Google Scholar]
- 36.Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127(7):1260–1268. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- 37.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol – a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 38.Day AJ, DuPont MS, Ridley S, et al. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436(1):71–75. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 39.Ronis MJ, Little JM, Barone GW, Chen G, Radominska-Pandya A, Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food. 2006;9(3):348–355. doi: 10.1089/jmf.2006.9.348. [DOI] [PubMed] [Google Scholar]
- 40.Prasain JK, Xu J, Kirk M, Smith Johnson M, Sfakianos J, Barnes S. Differential biliary excretion of genistein metabolites following intraduodenal and intravenous infusion of genistin in female rats. J Nutr. 2006;136(12):2975–2979. doi: 10.1093/jn/136.12.2975. [DOI] [PubMed] [Google Scholar]
- 41.Bjornsson TD, Callaghan JT, Einolf HJ, et al. The conduct of in vitro and in vivo drug-drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31(7):815–832. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- 42.Craig DA. The Cheng-Prusoff relationship: Something lost in the translation. Trends Pharmacol Sci. 1993;14(3):89–91. doi: 10.1016/0165-6147(93)90070-z. [DOI] [PubMed] [Google Scholar]
- 43.Helsby NA, Williams J, Kerr D, Gescher A, Chipman JK. The isoflavones equol and genistein do not induce xenobiotic-metabolizing enzymes in mouse and in human cells. Xenobiotica. 1997;27(6):587–596. doi: 10.1080/004982597240361. [DOI] [PubMed] [Google Scholar]
- 44.Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography- mass spectrometry. Drug Metab Dispos. 2000;28(3):298–307. [PubMed] [Google Scholar]
- 45.Roberts-Kirchhoff ES, Crowley JR, Hollenberg PF, Kim H. Metabolism of genistein by rat and human cytochrome P450s. Chem Res Toxicol. 1999;12(7):610–616. doi: 10.1021/tx9802320. [DOI] [PubMed] [Google Scholar]
- 46.Glue P, Clement RP. Cytochrome P450 enzymes and drug metabolism – basic concepts and methods of assessment. Cell Mol Neurobiol. 1999;19(3):309–323. doi: 10.1023/a:1006993631057. [DOI] [PubMed] [Google Scholar]
- 47.Foster BC, Vandenhoek S, Hana J, et al. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. Phytomedicine. 2003;10(4):334–342. doi: 10.1078/094471103322004839. [DOI] [PubMed] [Google Scholar]
- 48.Tolleson WH, Doerge DR, Churchwell MI, Marques MM, Roberts DW. Metabolism of biochanin A and formononetin by human liver microsomes in vitro. J Agric Food Chem. 2002;50(17):4783–4790. doi: 10.1021/jf025549r. [DOI] [PubMed] [Google Scholar]
- 49.Shelnutt SR, Cimino CO, Wiggins PA, Badger TM. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiol Biomarkers Prev. 2000;9(4):413–419. [PubMed] [Google Scholar]
- 50.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76(3):588–594. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- 51.Cassidy A. Factors affecting the bioavailability of soy isoflavones in humans. J AOAC Int. 2006;89(4):1182–1188. [PubMed] [Google Scholar]
- 52.Setchell KDR, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 53.Bloedon LT, Jeffcoat AR, Lopaczynski W, et al. Safety and pharmacokinetics of purified soy isoflavones:Single-dose administration to postmenopausal women. Am J Clin Nutr. 2002;76(5):1126–1137. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- 54.Izumi T, Piskula MK, Osawa S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130(7):1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 55.Ullmann U, Oberwittle H, Grossmann M, Riegger C. Repeated oral once daily intake of increasing doses of the novel synthetic genistein product Bonistein in healthy volunteers. Planta Med. 2005;71(10):891–896. doi: 10.1055/s-2005-864186. [DOI] [PubMed] [Google Scholar]
- 56.Metzner JE, Frank T, Kunz I, Burger D, Riegger C. Study on the pharmacokinetics of synthetic genistein after multiple oral intake in postmenopausal women. Arzneimittelforschung. 2009;59(10):513–520. doi: 10.1055/s-0031-1296435. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr. 1999;129(2):399–405. doi: 10.1093/jn/129.2.399. [DOI] [PubMed] [Google Scholar]