Abstract
Myoblasts isolated from duck embryonic muscle were purified and in vitro cultured. External characteristics were observed by using the immunofluorescence technique, and growth curve of duck embryonic myoblasts was established after measuring with the MTT method. Moreover, mRNA expression of three marker genes, the Desmin, the muscle creatine kinase (Mck) and the troponin C (Tnnc), which could reflect the development status of myofibers, were detected each 24 h for cultured cells by using the qPCR technique. Results showed that the in vitro cultured duck myoblasts went through a series of developmental stages, including the proliferation of myoblasts, the differentiation of multi-nuclei myotubes, and the formation of myofiber. The cultured duck embryonic myoblasts entered into a logarithmic stage approximately on the fourth day after seeding. Accompanying with its progressive growth before entering into the logarithmic phase, the myoblasts also showed some differentiation phenomena, reflected by a low expression level of Desmin and high expression level of the Mck and Tnnc genes. During the rapid growth of the logarithmic phase, there was a high expression of the Desmin gene, and a low expression level of the Mck gene and the Tnnc gene in the cultured myoblasts. The expression profiles of the three marker genes for muscle development could be used for distinguishing the different developmental stages of in vitro cultured myoblasts at the molecular level, which would be more accurate and more feasible than observing the external characteristics of the cultured cells.
Keywords: Gene expression, Embryonic myoblasts, Cell growth
Introduction
Peking duck (Anas platyrhynchos Domestica) is a world famous poultry species for its tender meat, which accounts for a large proportion of the world’s poultry meat production. Many phenotypic traits related to the muscle production have been selected for many years by breeding scientists, and lots of candidate genes have been confirmed to have close relationships with the potential ability of animal muscle growth by using SSCP, RFLP, RAPD techniques (Dolmatova et al. 2000; Jeroncic et al. 2010; Xu et al. 2007). These genes also could be used for molecular maker-assisted selection in duck (Dong et al. 2007; Kunhareang et al. 2009).
It’s reported that the potential abilities for muscle growth of adult livestock is determined at the embryonic stages, as the quantity of myofiber does not increase any more postnatal and it has been fixed during the fetal development for most of the animals. Hypertrophy of myofibers postnatally is only because of lengthening and thickening of myofibers (Cheng et al. 2005; Dhawan and Rando 2005. Myofiber originates from the myoblasts, and depends on their fusion and differentiation (Bischoff 1986). Therefore, researching on the proliferation and differentiation of embryonic myoblasts is significant to the muscle growth in the fields of medication and agriculture (McNally and Pytel 2007; Rando and Blau 1994; Matsuoka and Inoue 2008). The myoblasts growth characteristics in different organisms are different (Konieczny et al. 1982). Techniques to study the gene regulation mechanism on a molecular level, like RNA interference (RNAi) and gene transfection, could be performed for a better understanding of the myoblasts growth characteristics. Myoblasts have been isolated and cultured in many species including mouse, dog, chicken, and so on (Linkhart et al. 1980; Tonlorenzi et al. 2007), except for duck. The absent information of duck’s myoblasts is the main obstacles for further investigating the mechanisms of muscle development in duck.
Three marker genes, Desmin, muscle creatine kinase (Mck) and troponin C (Tnnc) have been used to identify the different states of myofiber development in vivo. Among them, Desmin will be highly expressed when myoblasts reentering into the cell cycle, Mck will be highly expressed when the multi-nucleotide myotubes begin differentiating and Tnnc will be expressed during myofiber formation and maturation (Yamane et al. 2000). However, the developmental states of in vitro cultured myoblasts are usually distinguished by their external characteristics, which will hardly give an exact result. Whether or not the expression of the three genes could be used as marker genes to identify the developmental states of in vitro cultured myoblasts is still not clear and valuable for study.
Therefore, in order to know the growth characteristics of in vitro cultured duck myoblasts at early stages and to validate the use of Desmin, Mck and Tnnc as marker genes for identifying developmental states of myoblasts, duck embryonic myoblasts were isolated, purified and in vitro cultured, and the developmental expression profiles of the three genes were detected using the qPCR method. These works would give new clues for the growth of myoblasts in duck, and might provide fundamental data for further research on the mechanisms of duck muscle development.
Materials and methods
Isolation, purification and primary culture of duck embryonic myoblasts
Muscle samples were isolated from the embryos of duck at 13 days of hatching. They were provided by Sichuan Agriculture University in China. The samples were first rinsed in D-Hanks (Gibco, China), and then were minced in small tissue pieces of 1 mm2 and digested in a mix of collagenase Type II 0.2% (w/v) (Gibco, China) and 0.25% trypsin (Sigma, China) in DMEM (Gibco, China) for 30 min at 37 °C. After digestion, the enzymatic reaction was terminated by the addition of growth medium with 15% FBS (Gibco, China). The liberated cells were suspended and filtrated by cell sieves. After that the cells were centrifuged at 1,500 rpm for 5 min, and the pellet was resuspended in growth medium. This was repeated for 3 times. The mixed cells containing both myoblasts and fibroblasts were transferred to culture flasks. As the fibroblasts are easy to adhere, the differential attachment technique was used to separate the myoblast cells using the following protocol: the mixed cells were cultured firstly at 37 °C and humified atmosphere containing 5% CO2 for 30 min to make the fibroblasts adhere first, then the un-adhered myoblasts were transferred into new flasks with 3 mL growth medium containing 15% FBS. After the flasks were maintained in an incubator with 5% CO2 at 37 °C for 12 h, the medium was changed to remove the contamination of blood cells, which can’t adhere on the flasks, and then medium was changed every other day. Cells viability was evaluated by Trypan Blue assay.
Identification of muscle myoblasts with Desmin
The Desmin protein is expressed specifically in the myoblast cells (Yablonka-Reuveni and Nameroff 1990), and in this research, the anti-Desmin antibody was used to check the homogeneity of myoblasts. The cultured myoblasts were rinsed with D-Hanks for one time, added 1 mL of 0.25% trypsin was added, and cells were detached at routine temperature. The liberated cells were transferred to new dishes as 1:2, and put in an incubator with 5% CO2 at 37 °C. Muscle myoblasts were identified by immunofluorescence method. When cells adhered on the dishes, they were fixed with 4% cold paraformaldehyde solution for 20 min, washed and penetrated with 0.1% trixton-100, blocked with PBS containing 4% BSA for 1 h, incubated with a mouse anti-swine Desmin monoclonal antibody (1:320, Boster, China) at 4°C overnight, rinsed with PBS twice, and then incubated in rabbit anti-mouse IgG, conjugated to fluorescein-isothiocyanate (FITC) (1:80, Boster, China) for 1 h. 40 μL DAPI (10 ug/ml in PBS; BiYunTian Biotechnology, China) was added to the sections for 20 minutes to label all nuclei. Cells were observed by using a Nikon Eclipse E-1000 microscope and photographed by using a Hamamatsu digital camera. The percentage of Desmin positive cells labeled in green was calculated in all cells to reflect the purity of myoblast cells.
MTT assay and duck myoblast Morphological Observations
Cells were seeded into 96-well microtiter plates at a density of 104 cells per well. Thirty wells of each plate were seeded. The cells were incubated in an incubator with 5% CO2 at 37°C. Each day, the culture medium of one plate was discarded and the cells were rinsed with PBS. The 3-(4,5-dimethylthiazol-2-yl)-2,5–diphenyltetrazolium bromide (MTT) assay was performed by adding 20 μL of 5 mg/mL MTT (Sigma, China) into the culture plates for 4 h. After that, the supernatants were removed and 150 μL of dimethyl sulfoxide (DMSO, Sigma, China) was added into each well. 15 min later, the absorbance value of each well was measured with a microplate reader with a wavelength of 490 nm. Before the detection of cells activities, the characteristics of myoblasts were observed with microscope.
QPCR of maker genes for myofiber development
Total RNAs from the cultured cells at different developmental stages were isolated with Trizol reagent according to the manufacturer’s protocol. RNAs were reverse-transcribed by using the reverse transcription system (Takara, Dalian, China). The mRNA expression levels were measured by real-time PCR by using the iCycler IQ5 (USA) and the Takara ExTaq RT–PCR kit (Takara, Dalian, China). The procedure for real-time PCR was as follows: 10 s of pre-denaturation reaction at 95 °C, followed by 45 cycles of 95 °C for 5 s and 62 °C for 30 s. Cells were collected from three wells at each stage and these experiments were repeated three times for each sample.
Sequence of duck β-actin gene from GenBank was used as an internal control. The real-time PCR primers (Table 1) used in this study (genes of interest and internal controls) were designed by using the primer 5 software (Primer Biosoft International, USA). The primers were synthesized by Shanghai Yingjun Biology Company. Amplicons corresponding to each target gene were examined by agarose gel electrophoresis to confirm the presence of a unique band with the expected size. The negative controls, corresponding to PCR amplification with non-reverse transcribed RNA, did not generate any signal. PCR products were diluted 16-fold and used to generate the calibration curve and to calculate the PCR amplification efficiency (Eff) for each gene (Mck, Tnnc, Desmin and β-actin ).
Table 1.
Primers used for qPCR
| Primers | Primer Sequence (5′ → 3′) | Product length (bp) | Annealing temperature ( °C) | Genbank accession number | |
|---|---|---|---|---|---|
| Mck | Forward | GATCTCCTCGATCTTCTTGAGG | 143 | 60 | HM487309 |
| Reverse | GCATCTGGCACAATGACAAC | ||||
| Tnnc | Forward | CGGTAGAGCAGTTGACAGAAGA | 122 | 61 | HM487308 |
| Reverse | CAGCATCCTCATCACCTTCC | ||||
| Desmin | Forward | GGTACAAGTCCAAGGTGAACGAC | 124 | 60.5 | HM460753 |
| Reverse | TCGATCTCGCAGGTGTAGGAC | ||||
| β-actin | Forward | GCTATGTCGCCCTGGATTTC | 168 | 55 | EF667345 |
| Reverse | CACAGGACTCCATACCCAAGAA | ||||
The relative mRNA expression levels of the Mck, Tnnc and Desmin gene were calculated by “normalized relative quantification” method with the BIO-RAD IQ5 software. Briefly, for each sample (three repeats/sample), mRNA expressions of the three genes were quantified. Average CT (threshold cycle) value of each gene in each sample was calculated. The calculation of the relative mRNA expression can be determined using the following formula (Livak and Schmittgen 2001)
 |
The Relative Quantity (Rel. Quantity) is the multiple of the differential expression of the gene of interest (GOI) between the samples and control samples. The normalizer (Norm) is the reference gene, in our case, the β-actin genes. The PCR amplification efficiency is represented by Eff.
Data analysis
The real-time PCR data were subjected to analysis of variance (ANOVA) and the means were compared for significance by Tukey’s test. The ANOVA and t test were performed by SAS (SAS Institute, Cary, NC, USA). A p value of less than 0.05 was considered statistically significant.
Results and analysis
Identification of myoblasts
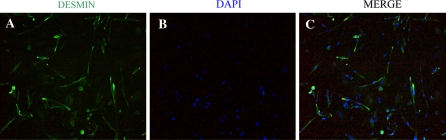
The duck embryonic myoblasts were isolated and identified (Fig. 1). The cytoplasmof duck myoblasts was labeled by secondary antibody in green (Fig. 1a), and all nucleus were stained by DAPI in blue (Fig. 1b). Results from these photos showed that the proportion of cells positively stained by the Desmin was higher than 95% in total, suggesting the high purity of the myoblasts obtained by us.
Fig. 1.
Identifications of duck myoblasts by using the immunofluorescence technique (×200). a Observation of the Desmin protein expressing in the duck myoblast. b Nucleus stained with DAPI. c Photos for overlapping
Dynamic observation and characterization of duck myoblast
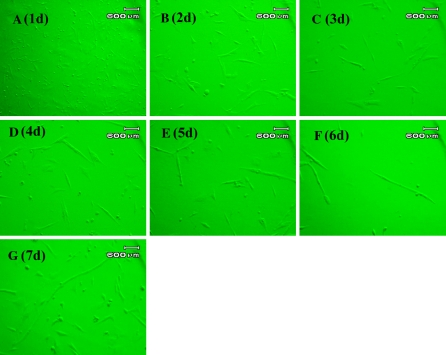
In vitro cultured duck embryonic myoblasts at different stages are shown in Fig. 2. The cells can be seen as bright spots with a round shape in the visual fields. Most of them were suspended in the medium, and only a little of them showed the capacity to adhere to the bottom of flasks. As soon as the cells adhered, they became spindle-shaped (Fig. 2a). After incubation for 2 days, the cultured cells expanded and a few of them began to fuse, shaped in ellipse with a single nuclei, and parts of them with dual nucleus (Fig. 2b). 3 days after incubation, the cells adhered together (Fig. 2c). When cells were cultured for 4 to 5 days, the number and volume of cells increased noticeably. And also they were found to be fused, spreading all over the bottom of flasks (Fig. 2d, e). When cells were cultured for 6 to 7 days, the myoblasts grew slowly because of a contact inhibition phenomenon (Fig. 2 f, g), and only a small quantity of sarcotubules could be found after this phase.
Fig. 2.
Morphologic characteristics of in vitro cultured duck myoblasts at different stages (By using microscope, at 200×). At the first day, the cells can be seen as bright spots with a round shape in the visual fields; At the second day, the cells expanded and a few of them began to fuse together, shaped in ellipse with a single nuclei, parts of them with dual nucleus; at the third incubation day, cells adhered together and interlaced as a reticulation shape. When cells were cultured for 4–5 days, the number and volume of cells increased noticeably, and they were found to be fused, spreading all over the bottom of flasks; When cultured for 6–7 days, the myoblasts grew slowly, and a small quantity of sarcotubules can be found after this proliferation phase
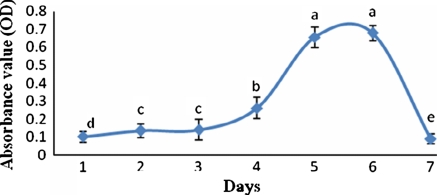
Growth curve of cells reflected that the myoblasts of duck underwent a latency period for about 3 days. After that, the cells entered into the logarithimic phase, which lasted for about 2 days. During this period, cells activities increased fast and noticeably. The cells enter into the plateau phase after they were cultured for six days. After this stage, the cells activities began to decrease gradually (Fig. 3).
Fig. 3.
Growth curve of duck myoblasts cultured in vitro. The different letters on each day represent the significant difference (p < 0.05)
Expression profiles of three maker genes for myofiber development
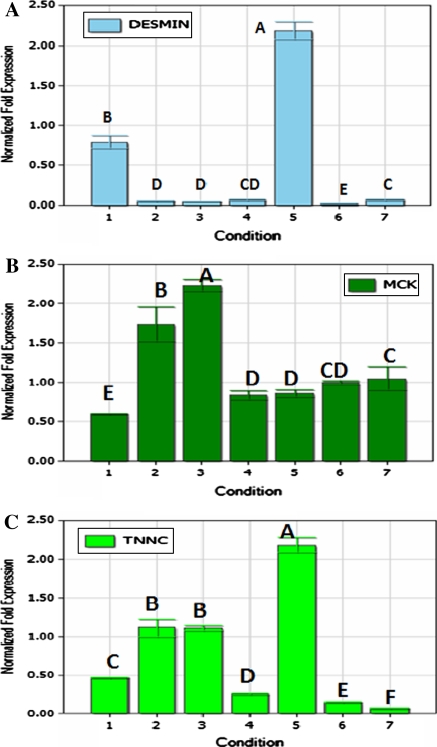
The expression profiles of the three maker genes for myofiber development are shown in Fig. 4. Generally, all of these genes, including Desmin, Mck and Tnnc, expressed during growth and differentiation of myoblasts. The Desmin gene was highly expressed on the fifth day after cells seeding, which is significantly higher than that on the first day (p < 0.05). Expressions of Desmin on the 1st and the fifth day were higher than that of other stages (p < 0.05). When the cells were incubated for 3 days, the expression of the Mck gene was significantly higher than 2 days after seeding (p < 0.05). After this period, the expression of Mck had an increasing tendency. The highest expression of Tnnc was observed on the fifth day, followed by high expression on the second and the third day after cells seeding.
Fig. 4.
The relative mRNA expression of three maker genes for the myofiber development. a Expression profile of the Desmin gene; b expression profile of the Mck gene; and c expression profile of the Tnnc gene during the growth of the cultured duck myoblasts. The different letters at the top of each bar represents the significant differences between the expression of the gene at the different stages (p < 0.05)
Discussions
Myoblasts proliferate and fuse with each other to form multinucleated myofibers, and mainly exist in the animal muscles in the embryonic stages (Le and Rudnicki 2007). In this research, we also got the duck myoblasts from the embryonal development stage. The released cells were mix of plenty of myoblasts and fibroblasts, and the external characteristics of both types of cells were so similar that they cannot be distinguished and well separated by their external characteristics. However, the fibroblasts could adhere on some plastic surface more easily than the myoblasts did (Fujiwara et al. 2009). Thus, we obtained plenty of duck myoblasts with high purity by using the differential attachment technique. We also found that several cycles of differential attachment lead to a much higher purity of myoblasts. Here, we used two cycles of differential attachment method to purify the myoblasts and received a good result.
Similar with other species, the duck myoblasts also go through a series of developmental stages including the proliferation stage, the myotubes differentiation stage and the myofiber maturation stage (Lan et al. 2005). However, there were 4 days in the latency stage for duck myoblasts to grow before they entered into the logarithmic phase. The proliferation and differentiation of myoblasts can be influenced by the choice of the serum concentration in the culture medium. Low concentration of serum in culture medium can induce cell differentiation, whereas high concentration serum can lead to a proliferation tendency of the cells (Fujiwara et al. 2009). In this study, we added serum with a concentration of 15% to the culture medium, resulting in a longer proliferation phase for the duck myoblasts. So, the time for the differentiation of myoblasts could be well controlled by the concentration of serum in the medium.
It is difficult to judge the growth state of cells through their external characteristics, as the differentiation of in vitro cultured myoblasts is so complex that they cannot reach a synchronous state (Huard et al. 2002). Desmin, Mck, and Tnnc are three marker genes for identifying the states of myofiber development. Yamane et al. (2000) detected the developmental expression profiles of these three maker genes in the tongue and limb muscle tissues in mice and compared the developmental differences of the two muscle tissues (Yamane et al. 2000). In our research, the developmental expression profiles of these three maker genes have been detected in the in vitro cultured duck myoblasts at different stages. The Desmin gene highly expressed in duck embryonic myoblasts at early stages, at which, the cells are more active than at other stages, indicating that the Desmin gene plays some roles in the myoblasts determination. The peak value of the expression of the Desmin gene in myoblasts was observed on the fifth day after cell seeding accompanied by an increase of the cell activities. These changes might relate to an adaptation to the new circumstances for myoblasts when they endured a long time latency period. During the latency period, the myoblasts fused to form myotubes, and in parallel, the expression of the Mck gene had an increasing tendency. The fusing phenomenon was suppressed when cells had a fast proliferation rate at the logarithmic phase, while the Mck gene expressed highly again when nutrients were exhausted. The expression profiles of the Tnnc gene are similar to that of the Mck gene during the latency period. Both of them were highly expressed when cells at the plateau phase. After obtention of the plateau phase, the in vitro cultured duck myoblasts grew gradually. In parallel, the expression of the Desmin gene decreased to a low level and that of the Mck and the Tnnc gene increased to a high level. During the rapid growing of the myoblasts in the logarithmic growth phase, there would be a high expression level of the Desmin gene, and low expression level of the Mck gene and the Tnnc genes.
In summary, we obtained the duck embryonic myoblasts with high purity by using the differential attachment technique for the first time. It needs about 4 days for the in vitro cultured duck myoblasts entering into the logarithmic growth phase. After obtention of the plateau phase, the in vitro cultured duck myoblasts grew gradually. In parallel, the expression of the Desmin gene decreased to a low level and that of the Mck and the Tnnc gene increased to a high level.
Acknowledgments
The work was supported by the National High Technology Research and Development Program of China (No.2010AA10A109), and Chinese Agriculture Research Service (CARS-43-6).
References
- Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Cheng L, Lai MD, Sanderson JE, Yu CM, Li M. Enhanced fusion of myoblasts with myofibers for efficient gene delivery induced by a partially purified protein fraction from rat muscle extract. Arch Biochem Biophys. 2005;441:141–150. doi: 10.1016/j.abb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Dolmatova II, Saitbatalov TF, Gareev FT. RAPD-analysis of duck genetic polymorphisms. Interlineal differences in a Peking duck species. Genetika. 2000;36:805–812. [PubMed] [Google Scholar]
- Dong B, Gong DQ, Meng H, Yu JF, Zhao XT, Duan XJ, Gu ZL. Identification and genetic analysis of SNPs in duck adiponectin gene. Yi Chuan. 2007;29:995–1000. doi: 10.1360/yc-007-0995. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Tsukada R, Shioya I, Takagi M. Effects of heat treatment and concentration of fish serum on cell growth in adhesion culture of Chinese hamster ovary cells. Cytotechnology. 2009;59:135–141. doi: 10.1007/s10616-009-9199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg. 2002;84-A:822–832. [PubMed] [Google Scholar]
- Jeroncic I, Mulic R, Klismanic Z, Rudan D, Boban M, Zgaga L. Interactions between genetic variants in glucose transporter type 9 (SLC2A9) and dietary habits in serum uric acid regulation. Croatian Med J. 2010;51:40–47. doi: 10.3325/cmj.2010.51.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny SF, Mckay J, Coleman JR. Isolation and characterization of terminally differentiated chicken and rat skeletal muscle myoblasts. Dev Biol. 1982;91:11–26. doi: 10.1016/0012-1606(82)90003-3. [DOI] [PubMed] [Google Scholar]
- Kunhareang S, Zhou H, Hickford JG. Allelic variation in the porcine MYF5 gene detected by PCR-SSCP. Mol Biotechnol. 2009;41:208–212. doi: 10.1007/s12033-008-9122-z. [DOI] [PubMed] [Google Scholar]
- Lan MA, Gersbach CA, Michael KE, Keselowsky BG, Garcia AJ. Myoblast proliferation and differentiation on fibronectin-coated self assembled monolayers presenting different surface chemistries. Biomaterials. 2005;26:4523–4531. doi: 10.1016/j.biomaterials.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Le GF, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart TA, Clegg CH, Hauschka SD. Control of mouse myoblast commitment to terminal differentiation by mitogens. J Supramol Struc. 1980;14:483–498. doi: 10.1002/jss.400140407. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Inoue A. Controlled differentiation of myoblast cells into fast and slow muscle fibers. Cell Tissue Res. 2008;332:123–132. doi: 10.1007/s00441-008-0582-z. [DOI] [PubMed] [Google Scholar]
- McNally EM, Pytel P. Muscle diseases: the muscular dystrophies. Annu Rev Pathol. 2007;2:87–109. doi: 10.1146/annurev.pathol.2.010506.091936. [DOI] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Kurokawa M, Yamauchi N, Hattori MA. Gene silencing of myostatin in differentiation of chicken embryonic myoblasts by small interfering RNA. Am J cell Physiol. 2006;291:C538–C545. doi: 10.1152/ajpcell.00543.2005. [DOI] [PubMed] [Google Scholar]
- Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M (2007) Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol 3:2B.1.1–2B.1.29 [DOI] [PubMed]
- Xu SH, Bao WB, Huang J, Cheng JH, Shu JT, Chen GH. Polymorphic analysis of intron 2 and 3 of growth hormone gene in duck. Yi Chuan. 2007;29:438–442. doi: 10.1360/yc-007-0438. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Nameroff M. Temporal differences in Desmin expression between myoblasts from embryonic and adult chicken skeletal muscle. Differentiation. 1990;45:21–28. doi: 10.1111/j.1432-0436.1990.tb00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane A, Mayo M, Shuler C, Crowe D, Ohnuki Y, Dalrymple K, Saeki Y. Expression of myogenic regulatory factors during the development of mouse tongue striated muscle. Arch Oral Biol. 2000;45:71–78. doi: 10.1016/S0003-9969(99)00105-3. [DOI] [PubMed] [Google Scholar]