Abstract
The pig is the non-primate species that is immunologically closest to humans, and has been considered as an alternative source to human allografts for transplantation. In fact, there has been recent interest in identifying and culturing porcine neural progenitor cells (PNPCs) in vitro, but the long-term culturing has not yet been characterized. Here, we reported the spontaneous differentiation of PNPCs into neuronal and glial cells. For in vitro cultures, the primary cells of the subventricular zone of the forebrain striatum were cultured in the presence of epidermal growth factor and basic fibroblast growth factor to allow the growth of spherical masses that exhibit sustained growth and self-renewal capacity. After growth factor removal, the neurospheres with 10 and 130 days of culture spontaneously differentiated into Tuj1-positive neurons and GFAP-positive astrocytes as seen by double immunocytofluorescence. Molecular characterization using reverse transcription-polymerase chain reaction showed that neurospheres expressed nestin, neuron-specific enolase, and glial fibrillary acidic protein (GFAP). In addition, after cultured in the differentiation medium for 3 months, the growth of neurosphere became slow and displayed cystic structures with the same morphology as that of embryonic bodies derived from embryonic stem cells. It is concluded that PNPCs have the ability to provide an expandable source of neural cells that can develop into neuronal and glial subtypes.
Keywords: Porcine, Neural progenitors, Differentiation, Neurons, Astrocytes
Introduction
Neural stem cells (NSCs) are multipotent and self-renewing precursors of the nervous system. Under appropriate conditions in vitro, NSCs can differentiate into specific cell types such as neurons, astrocytes and oligodendrocytes. In the presence of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), NSCs can be expanded into either neurospheres or single cells. Therefore, NSCs have been considered a promising source of cells for the treatment of central nervous system (CNS) diseases (Reynolds and Weiss 1992; Weiss et al. 1996). The neural progenitor cells have a limited potential for self renewal and differentiation. In this study, our use of porcine neural progenitor cells (PNPCs) to describe the cultured cells focus on the fact that the mixed cells populations consisted of stem cells and lineage-restricted precursor cells.
A basic limitation upstream of purported neurological applications is the choice of the model system used for study. It has been shown that species-specific differences in the properties of NCSs prevent the assured extrapolation of findings from rodent cell cultures to humans (Ray and Gage 2006). Regulatory mechanisms of neurogenesis should therefore be investigated using neural stem cells taken from adult humans, or at least, from the most closely related non-primate animal species (Armstrong et al. 2001). The pig is the non-primate species that is immunologically closest to humans, and has been considered as an alternative source to human allografts for transplantation (Armstrong et al. 2001; Barker et al. 2000). Its use can also overcome the ethical and practical difficulties associated with using human fetal neural tissue.
Neural progenitor cells (NPCs) have now been derived from the brain or spinal cord of mammalian species, including mouse (Vukicevic et al. 2010; Lindstrom et al. 2009; Hernandez-Benitez et al. 2010), dog (Walton and Wolfe 2008; Milward et al. 1997), rat (Go et al. 2009), human (Luo et al. 2010; Liu et al. 2010) and pig (Schwartz et al. 2005). PNPCs seems to be less immunogenic and thus survive better than porcine neural xenografts (Armstrong et al. 2001; Barker et al. 2000), up to 5 months in rats (Harrower et al. 2006). Some recent studies have demonstrated that PNPCs derived from the fetal brain can be expanded in culture with EGF and bFGF in vitro (Armstrong et al. 2001; Smith and Blakemore 2000; Isacson and Deacon 1996; Uchida et al. 2003). Importantly, the porcine expanded NPCs displayed long-term survival with maturation and integration into the host brain in a rat model of Parkinson’s disease (Harrower et al. 2006). This raises the possibility that porcine expanded NPCs is a promising alternative source to human allografts for transplantation in neurological disorders.
In this report, we described the isolation and differentiation of PNPCs taken from fetal pigs. Long-term culture of clonal populations from individual PNPCs can proliferate and differentiate to form cells with characteristics of neurons and astrocytes. Our findings represent the first report of spontaneous differentiation of PNPCs into neuronal and glial subtypes in vitro.
Methods
Reagents
Neurobasal™ medium supplemented with l-glutamine (0.5 mM), penicillin/streptomycin (100 μg/mL), B27, EGF, bFGF, and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, NY). Trizol and SuperScript™ III First-Strand Synthesis System were from Invitrogen (Carlsbad, CA, USA). Mouse monoclonal Nestin was purchased from Chemicon (Temecula, CA, USA). Poly-l-lysine (PLL), mouse monoclonal glial acidic fibrillary protein (GFAP) and mouse monoclonal CNPase were obtained from Sigma (St. Louis, MO, USA). Rabbit Neuronal Class III β-Tubulin (Tuj1) polyclonal antibody was from Co-vance (Princeton, NJ, USA). Alexa Fluor 488-congugated goat anti-mouse IgG and Alexa Fluor 594-congugated goat anti-rabbit IgG and DAPI were supplied by Molecular Probes (Eugene, OR, USA).
Animals
A pregnant sow was placed under general anesthesia, the uterine horns and fetuses were removed, and the sow was terminated prior to waking. Anaesthesia and surgery protocols were approved by the Experimental Animals Administration Committee of Jilin Province.
Culture of PNPCs
Cells were isolated using a previously described method (Schwartz et al. 2005) with modifications. Briefly, embryonic day 65–80 fetal pigs were removed from pregnant sows. The subventricular zone of the forebrain was separated from the cerebellum and brainstem, cut up into small pieces. These were digested in 0.08% trypsin at 37 °C for 30 min. Following three washes in sterile phosphate buffered saline (PBS, pH7.4), the cell suspension was gently triturated in the same solution using a fine polished Pasteur pipette, and then was passed through the 70 μm cell strainer (BD Falcon, Franklin Lakes, NJ, USA). The number of viable cells was determined using 0.4% typan blue. After single cells were dissociated, they were seeded in flasks containing neural progenitor growth medium at a final cell concentration of approximately 2.0 × 105 cells/ml and incubated at 37 °C, 5% CO2. The growth medium was composed of NeurobasalTM Medium supplemented with l-glutamine (0.5 mM), penicillin/streptomycin (100 μg/mL), B27 (1:50), EGF (20 ng/mL), and bFGF (20 ng/mL). Half of the medium was changed every 3–4 days. Neural progenitors were passaged using a fine polished Pasteur pipette, triturated 10–20 times and the mixture of single cells and intact spheres were re-seeded into the new plate with fresh medium as above.
Differentiation of NPCs in 10 and 130 days’ culture
To induce the differentiation of PNPCs, neurospheres cultured for 10 and 130 days (d) in growth medium were plated in either 24-well plates with coverslips coated with poly-l-lysine (PLL) or 6-well plates coated with PLL. These were then cultured for an additional 5 days and 3 months in differentiation medium composed of Neurobasal™ Medium supplemented with l-glutamine, penicillin/streptomycin and B27. The adherent cells grown on coverslips were used for immunofluorescence analysis, while the cells and neurospheres grown in 6-well plates were gently harvested and used for mRNA analysis.
Immunofluorescence staining
The expressions of the nestin, GFAP, Tuj1 and CNPase were evaluated by double or single immunofluorescence staining. Briefly, culture dishes were washed with PBS, fixed for 20 min in 4% paraformaldehyde, washed 3 times with PBS, permeabilized with 0.1% Triton X-100 PBS for 20 min, and blocked with PBS containing 5% goat serum (GS) for 1 h at room temperature. Dishes were incubated with one or two primary antibodies as mouse monoclonal Nestin (1:200), mouse monoclonal GFAP (1:400), Rabbit Tuj1 polyclonal antibody (1:1,000), mouse monoclonal CNPase (1:100). Incubations with primary antibodies were carried out for 1 h in 1% (vol/vol) goat serum (GS) in PBS in a humidified atmosphere at room temperature and the sections were then washed three times in PBS. For mouse and rabbit primary antibodies, the secondary antibody was Alexa Fluor 488 goat anti-mouse at 1:400 dilution and Alexa Fluor 594 goat anti-rabbit at 1:1,000 dilution, respectively. Secondary antibodies were applied in 1% (vol/vol) GS in PBS at room temperature for 40 min. The coverslips were then washed three times in PBS, and then mounted by ProLong® Gold antifade reagent with DAPI. Images were collected using a spot digital camera mounted onto a Zeiss microscope and analyzed via Axiovision image software. Quantification was done by counting the number of cells immunoreactive for either Tuj1 or GFAP on double immunocytochemistry analysis, followed by calculation of the percentages over the number of total cells counted by blue nuclei stained by DAPI.
RNA extraction and reverse transcription-polymerase chain reaction (RT–PCR)
Total RNA was extracted from cultured neural progenitors using Trizol according to the manufacturer’s instructions. The isolated RNA was subjected to DNase Ι treatment to remove any residual genomic DNA. RNA was reverse-transcribed with the SuperScript™ III First-Strand Synthesis System. PCR was preformed using a Hybaid PCR Gradient Thermal Cycler. The PCR components in a final volume of 20 μl reaction included PCR buffer (10×), MgCl2 (50 mMol/L), forward primer, reverse primer, Platinum® Taq DNA Polymerase (5 U/μL) and cDNA template. The sequence of the primer and other parameters of PCR are given in Table 1 (Shanghai Sangon Biotech, Shanghai, China). The amplified PCR products were run on 1.5% agarose gels along with the 1 kbp plus ladder and visualized with ethidium bromide.
Table 1.
Reverse transcription-polymerase chain reaction primers
| Gene | 5′Primer | 3′Primer | Annealing temperature (°C) | Size (bp) | Ref. | Cycle (nb) |
|---|---|---|---|---|---|---|
| GFAP | GATGAAACCAACCTGAGGCTGG | TGCTGTGCCAGCTGCTCC T | 62 | 185 | McKay et al. (2004) | 30 |
| NSE | CTTGGAGCTGGTGAAGGAAG | TTTTGGGTTGGTCACTGTCA | 60 | 310 | Liard et al. (2009) | 35 |
| nestin | GGCTTCTCTCAGCATCTTGG | AAGGCTGGCATAGGTGTGTC | 65 | 150 | Dyce et al. (2004) | 30 |
Data analysis
Quantitative data are expressed as the mean ± SE, and statistical significance was determined by the two-tailed Student t test, with statistical significance set at p < 0.05.
Results
The morphology of the neurosphere of PNPCs
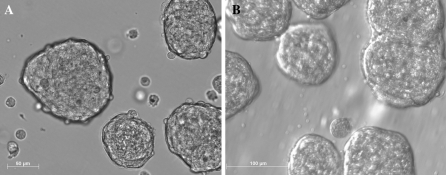
PNPCs isolated from fetal porcine brains displayed floating spherical masses of cells with similar morphology and different size distribution in serum-free medium culture (Fig. 1a, b). After passage, a mixed suspension of single cells and sphere remnants reformed new neurospheres. There was morphological evidence of active proliferation, seen as numerous dividing profiles and a rapid increase in the density of the spherical masses of cells. With long-term culture for 130 days, the PNPC cultures displayed few new neurospheres.
Fig. 1.
The morphology of porcine neural cells (PNPCs) cultured in serum-free medium for 10 days (a) and 130 days (b). Note that PNPCs aggregate to form free-floating neurospheres
Differentiation of PNPCs
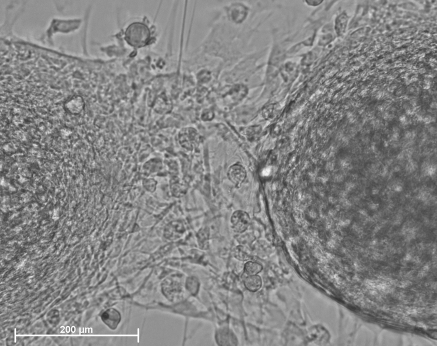
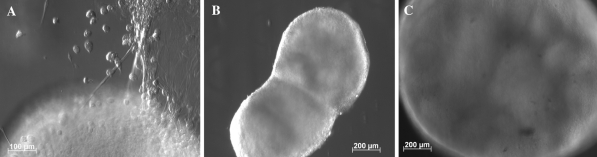
Removal of the growth factors EGF and bFGF led to spontaneous differentiation of NPCs in the medium composed of Neurobasal™ and B27. After 10 days of primary culture in the medium supplemented with EGF and bFGF, the neurospheres were reseeded onto coverslips coated with PLL in Neurobasal™ and B27 medium. After several hours, the neurospheres attached to coverslips were surrounded by cells which migrated onto the substrate. The following days, radiating processes from the edge of the neurospheres extended to contact adjacent neurospheres to form a network and some cells spread from the neurospheres. As shown in Fig. 2, the culture network became larger and more cells spread from the nerospheres after another 5 days culture. However, when neural progenitors were cultured in the differentiation medium for 3 months in a flask which had not been precoated with PLL, the neurospheres almost ceased to proliferate. The morphology of neurospheres became cystic structures, similar to that of an embryonic body derived from embryonic stem cells. Some neurospheres attached to the flask, and some cells spread and a neurite network formed (Fig. 3).
Fig. 2.
The morphology of differentiated cells derived from primary porcine neural progenitors. The primary 10 days of culture in the form of neurosphere were re-seeded in differentiated medium for additional 5 days. Note that radiating processes developed from the edge of the neurosphere were extended to contact with the adjacent attached neurospheres to form the network and some cells spread from the neurospheres
Fig. 3.
The morphology of neural progenitors cultured for 3 months in differentiated medium. Note that some cells spread from the neurosphere (a), the morphology of some neurospheres became the cystic structures with the morphology familiar with embryonic body derived from the embryonic stem cells (b, c)
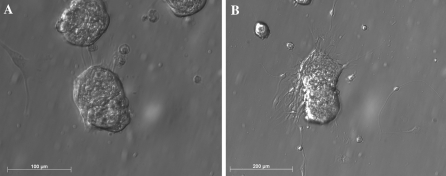
The additional 5 days differentiation of NPCs after the long-term culture in growth medium for 130 days displayed a similar morphology to those observed in the short-term primary culture for 10 days (Fig. 4).
Fig. 4.
The morphology of differentiated porcine neural progenitors with 130 days of culture in medium supplemented with EGF and bFGF. Note that some cells migrated onto the substrate to contact with the adjacent neurospheres (a) and some radiating process developed from the edge of the neurosphere (b)
Marker expression in neurospheres
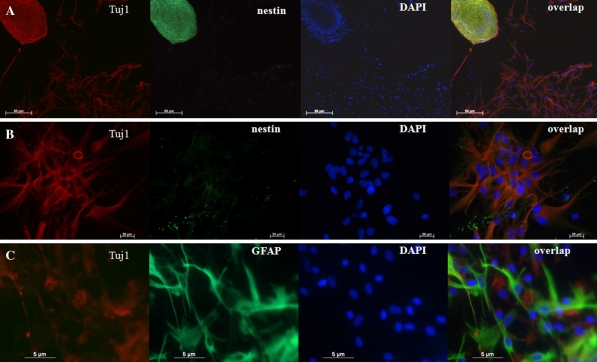
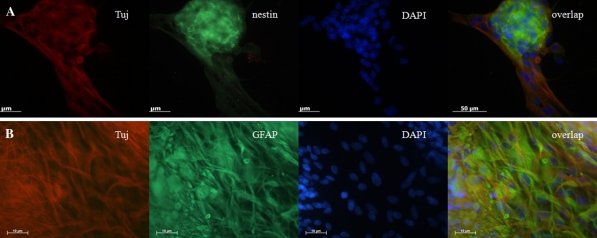
The differentiated neural progenitors with 10 days (Fig. 5) and 130 days (Fig. 6) of culture were passaged and seeded for an additional 5 days. The neurospheres were then fixed and detected by immunocytochemistry staining. As shown in Fig. 5, the neurospheres within cultures displayed positive staining for nestin, a NSC-specific marker. The differentiated cells derived from neurospheres expressed the markers for neurons and astrocytes as Tuj1 and GFAP, respectively. However, double labeling for nestin and Tuj1 showed that the differentiated cells did not express nestin (Fig. 5b), further confirmed by the spontaneous differentiation of porcine neural progenitors into neuronal cells after removal of EGF and bFGF. In addition, the differentiated cells derived from neural progenitors did not express CNPase, an oligodendrocyte marker (data not shown). The percentage of neurons derived from the neural progenitors with 10 days of culture (34.23 ± 5.80) was significantly higher than those derived from 130 days (18.98 ± 8.65, t = 3.276, p < 0.05). The percentage of astrocytes derived from the neural progenitors with 10 days of culture (43.25 ± 4.04) was lower than that derived from 130 days (54.41 ± 13.13), but there was no statistical significance.
Fig. 5.
Marker expression by neurosphere or differentiated cells with 10 days of culture. a Primary cultured neurospheres in differentiation medium showed positive staining of nestin (green) and Tuj1 (red). b The differentiated cells derived from primary cultured neurospheres showed negative staining of nestin (green) and positive staining of Tuj1 (red). c The differentiated cells derived from primary cultured neurospheres showed positive staining of Tuj1 (red) and glial acidic fibriallary protein (GFAP) (green)
Fig. 6.
Marker expression by neurosphere or differentiated cells with 130 days of culture. a Primary cultured neurospheres in differentiation medium showed positive staining of nestin (green) and Tuj1 (red). b The differentiated cells derived from primary cultured neurospheres showed positive staining of Tuj1 (red) and glial acidic fibriallary protein (GFAP) (green)
RT–PCR profiling of neurospheres
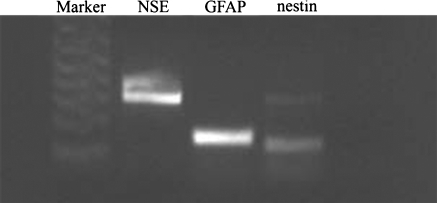
Multipotency was confirmed at the differentiated and proliferated stage of neurospheres by RT–PCR detection of phenotypic markers characterizing the neural cell lineages. The neurospheres in the additional 5 days differentiation culture following 10 days of primary culture displayed mRNA expression of neuron-specific markers (NSE) and of astrocytes-specific markers (GFAP), whereas the expression for nestin was faint (Fig. 7).
Fig. 7.
RT-PCR analysis of gene expression in porcine neural progenitor cells (PNPCs). Evidence was found for the expression of NSE, GFAP and nestin (faint). Ladders (1 kb plus ladder) provided for reference
Discussion
The data presented here indicate that PNPCs have spontaneous differentiation potential for at least two different lineages. These cells express marker genes for PNPCs of neural lineages. In the absence of EGF and bFGF, the neurospheres can spontaneously differentiate into Tuj1-positive neurons and GFAP-positive astrocytes, indicating that the PNPCs have the ability to provide an expandable source of neural cells that can develop into neuronal and glial subtypes.
The result of RT–PCR showed that the neurospheres in the additional 5 days differentiation culture following 10 days of primary culture displayed mRNA expression of neuron-specific markers (NSE) and of astrocytes-specific markers (GFAP), whereas the expression for nestin was faint (Fig. 7). The result suggested the decreasing of neural progenitors number because some progenitors differentiated into neuron and glia, which did not express nestin, a marker of neural stem cells or progenitos. The immunofluorescence staining results showed that the percentage of neurons derived from the neural progenitors with 10 days of culture was significantly higher than that derived from 130 days; the percentage of astrocytes derived from the neural progenitors with 10 days of culture was lower than that derived from 130 days, but there was no statistic significance, which suggested that the ability of long-term culture PNPCs to differentiate into neuron decreased.
Multipotent and self-renewing neural precursor cells termed NPCs possess the capability to differentiate into neurons, astrocytes and oligodendrocytes under appropriate conditions in vitro, and can be expanded in a primary culture treated with EGF and bFGF into neuroshperes or single cells (Skalnikova et al. 2008). Porcine neural progenitors have been proven to be less immunogenic and survive better than porcine neural xenografts (Armstrong et al. 2001; Barker et al. 2000). Therefore, it is possible that at some point in the future, they could become a promising source of cells for the treatment of many devastating acquired and hereditary diseases of CNS (Weiss et al. 1996; Ray and Gage 2006).
Some previous studies have shown that NPCs-derived neurospheres can be differentiated into neurons, astrocytes and oligodendrocytes when cultured in a medium supplemented with 1 mM all-trans retinoic acid (Skalnikova et al. 2008) or with DMSO, butylated hydroxyanisole (BHA) and NGF (25 ng/mL) (Zheng et al. 2010) However, the present study demonstrated that, with a long-term culture, porcine neurospheres still can spontaneously differentiate into neurons and astrocytes without supplemental bFGF and EGF without the aid of the other inducer. Skalnikova et al. (2008) found that differentiated neural cells expressed βIII-tubulin, GFAP or CNPase in a differentiation medium supplemented with all-trans retinoic acid. Moreover, Zheng et al. (2010) reported that both enhanced green fluorescence protein gene-transfected and wild-type NSCs could be differentiated into astrocytes (GFAP+), oligodendrocytes (GalC+) and neurons (NF+, NSE+ and MAP2+) in a differentiation medium supplemented with DMSO, butylated hydroxyanisole (BHA) and NGF. This indicates that the differentiation of oligodendrocytes derived from neurospheres needs a specific induction condition.
In conclusion, the present study demonstrates that the spontaneous in vitro differentiation potential of PNPCs includes cells of neuronal and glial phenotypes. As the PNPCs have been considered as an alternative source to human allografts for transplantation used to treat patients with neurological disorders, these findings may provide new insight for developing the clinical application of PNPCs.
References
- Armstrong RJ, Harrower TP, Hurelbrink CB, McLaughin M, Ratcliffe EL, Tyers P, Richards A, Dunnett SB, Rosser AE, Barker RA. Porcine neural xenografts in the immunocompetent rat: immune response following grafting of expanded neural precursor cells. Neuroscience. 2001;106:201–216. doi: 10.1016/S0306-4522(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Barker RA, Ratcliffe E, McLaughlin M, Richards A, Dunnett SB. A role for complement in the rejection of porcine ventral mesencephalic xenografts in a rat model of parkinson’s disease. J Neurosci. 2000;20:3415–3424. doi: 10.1523/JNEUROSCI.20-09-03415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyce PW, Zhu H, Craig J, Li J. Stem cells with multilineage potential derived from porcine skin. Biochem Biophys Res Commun. 2004;316:651–658. doi: 10.1016/j.bbrc.2004.02.093. [DOI] [PubMed] [Google Scholar]
- Go HS, Shin CY, Lee SH, Jeon SJ, Kim KC, Choi CS, Ko KH. Increased proliferation and gliogenesis of cultured rat neural progenitor cells by lipopolysaccharide-stimulated astrocytes. Neuroimmunomodulation. 2009;16:365–376. doi: 10.1159/000228911. [DOI] [PubMed] [Google Scholar]
- Harrower TP, Tyers P, Hooks Y, Barker RA. Long-term survival and integration of porcine expanded neural precursor cell grafts in a rat model of parkinson’s disease. Exp Neurol. 2006;197:56–69. doi: 10.1016/j.expneurol.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Hernandez-Benitez R, Pasantes-Morales H, Saldana IT, Ramos-Mandujano G. Taurine stimulates proliferation of mice embryonic cultured neural progenitor cells. J Neurosci Res. 2010;88:1673–1681. doi: 10.1002/jnr.22328. [DOI] [PubMed] [Google Scholar]
- Isacson O, Deacon TW. Specific axon guidance factors persist in the adult brain as demonstrated by pig neuroblasts transplanted to the rat. Neuroscience. 1996;75:827–837. doi: 10.1016/0306-4522(96)00305-3. [DOI] [PubMed] [Google Scholar]
- Liard O, Segura S, Pascual A, Gaudreau P, Fusai T, Moyse E. In vitro isolation of neural precursor cells from the adult pig subventricular zone. J Neurosci Methods. 2009;182:172–179. doi: 10.1016/j.jneumeth.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Lindstrom S, Eriksson M, Vazin T, Sandberg J, Frisén J, Andersson-Svahn H. High-density microwell chip for culture and analysis of stem cells. PLoS One. 2009;4:e6997. doi: 10.1371/journal.pone.0006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HK, Wang Y, Belz T, Bock D, Takacs A, Radlwimmer B, Barbus S, Reifenberger G, Lichter P, Schutz G. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 2010;24:683–695. doi: 10.1101/gad.560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MH, Hannemann H, Kulkarni AS, Schwartz PH, O’Dowd JM, Fortunato EA. Human cytomegalovirus infection causes premature and abnormal differentiation of human neural progenitor cells. J Virol. 2010;84:3528–3541. doi: 10.1128/JVI.02161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay GJ, Campbell L, Oliver M, Brockbank S, Simpson DA, Curry WJ. Preparation of planar retinal specimens: verification by histology, mRNA profiling, and proteome analysis. Mol Vis. 2004;10:240–247. [PubMed] [Google Scholar]
- Milward EA, Lundberg CG, Ge B, Lipsitz D, Zhao M, Duncan ID. Isolation and transplantation of multipotential populations of epidermal growth factor-responsive, neural progenitor cells from the canine brain. J Neurosci Res. 1997;50:862–871. doi: 10.1002/(SICI)1097-4547(19971201)50:5<862::AID-JNR22>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ray J, Gage FH. Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol.Cell. Neurosci. 2006;31:560–573. doi: 10.1016/j.mcn.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Schwartz PH, Nethercott H, Kirov II, Ziaeian B, Young MJ, Klassen H. Expression of neurodevelopmental markers by cultured porcine neural precursor cells. Stem Cells. 2005;23:1286–1294. doi: 10.1634/stemcells.2004-0306. [DOI] [PubMed] [Google Scholar]
- Skalnikova H, Vodicka P, Pelech S, Motlik J, Gadher SJ, Kovarova H. Protein signaling pathways in differentiation of neural stem cells. Proteomics. 2008;8:4547–4559. doi: 10.1002/pmic.200800096. [DOI] [PubMed] [Google Scholar]
- Smith PM, Blakemore WF. Porcine neural progenitors require commitment to the oligodendrocyte lineage prior to transplantation in order to achieve significant remyelination of demyelinated lesions in the adult cns. Eur J Neurosc. 2000;12:2414–2424. doi: 10.1046/j.1460-9568.2000.00137.x. [DOI] [PubMed] [Google Scholar]
- Uchida K, Okano H, Hayashi T, Mine Y, Tanioka Y, Nomura T, Kawase T. Grafted swine neuroepithelial stem cells can form myelinated axons and both efferent and afferent synapses with xenogeneic rat neurons. J Neurosci Res. 2003;72:661–669. doi: 10.1002/jnr.10628. [DOI] [PubMed] [Google Scholar]
- Vukicevic V, Jauch A, Dinger TC, Gebauer L, Hornich V, Bornstein SR, Ehrhart-Bornstein M, Muller AM. Genetic instability and diminished differentiation capacity in long-term cultured mouse neurosphere cells. Mech Ageing Dev. 2010;131:124–132. doi: 10.1016/j.mad.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Walton RM, Wolfe JH. In vitro growth and differentiation of canine olfactory bulb-derived neural progenitor cells under variable culture conditions. J Neurosci Methods. 2008;169:158–167. doi: 10.1016/j.jneumeth.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YM, An ZX, Zhao XE, Quan FS, Zhao HY, Zhang YR, Liu J, He XY, He XN. Comparation of enhanced green fluorescent protein gene transfected and wild-type porcine neural stem cells. Res Vet Sci. 2010;88:88–93. doi: 10.1016/j.rvsc.2009.06.002. [DOI] [PubMed] [Google Scholar]