Abstract
Antigen-presenting cells can capture antigens that are deposited in the skin, including vaccines given subcutaneously. These include different dendritic cells (DC) such as epidermal Langerhans cells (LC), dermal DC and dermal langerin+ DC.
To evaluate access of dermal antigens to skin DC, we used mAb to two C-type lectin endocytic receptors, DEC-205/CD205 and langerin/CD207. When applied to murine and human skin explant cultures, these mAb were efficiently taken up by epidermal LC. Additionally, anti-DEC-205 targeted langerin+ CD103+ and langerin− CD103− mouse dermal DC. Unexpectedly, intradermal injection of either mAb, but not isotype control, resulted in strong and rapid labelling of LC in situ, implying that large molecules can diffuse through the basement membrane into the epidermis. Epidermal LC targeted in vivo by ovalbumin-coupled anti-DEC-205 potently presented antigen to CD4+ and CD8+ T cells. Thus, epidermal LC play a major role in uptake of lectin-binding ligands under standard vaccination conditions.
Keywords: Skin, Dendritic Cells, Langerhans Cells, Langerin, Vaccination
INTRODUCTION
Dendritic cells (DC) are specialized to take up and present antigens, a feature now being considered in the design of vaccines (Steinman and Banchereau, 2007). Cutaneous DC, including epidermal Langerhans cells (LC) and dermal DC, are ideally positioned to take up skin-administered vaccines, process them and carry them to the draining lymph nodes, where they stimulate antigen-specific T cells (Romani et al., 2006). The immunogenic potential of LC in vivo depends on the dose and localization of the antigen (Stoitzner et al., 2008; Wang et al., 2008; Bennett et al., 2007).
C-type lectin receptors facilitate uptake and processing of antigenic proteins, and this ability has been exploited to improve immune responses by targeting antigens to DC (Tacken et al., 2007). The best-studied example is DEC-205/CD205, which is expressed at highest levels by select subsets of DC (Henri et al., 2001). When protein antigens are coupled to anti-DEC-205 mAb and mice are immunized with these conjugates in the presence of DC-activating agents, T cell-dependent immune responses (Bonifaz et al., 2004; Boscardin et al., 2006) are dramatically enhanced in vivo.
Langerin/CD207 is another C-type lectin, specifically expressed in the skin by epidermal LC, and by a recently described subset of mouse dermal DC (Valladeau et al., 2000; Valladeau et al., 2002; Kaplan et al., 2008). Antigen targeting via anti-langerin mAb also results in efficient presentation to CD4+ and CD8+ T cells (Idoyaga et al., 2008).
In many of the above-cited studies, immunization with anti-DEC-205 conjugates was performed by subcutaneous injection into the footpad. However, despite extensive research performed with anti-DEC-205 mAb, it has not been studied whether cutaneous DEC-205+ DC participate in uptake and transport of targeting mAb (Bonifaz et al., 2004; Carter et al., 2006). This question appears important in view of the differential roles that epidermal LC, dermal DC, and lymph node-resident DC seem to play (Kissenpfennig and Malissen, 2006; Allan et al., 2006). Therefore, we examined in more detail in situ uptake and handling of mAb against C-type lectins by skin DC, and, surprisingly, observed that intradermal mAb are captured by epidermal LC.
RESULTS
Langerhans cells in situ are specifically targeted by mAb in murine skin explant cultures
Epidermal LC and dermal langerin+ DC each express DEC-205 and langerin (Kraal et al., 1986; Stoitzner et al., 2003; Valladeau et al., 2002; Kaplan et al., 2008), while other dermal DC lack langerin and display low levels of DEC-205 (Henri et al., 2001). The density of langerin+ cells in the undisturbed dermis was about 5% of LC density in the epidermis (~50–80 versus ~1000 per mm2 in BALB/c). Dermal langerin+ cells are emigrating epidermal LC (Stoitzner et al., 2003) as well as DC that reside in the dermis (Kaplan et al., 2008).
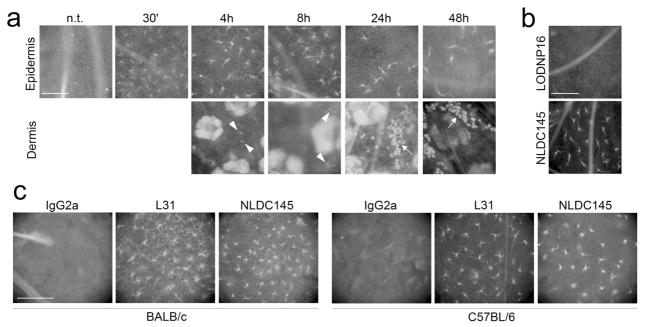
To determine if these DC subsets would capture anti-receptor antibodies, whole skin explants from BALB/c mice were cultured for different times in hybridoma supernatant NLDC145 (anti-DEC-205) or control nonreactive IgG2a, LODNP16. Then, epidermal and dermal sheets were prepared. Labelling was evident on epidermal LC within 30 min with NLDC145, was brightest after 2–4 h, and persisted at least 48 h (Fig. 1a). Conversely, no binding to LC was evident with LODNP16 (Fig. 1b). Similar rapid targeting was observed when anti-langerin Ab L31 were used, and equivalent labelling was seen with whole skin from BALB/c and C57BL/6 mice (Fig. 1c).
Figure 1. Langerhans cells in situ are specifically targeted by mAb in murine skin explant cultures.
(a): Whole skin explants from BALB/c mice were incubated for 30′ to 48h in culture medium containing NLDC145 (anti-mouse DEC-205) hybridoma supernatant. Epidermis was then separated from dermis, fixed, and targeting mAb was revealed with anti-rat IgG secondary Ab. Arrowheads indicate dermal cells targeted in situ. Arrows point at DC-filled dermal lymphatic vessels. (b): Whole skin explants from BALB/c mice were incubated for 18 h in culture medium containing NLDC145 or LODNP16 (isotype control) hybridoma supernatants, then epidermis was separated, fixed, and stained with anti-rat IgG Ab. (c): Whole skin explants from BALB/c or C57BL/6 mice were incubated for 4h with 5μg/mL of the indicated purified mAb, then epidermis was separated, fixed, and stained with anti-rat IgG Ab. Scale bars = 100μm. Results are representative of three independent experiments.
In keeping with the absence of DEC-205 staining in the dermis (Kraal et al., 1986), dermal cells that had taken up anti-DEC-205 mAb were rarely observed before 18h of culture (Fig. 1A, bottom; arrowheads). At time points beyond 18h, NLDC145 was detected in considerably more dermal cells. Many of them were arranged in typical “cords”, which represent dermal lymphatic vessels filled with migratory LC (Stoitzner et al., 2003) (Fig. 1a, bottom; arrow).
Migratory skin DC transport anti-DEC-205 and anti-langerin mAb following ex vivo targeting
To extend our in situ observations, we analysed by FACS migratory cells from whole skin explants that had been pre-incubated in targeting mAb for 3h, washed extensively, then returned to culture to allow DC to “crawl out” of the explants. In addition to anti-DEC-205, we used L31, a new mAb to langerin. As opposed to 929F3 mAb, which recognizes an intracellular domain of langerin, L31 binds to the extracellular carbohydrate-recognition domain (Cheong et al., 2007; Idoyaga et al., 2008).
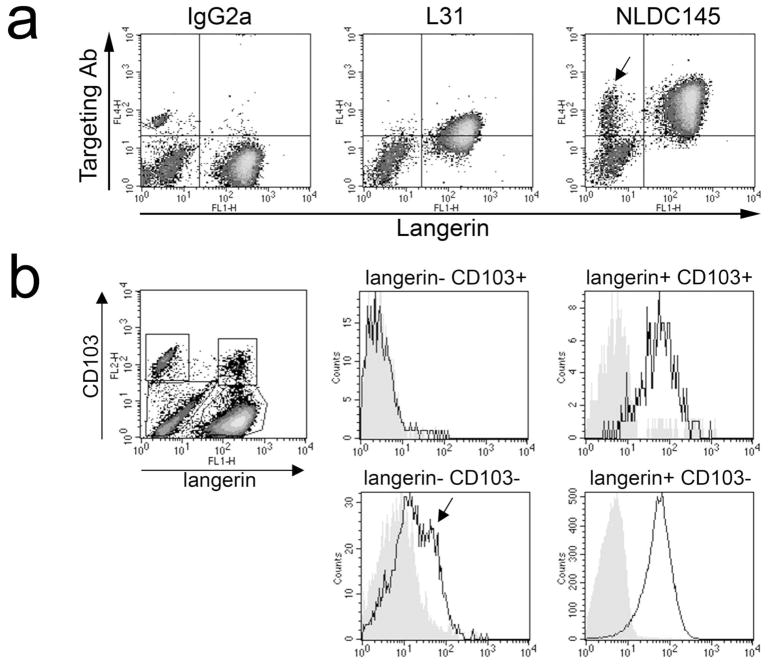
More than 85% of the migratory cells expressed langerin (Fig. 2a). NLDC145 was primarily taken up by langerin+ cells, with the exception of a small langerin− population (~5% of all migratory cells; Fig. 2a, right panel, arrow). As expected, L31 targeting mAb was exclusively detected on langerin+ cells, albeit at lower levels than NLDC145. Of note, the levels of the targeting mAb decreased with time in culture, possibly resulting from a degradation of endocytosed mAb (day 2 vs. day 4; data not shown).
Figure 2. Langerhans cells and dermal DC migrating out of murine skin explants transport the targeting mAb.
Whole skin explants were incubated for 3h in medium containing L31 (anti-langerin), NLDC145 (anti-DEC-205), or IgG2a isotype control, washed in PBS and cultured for 3 days. Targeting mAb was detected in permeabilised migratory cells by anti-rat IgG Ab. Cells were counterstained with 929F3 mAb to an epitope on the cytosolic domain of langerin in (a) or 929F3 anti-langerin plus anti-CD103 in (b). Histograms in (b) show expression of the targeting mAb on subsets gated as depicted in the density plot on the left. Filled histograms: IgG2a; empty histograms: anti-DEC-205. Results are representative of three independent experiments.
To investigate the relative contributions of epidermal LC and dermal langerin+ DC, we also studied expression of CD103, an integrin selectively expressed by the latter subset (Kaplan et al., 2008). A small proportion (< 3%) of langerin+ migratory DC expressed CD103 and were efficiently targeted by anti-DEC-205 (Fig. 2b). Among langerin− migratory cells, which most likely originate in the dermis, only CD103− DC bore the targeting mAb to DEC-205.
Epidermal LC are specifically targeted in vivo by mAb injected intradermally
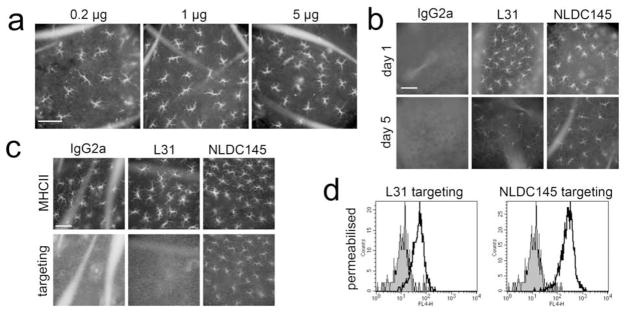
To examine whether targeting mAb would also reach epidermal LC in a standard immunisation procedure, we prepared epidermal sheets 18h after intradermal injection. As little as 0.2 μg of purified NLDC145 was sufficient to visualize epidermal LC in situ 18h after intradermal injection (Fig. 3a). For further experiments, we injected 1 μg to obtain a brighter staining. No staining could be observed with IgG2a isotype control (Fig. 3b). Interestingly, L31 and NLDC145 were still detectable on LC for up to 5 days, although labelling was clearly weaker at this late time point (Fig. 3b).
Figure 3. Langerhans cells are targeted within the epidermis following intradermal injection of anti-lectin antibodies in vivo.
Different targeting mAb were injected intradermally into the ear skin of C57BL/6 mice. Epidermal sheets were prepared 18 h after injection of 0.2, 1, or 5 μg of NLDC145 (a) and 24h or 5 days after injection of 1 μg IgG2a, L31, or NLDC145 (b). The capture of the injected mAb was visualized with anti-rat IgG Ab. (c): 18h after injection of 1μg targeting mAb, MHC class II (upper panel) and targeting mAb (lower panel) were revealed on epidermal sheets from langerin−/− mice. (d): 4 h after injection of 1μg targeting mAb into ear skin of BALB/c mice, whole skin explants were cultured for 3 days. The targeting mAb was detected in permeabilised migratory cells (gated on langerin+ cells; mAb 929F3). Filled histograms: IgG2a; empty histograms: mAb as indicated. Scale bars = 100μm. Results are representative of three independent experiments.
Next, we used langerin−/− mice (Kissenpfennig et al., 2005), which have a normal distribution of epidermal MHC class II+ LC (Fig. 3c, top). As anticipated, langerin−/− LC showed normal binding of intradermally injected anti-DEC-205 mAb, but failed to be targeted by anti-langerin L31 mAb (Fig. 3c, bottom).
Finally, whole skin explants were prepared 4 h after intradermal injection of targeting mAb, washed in PBS, and cultured for 3 days. Langerin+ DC that had been targeted in vivo retained detectable amounts of the L31 and NLDC145 targeting mAb after migrating out of skin explants (Fig. 3d). Targeting intensity was more pronounced for NLDC145, and it declined markedly with time for both targeting mAb (day 2 vs. day 4; data not shown).
Epidermal LC present DEC-205–targeted antigen following in vivo uptake
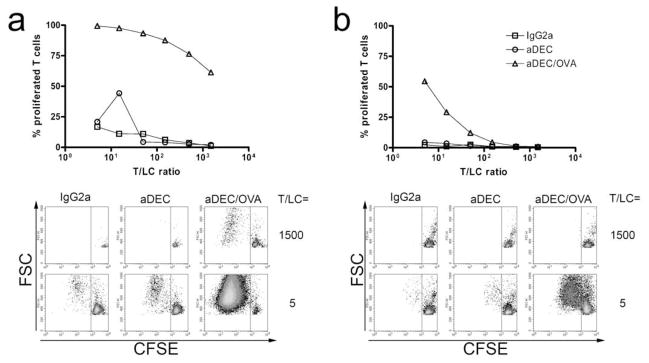
We next used anti-DEC-205 coupled to the model antigen ovalbumin (anti-DEC/OVA) to evaluate antigen presentation by epidermal LC targeted in vivo. 4h after intradermal injection of anti-DEC/OVA or control antibodies, we prepared LC by culturing epidermal sheets for 3 days. Migratory LC were then cultured at different ratios with CFSE-labelled, OVA-specific CD4+ or CD8+ T cells from OT-II or OT-I mice, respectively. Proliferation was evaluated by the percentage of T cells exhibiting low CFSE stainings. We observed efficient presentation of OVA by anti-DEC/OVA-targeted epidermal LC to CD8+ T cells (Fig. 4a). CD4+ T cells also proliferated, although to a considerably lower extent (Fig. 4b).
Figure 4. Langerhans cells present DEC-205–targeted antigen following in vivo uptake.
4h after i.d. injection of 0.5μg of OVA-coupled anti-DEC-205, or indicated control reagents, epidermal sheets were prepared and cultured for 3 days. Migratory epidermal LC were then cultured with CFSE-labelled CD8+ (a) or CD4+ (b) T cells purified from OT-II or OT-I mice, respectively. After 4 days, T cell proliferation was evaluated by dilution of CFSE. Upper panels show T cell proliferation observed at different T/LC ratios, and lower panels display typical FACS stainings. Results are representative of two independent experiments.
Human skin DC bind and transport anti-DEC-205 and anti-langerin mAb
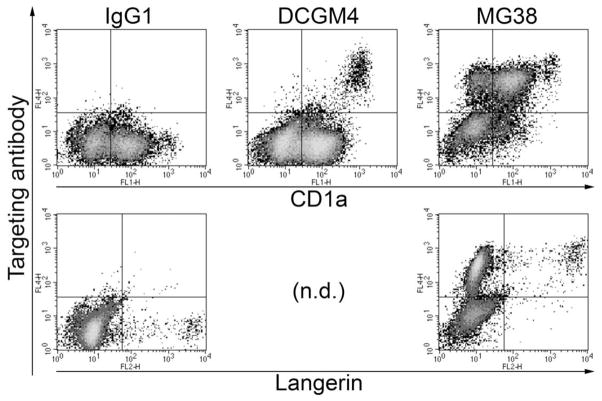
Finally, we extended our observations to human skin DC subsets, which we targeted in situ in whole skin explant cultures. After 4 days, we observed 3 distinct populations of migratory DC targeted by anti-human DEC-205 mAb, CD1aneg/low, CD1a+ and CD1ahigh cells (Fig. 5, top). Anti-DEC-205 was absent (or very low) on a proportion of the CD1aneg/low cells. Only CD1ahigh DC could bind anti-langerin mAb, indicating that they are actually LC, while CD1a+ langerin− and CD1a− langerin− DC (Fig. 5, bottom) probably originate in the dermis (Ebner et al., 2004).
Figure 5. Human skin DC bind and transport targeting mAb.
Human whole skin explants were incubated for 4h in medium containing DCGM4 (anti-langerin), MG38 (anti-DEC-205), or IgG1 isotype control, then cultured for 4 days. Targeting mAb were detected in permeabilised migratory cells by means of anti-mouse IgG secondary Ab. Cells were counterstained with anti-CD1a or anti-langerin. n.d., not determined. Results are representative of two independent experiments.
DISCUSSION
Targeting antigens to DC with the help of specific mAb has great potential for the development of effective vaccines (Tacken et al., 2007). We found that anti-langerin and anti-DEC-205 mAb are captured in a selective manner by mouse and human skin DC, and that targeted DC carry mAb when leaving the skin. Fc receptor-mediated uptake by LC could be formally excluded, as demonstrated by the lack of binding of non-specific control mAb in wild-type mice, and of anti-langerin mAb in langerin−/− mice.
Both anti-langerin and anti-DEC-205 were taken up by epidermal LC within minutes after intradermal injection. This indicates that even large immunoglobulins (150 kDa) can easily diffuse from the dermis through the basement membrane into the epidermis and gain access to LC. Of note, the density of LC in the epidermal sheets remained roughly unchanged, showing that intradermal injection of targeting mAb in PBS did not result in a degree of inflammation that would have enhanced emigration of LC. Moreover, targeting mAb persisted on LC for several days, suggesting that their degradation occurs very slowly following in vivo uptake. This remarkable property implies that targeted DC constitute a pool of antigen-presenting cells that could be exploited throughout an extended period.
In the mouse, the main target of anti-DEC-205 was epidermal LC. In skin explant cultures, targeting mAb were able to access LC in the epidermis, while relatively few dermal cells acquired mAb. However, due to the relatively high background staining of the dermis, we could not exclude the existence of targeted dermal DC displaying only low levels of NLDC145 in situ. In fact, when migratory cells were analysed by flow cytometry, langerin− CD103− and langerin+ CD103+ dermal DC subsets had also internalised anti-DEC-205, albeit to a much lower extent in terms of quantity and cell numbers, respectively.
In parallel, human skin explant cultures also revealed efficient labelling of human skin DC by anti-lectin mAb. Targeting of epidermal LC was achieved by both anti-langerin and anti-DEC-205 mAb, while two distinct DC populations exclusively bound anti-DEC-205. We did not further investigate these two subsets, since human dermal DC remain less well defined, and their phenotype and distribution differ markedly from what is observed in a murine system (Zaba et al., 2008; Tacken et al., 2007; Ebner et al., 2004). Nevertheless, our findings suffice to indicate that mAb injected into human skin would efficiently target skin DC, including LC, in situ.
Finally, in vivo targeted epidermal LC were able to present the model antigen ovalbumin coupled to anti-DEC-205. Interestingly, targeting through DEC-205 appeared to preferentially lead to cross-presentation to CD8+ T cells, while only limited CD4+ T cell proliferation could be induced, as already proposed in the literature (Dudziak et al., 2007). Future investigations will focus on defining the functional roles of skin DC targeted in vivo in cutaneous immune responses.
MATERIALS AND METHODS
Mice
Inbred BALB/c, C57BL/6, OT-I and OT-II mice were purchased from Charles River Laboratories (Sulzfeld, Germany) and used at 2–10 months of age. Experiments were performed according to governmental guidelines. Langerin-deficient mice (Kissenpfennig et al., 2005) were kindly supplied by Dr. S. Saeland.
Media and reagents
Complete culture medium was RPMI-1640 supplemented with 10% heat-inactivated FCS, 2mM L-glutamine (Sigma, St. Louis, MO), 50 μg/ml gentamicin (PAA, Linz, Austria), and 50μM beta-mercaptoethanol. Targeting by rat mAb was detected in situ by goat anti-rat IgG/FITC (BD-Biosciences, San Diego, CA), or chicken anti-rat IgG/Alexa 594 (Invitrogen, Eugene, OR). For FACS analyses, permeabilisation was performed with Cytofix/perm (BD), and targeting mAb was detected with goat anti-rat IgG/APC in murine samples, or rat anti-mouse IgG/APC in human samples (BD). For analyses of murine cells, we used mAb to MHC class II (anti-I-A/I-Ediverse, clone 2G9), CD103 (clone M290) (BD), and langerin (clone 929F3; Dendritics, Lyon, France), while human cells were stained with mAb to CD1a (clone HI149; BD) or langerin (clone DCGM4; Dendritics).
Targeting mAb
L31 mAb recognizing an extracellular portion of murine langerin was used to target langerin as described (Cheong et al., 2007), and NLDC145 mAb (BMA Biomedicals, Augst, Switzerland) to target murine DEC-205 (Kraal et al., 1986). Ovalbumin-coupled anti-DEC-205 (aDEC/OVA) was used in OVA-specific T cell proliferation assays (Boscardin et al., 2006). Control mAb was rat IgG2a (R&D Systems, Minneapolis, MN). Human skin cells were targeted by anti-human DEC-205 (clone MG38) (Guo et al., 2000) or anti-human langerin (clone DCGM4) (Valladeau et al., 2000). Mouse IgG1 (BD) was used as isotype control.
Counts of dermal langerin+ cells density
Numbers were determined on dermal sheet specimens from several different C57BL/6 and BALB/c mice, stained by anti-langerin mAb (929F3) and revealed by secondary goat-anti-rat Ig conjugated to Alexa 594. In total, langerin+ cells in 40 randomly selected microscopical fields were counted and areal density determined by means of a calibrated ocular grid.
Mouse skin explant culture and in vitro targeting experiments
Briefly, mice were killed, ears were cut off at the base, and ear skin was split into dorsal and ventral halves (Stoitzner et al., 2003). The dorsal halves were cultured in 24-well plates (one per well), for the indicated periods, in complete culture medium containing 10% (v/v) of hybridoma supernatants (NLDC145, or LODNP16/IgG2a isotype control). Finally, epidermal and dermal sheets were separated with ammonium thiocyanate and fixed in acetone. For cell migration experiments, ear skin explants from BALB/c mice were exposed to 5μg/mL targeting mAb (IgG2a, L31 or NLDC145) for 3h at 37°C, then extensively washed in PBS, to avoid binding of targeting mAb to migratory cells outside of the skin. Explants were further cultured in complete medium without chemokines. After 2 to 4 days, emigrant DC were harvested and investigated by flow cytometry. Alternatively, 4h after in vivo targeting (see below), epidermis was separated from dermis with dispase and cultured for 3 days. Targeted migratory epidermal LC were then used in T cell proliferation assays.
In vivo targeting experiments
0.2–5μg of purified rat IgG2a, L31, NLDC145 or aDEC/OVA diluted in 25μL PBS were injected into the ear pinna of anaesthesized C57BL/6 or langerin−/− mice.
In vitro T cell proliferation assays
OVA-specific T cells were obtained by MACS purification (Miltenyi-Biotec, Bergisch Gladbach, Germany) of CD4+ and CD8+ T cells from lymphoid organs of OT-II and OT-I mice, respectively. Purified T cells were labelled by CFSE, and cultured in flat-bottom microwells with in vivo targeted epidermal LC for 4 days, at different T/LC ratios (150000 T cells per well). CFSE dilution, monitored by FACS, allowed to evaluate T cell proliferation.
Humanskin explant culture and in vitro targeting experiments
Healthy human split-thickness skin was obtained from plastic surgery (Ebner et al., 2004), and floated on culture medium containing 5μg/mL targeting mAb (IgG1, DCGM4 and MG38) for 3h at 37°C. Then, explants were thoroughly washed in PBS, and cultured in 6-well plates. 4 days later, emigrant DC were harvested and investigated by FACS.
Statistical analyses
Experiments were performed at least three times with similar results. Error bars are from standard deviations. P-values are from two-tailed Student’s t-tests.
Acknowledgments
We are particularly indebted to Dr. Barbara del Frari for providing us with human skin, and to Pr. Peter Fritsch, Chairman of the Department of Dermatology, for his continued support. VF and CHT are supported by the Austrian Science Fund (FWF L120-B13 to NR), and SE by the Kompetenzzentrum Medizin Tirol (CEMIT-03b to NR). PS is recipient of a research grant from Innsbruck Medical University (MFI-9442). CGP is a recipient of National Institutes of Health grant AI 057158. RMS and JI are supported by the Center for AIDS Vaccine Discovery, National Institutes of Health (Grants AI 13013, AI 40874, and AI 057158 to RMS) and the Canadian Histiocytosis Association.
Abbreviations
- LC
Langerhans cell(s)
- DC
dendritic cell(s)
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Reference List
- 1.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 3.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii SI, Soares H, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boscardin SB, Hafalla JCR, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol. 2006;177:2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 6.Cheong C, Idoyaga J, Do Y, Pack M, Park SH, Lee H, et al. Production of monoclonal antibodies that recognize the extracellular domain of mouse Langerin/CD207. J Immunol Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 8.Ebner S, Ehammer Z, Holzmann S, Schwingshackl P, Forstner M, Stoitzner P, et al. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int Immunol. 2004;16:877–887. doi: 10.1093/intimm/dxh088. [DOI] [PubMed] [Google Scholar]
- 9.Guo M, Gong SC, Maric S, Misulovin Z, Pack M, Mahnke K, et al. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Human Immunol. 2000;61:729–738. doi: 10.1016/s0198-8859(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 10.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 11.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, et al. Cutting Edge: Langerin/CD207 Receptor on Dendritic Cells Mediates Efficient Antigen Presentation on MHC I and II Products In Vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 13.Kissenpfennig A, Aït-Yahia S, Clair-Moninot V, Stössel H, Badell E, Bordat Y, et al. Disruption of the langerin/CD207 gene abolishes Birbeck granules without a marked loss of Langerhans cell function. Mol Cell Biol. 2005;25:88–99. doi: 10.1128/MCB.25.1.88-99.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kissenpfennig A, Malissen B. Langerhans cells - revisiting the paradigm using genetically engineered mice. Trends Immunol. 2006;27:132–139. doi: 10.1016/j.it.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Kraal G, Breel M, Janse M, Bruin G. Langerhans’ cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells - changing views on their function in vivo. Immunol Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 18.Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, et al. Tumor immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 19.Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stössel H, Kapp M, et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 20.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 21.Valladeau J, Clair-Moninot V, Dezutter-Dambuyant C, Pin JJ, Kissenpfennig A, Mattéi MG, et al. Identification of mouse Langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J Immunol. 2002;168:782–792. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- 22.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin Expressing Cells Promote Skin Immune Responses under Defined Conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 24.Zaba LC, Krueger JG, Lowes MA. Resident and “Inflammatory” Dendritic Cells in Human Skin. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]