Abstract
Compared to other developed countries, the United States ranks poorly in terms of life expectancy at age 50. We seek to shed light on the US’s low life expectancy ranking by comparing the age-specific death rates of 18 developed countries at older ages. A striking pattern emerges: between ages 40 and 75, US all-cause mortality rates are among the poorest in the set of comparison countries. The US position improves dramatically after age 75 for both males and females. We consider four possible explanations of the age patterns revealed by this analysis: (1) access to health insurance; (2) international differences in patterns of smoking; (3) age patterns of health care system performance; and (4) selection processes. We find that health insurance and smoking are not plausible sources of this age pattern. While we cannot rule out selection, we present suggestive evidence that an unusually vigorous deployment of life-saving technologies by the US health care system at very old ages is contributing to the age-pattern of US mortality rankings. Differences in obesity distributions are likely to be making a moderate contribution to the pattern but uncertainty about the risks associated with obesity prevent a precise assessment.
Compared to other developed countries, the United States ranks poorly in terms of life expectancy at older ages. In terms of life expectancy at age 50, it trails the world leader, Japan, by 3.3 years and the 29 countries ahead of the US by an average of 1.3 years (WHO 2009). A majority of the difference in life expectancy at birth between the US and other developed countries is attributable to differences in life expectancy at age 50.1
The US’s poor performance is often blamed on its health care system. In a previous analysis, we reviewed a number of studies comparing the efficacy of the US health care system with that of several other developed countries (Preston and Ho 2009). We found that, by the standards of other OECD countries, the US health care system typically functions well in the identification and treatment of cancer and heart disease, the two leading causes of death at older ages. We examined in greater depth death rates from prostate and breast cancer, diseases for which effective methods of identification and treatment have been developed and where behavioral factors do not play a dominant role. We found that the US experienced a significantly faster decline in prostate and breast cancer mortality than the comparison countries between 1994 and 2005. On the basis of this analysis we concluded that the health care system is not likely to be responsible for the US’s low life expectancy at age 50 (Ibid).
Only one broad age range, 50+, was considered in that analysis. We now supplement that analysis by considering the relative ranking of the US at different ages among a comparison set of 17 OECD countries. Research related to this topic is scarce. One exception is an article by Manton and Vaupel (1995), who documented that the US had unusually favorable survival after age 80 relative to Sweden, France, England, and Japan. Using extinct-cohort methods, they found that US life expectancy at age 80 significantly exceeded life expectancy in the comparison countries. They speculated that this phenomenon may reflect more effective medical care and more favorable personal behaviors among the elderly in the US compared to the elderly in Europe and Japan. They also noted the possibility of persistent cohort effects involving education, immigration, selection, and adverse health conditions at younger ages. Nolte and McKee (2008) found that the US has unusually high death rates below age 75 from a number of diseases including heart disease and stroke. In this paper, we consider the international ranking of the US in greater detail by focusing on five-year age groups beginning at age 40, and attempt to shed light on the sources of the unusual age pattern that emerges.
Age Patterns of International Rankings
We use two primary measures to characterize the international variation in mortality rates by age in 2005: the ranking of US age-specific mortality rates among 18 OECD countries; and the ratio of US age-specific mortality rates to the unweighted mean of the other 17 OECD countries. Employing rankings as a summary measure allows us to gauge the US position among the comparison countries; necessarily, the use of rankings involves some loss of information. The use of ratios presents a complementary picture indicating the magnitude of the difference in death rates between the US and the 17 other countries. The comparison countries are Australia, Austria, Belgium, Canada, Denmark, Finland, France, Germany, Italy, Japan, the Netherlands, Norway, Spain, Sweden, Switzerland, Portugal, and the United Kingdom. Calculations are done for males and females separately. Death rates by country, age group, and sex are taken from the Human Mortality Database (HMD) (2008) for the comparison countries. US death rates were calculated from the most recent US National Center for Health Statistics life tables (Arias, Rostron and Tejada-Vera 2010). They reflect an increase in mortality at ages above 85 relative to previously-issued life tables. This increase brings them into close alignment with life tables produced by the Social Security Administration.
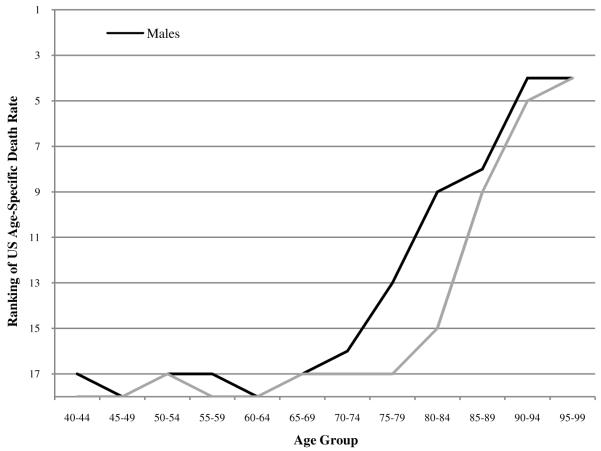
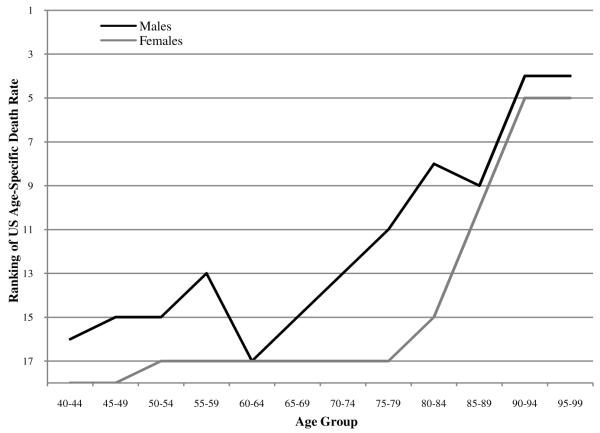
Figure 1 shows that, between ages 40 and 75, US all-cause mortality rates are among the poorest in the set of comparison countries. The US position improves dramatically after age 70 for males and after age 75 for females. Lest it be thought that what happens above age 75 is relatively unimportant for measures of longevity, it should be pointed out that two-thirds of newborns (67.3%) survive to age 75 in the published US life tables for 2006 (United States National Center for Health Statistics 2009: 26).
FIGURE 1.
Ranking of US age-specific death rates among 18 OECD countries in 2005
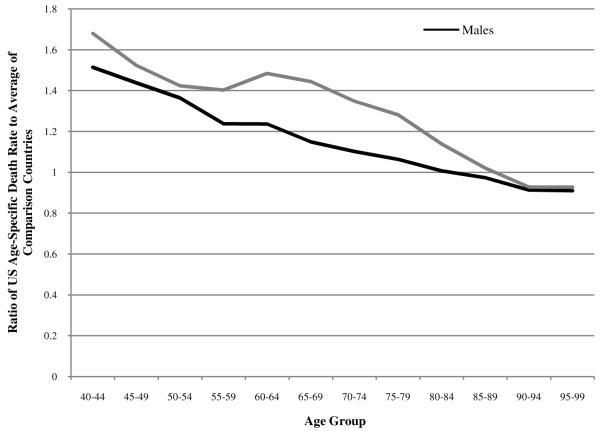
Males do relatively better than females in every age group except 95-99. US males rank fourth out of eighteen countries in the age groups 90-94 and 95-99, while US females rank fifth and fourth in these age groups. Clearly, we observe a very striking pattern of improvement in US rankings from near the bottom to near the top as age advances. This sharp upward slope in US mortality rankings with age has not been previously documented and constitutes a dramatic feature of the US mortality profile. The pattern shown in Figure 1 is echoed in Figure 2, where the ratio of US age-specific death rates to the average of the comparison countries systematically declines with age and is higher at all ages for women than for men.2
FIGURE 2.
Ratio of US age-specific death rates to the average of a comparison set of 17 OECD countries in 2005
We now consider four possible explanations of the age patterns revealed by this analysis: (1) access to health insurance; (2) smoking patterns; (3) age gradients in health care system performance; and (4) selection.
Access to Health Care
Perhaps the most obvious place to look for an explanation of the pattern that we have demonstrated is the age-pattern of entitlement to medical services in the US. About 15.4% of the population was estimated to be uninsured in 2008 (DeNavas-Walt, Proctor, and Smith 2009). In contrast, about 98% of residents qualify for Medicare at age 65, which provides virtually full coverage for hospitalization and subsidized coverage for other medical services. This abrupt age threshold for health insurance is not present in other OECD countries (Commonwealth Fund 2007). Thus, it is plausible that the US performs well at older ages because of older individuals’ expanded access to the health care system. Card et al. (2008) used the age threshold of Medicare eligibility to conduct a quasi-experimental study of the effects of reaching age 65 on access to and utilization of health care services. They found that Medicare eligibility causes a sharp increase in the use of health care services. Routine doctor visits increased, an increase that was concentrated among groups that previously lacked coverage, and overall hospitalizations increased sharply. For patients with conditions mainly treated by drugs, such as heart failure, all groups showed a very small increase in hospitalization rates at 65 (Ibid).
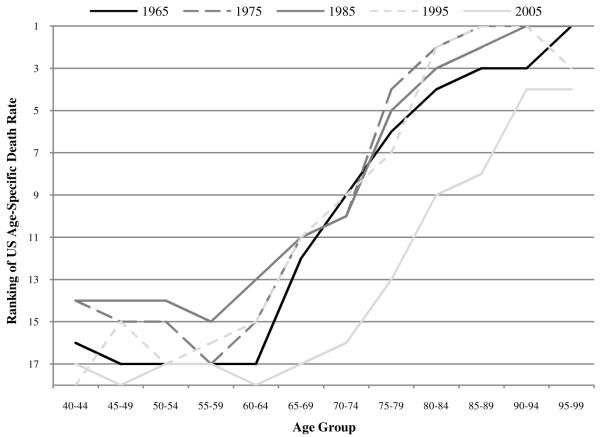
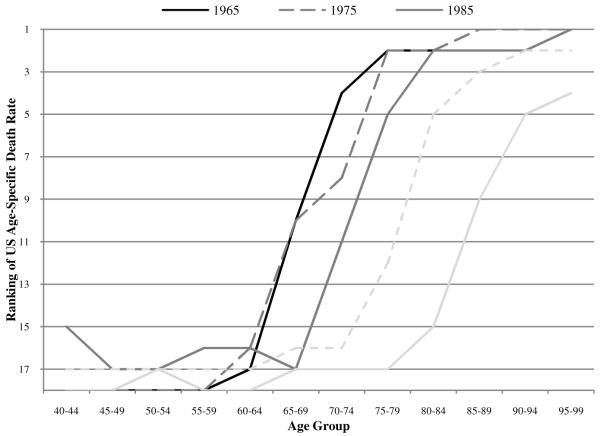
We explore the merits of the increased access explanation by examining equivalent rankings and ratios in 1960, a year that predates the implementation of Medicare in 1968.3 Contrary to the hypothesis, Figure 3 shows that the upward-sloping age patterns already existed in 1960. In fact, a comparison of Figures 1 and 3 shows that the US position among the comparison countries has in fact systematically worsened since 1960. This deterioration is particularly clear for females. Thus, it does not appear that Medicare entitlements are responsible for the upward slope in current US rankings, since the pattern was clearly established before Medicare was introduced. In fact, US rankings at ages 65-69 to 80-84 have substantially deteriorated since the introduction of Medicare. This deterioration is evident in Figures 4 and 5, which show the rankings of US age-specific death rates in 10-year intervals, starting with 1965 and ending with 2005.
FIGURE 3.
Ranking of US age-specific death rates among 18 OECD countries in 1960
FIGURE 4.
Ranking of US male age-specific death rates among 18 OECD countries in 1965, 1975, 1985, 1995, and 20054
FIGURE 5.
Ranking of US female age-specific death rates among 18 OECD countries in 1965, 1975, 1985, 1995, and 2005
The position of US males in 2005 has deteriorated relative to the earlier years, which are clustered together. In contrast, the female rankings have worsened steadily between 1975 and 2005. The existence of this pattern in 1960 suggests factors intrinsic to the US population may be responsible. Higher mortality rates among younger US men in 1960 could be due to unusually heavy smoking; however, the same is not true of US women, who did not smoke heavily in earlier years.
Smoking
A second plausible hypothesis is that the American profile in 2005 has been distorted by its history of heavy smoking. The major effect of smoking on population levels of mortality has been well-documented.5 In recent decades, the smoking histories of OECD countries have differed substantially by country and by sex. During the period 1945-1985, the US had the highest per adult consumption of manufactured cigarettes outside of Eastern Europe (Forey et al. 2002), with a very rapid increase in smoking registered among women.
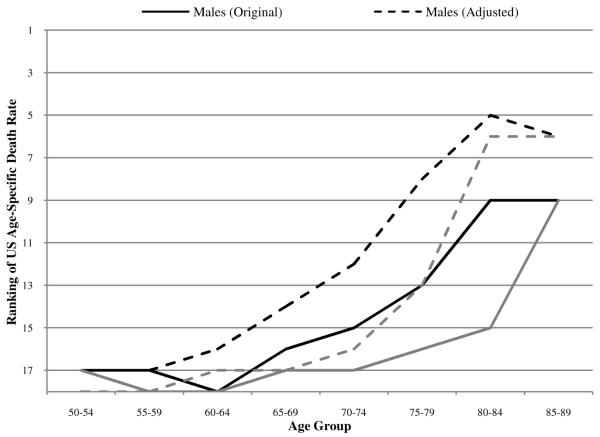
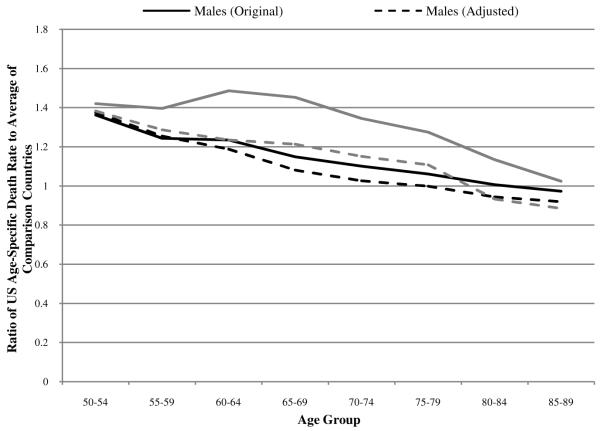
To investigate the impact of smoking on these rankings, we use a procedure developed in Preston, Glei, and Wilmoth (2010). The procedure is based on a macro-statistical analysis of the relation between lung cancer mortality, used as an indicator of damage from smoking, and mortality from other causes of death in a data set covering 21 developed countries from 1950 to 2006. In the absence of smoking, lung cancer mortality is assumed to be that observed among non-smokers in a large American Cancer Society cohort study. We draw lung cancer deaths and deaths from all causes by age group and sex for each country from the World Health Organization Mortality Database (2008) to obtain the fraction of total deaths that are due to lung cancer. The HMD all-cause death rates are multiplied by this fraction to obtain lung cancer death rates, which allows us to estimate the fraction of deaths attributable to smoking by sex, age group, and country.6 We then remove smoking-attributable deaths from the overall death rates in each country to assess the impact of smoking on the 2005 international mortality rankings and ratios. Results are shown in Figures 6 and 7.
FIGURE 6.
Ranking of US age-specific death rates among 18 OECD countries with and without adjustment for smoking attributable deaths
FIGURE 7.
Ratio of US age-specific death rates to the average of 17 OECD countries with and without adjustment for smoking-attributable deaths
Far from accounting for the upward slope in US rankings, adjustment for smoking actually makes the slope steeper for both men and women. At its peak, the improvement in rankings is five positions for men (at age 75-79) and nine positions for women (at age 80-84). At ages 50-64, on the other hand, improvements are minimal. On the basis of rankings, it appears that, relative to their counterparts elsewhere, the costs of heavy smoking in the US are being borne primarily by people above age 75.
Mortality ratios tell a somewhat different story. As shown in Figure 7, removing smoking-attributable deaths for women has a very large effect on mortality ratios at younger ages; the ratio of US female death rates to death rates in the composite declines by >0.2 at ages 60-64 and 65-69. These ages coincide with the heaviest smoking birth cohorts of American women, those born around World War II (Preston and Wang (2006). Removal of smoking-attributable deaths reduces mortality ratios much more for women than for men, resulting in an age-patterns of ratios that are quite similar for men and women.
Despite the major effect of smoking on the mortality of US women aged 60-69, their ranking doesn’t improve relative to the composite because US death rates were so much higher to start with. The “outlier” status of US women at ages under 70 means that the large changes in ratios that result from removing smoking-attributable deaths have little if any effect on the US ranking. On the other hand, male death rates are more closely clustered than female death rates, so that the relatively modest effect of removing smoking-attributable deaths on the US male mortality ratios translates into a perceptible effect on male rankings beyond age 64.
Given the history of cigarette consumption in the US relative to other developed countries, we expected that smoking might be an important part of the explanation for the US disadvantage at all ages, and especially so for younger females. Although the adjustment for smoking has a very large effect on American women’s mortality at ages 55-74 relative to the composite, it is not sufficient to improve women’s rankings substantially. Instead, sizeable improvements in rankings are produced at older ages for both men and women. Thus, allowance for smoking actually makes the age pattern of rankings sharper and steeper.
Age Patterns of Health Care System Performance
We now investigate whether the health care system, quite apart from health insurance issues, may be responsible for the unusual age pattern of American mortality. For example, it is possible that younger patients in the US receive less effective medical care than younger patients in other developed countries, while the US position is much better among older persons. Even though the focus of this section is on age-patterns of care, it should be noted that the level of care itself may have cumulative effects on the age-pattern of mortality. Table 1 contains a concise summary of the studies that we review and indicates whether a US advantage exists and whether it is greater at older ages.
TABLE 1.
Summary of Studies Performing International Comparisons of Medical Treatment at Older Ages
| Study | Study Population14 | Variable Examined | US Advantage?* | US Advantage Greater at Older Ages** (75+, 80+, 85+)? |
|---|---|---|---|---|
| Crimmins, Garcia, and Kim (2009) | Adults aged 20+ in 4 OECD countries |
Percent of population taking lipid-lowering drugs |
Yes | Mixed |
|
| ||||
| Howard, Richardson, and Thorpe (2009) | Adults aged 50+ in 17 OECD countries |
Screening rates: | ||
| Breast cancer | Yes | Yes | ||
| Cervical cancer | Yes | Yes | ||
| Colorectal cancer15 | Yes | Yes | ||
| Prostate cancer | Yes | Yes | ||
|
| ||||
| Hughes (2003) | Patients aged 40+ diagnosed with breast cancer in 1989-1995 in 9 OECD countries16 |
Five-year relative breast cancer survival rates |
Yes | No |
|
| ||||
| Moise (2003) | Patients aged 40+ hospitalized for AMI, 1997, in 7 OECD countries |
Proportion of AMI patients undergoing CABG and PTCA |
Yes | Yes |
| 1996, in 7 OECD countries17 |
One year case fatality rates from AMI |
Yes | Yes | |
|
| ||||
| Moon et al (2003) | Patients aged 40+ hospitalized with ischaemic stroke, 1998, in 9 OECD countries |
7-day hospital fatality rate | Males: Mixed Females: Yes |
Males: Yes Females: No |
| in 8 OECD countries18 | 30-day hospital fatality rate | Yes | Males: Yes Females: No |
|
|
| ||||
| O’Neill and O’Neill (2007) | Adults 18+ diagnosed with high blood pressure |
Percent receiving treatment | Yes | No |
| Adults 18+ diagnosed with diabetes in US and Canada |
Percent taking insulin or pills | Yes | Yes | |
|
| ||||
| Technological Change in Health Care Research Network (2001) | Patients hospitalized with AMI, 1989-1998, in 6 OECD countries and Israel and Scotland |
Use of cardiac procedures19 | Yes | Yes |
|
| ||||
| Wolf-Maier et al (2004) | Adults aged 35+ in 7 OECD countries20 |
Hypertension treatment rates | Yes | No |
Ratio of favorable outcomes per person, US versus mean or median of other countries for all ages
Ratio of favorable outcomes per person grows with age
Based on national probability samples unless otherwise indicated.
Colonoscopy, sigmoidoscopy, and fetal occult bloodtest.
Population-based cancer registries and data collected as part of OECD Aging-Related Diseases Study.
Data collected as part of OECD Aging-Related Diseases Study.
Data collected as part of OECD Aging-Related Diseases Study.
This includes cardiac catheterization one year after heart attack, bypass surgery one year after heart attack, and one-day primary angioplasty.
All surveys based on national probability sample except for Sweden, which had a regional sample.
Cancer
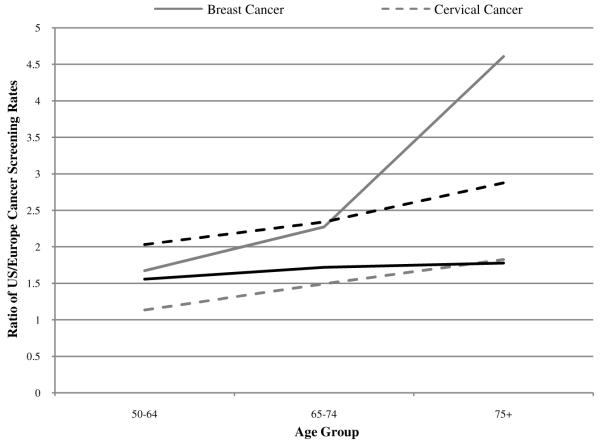
Compared to European countries, the US performs particularly well in terms of cancer screening and cancer survival. Howard, Richardson, and Thorpe (2009) have conducted the most comprehensive study of screening differences between the US and Europe. They use the 2004 Health and Retirement Survey (HRS) and Medical Expenditure Panel Surveys (MEPS) to calculate US screening rates. They use the 2004 Survey of Health, Ageing and Retirement in Europe (SHARE) and the 2006 Eurobarometer survey to calculate European screening rates. Four screening indicators were considered: mammography in the past two years, colorectal screening in the past 10 years in Europe and in the past 5 years in the US7, pap smear in the past year, and PSA test in the past year.
As shown in Figure 8, US screening rates were substantially higher at all ages and the US advantage grew with age. Overall, European screening rates were 22-88% of the corresponding US rates. Although screening rates are inversely related to age in all countries, the decline in age was sharper in European countries than in the US for all forms of cancer considered. For mammography, the ratio of US to European screening rates was 1.67 in the age group 50-64, 2.27 in the age group 65-74, and 4.61 in the age group 75+. The ratios for the other three screening tests showed a similar increase with age; the US performed well at all ages and relatively better at older ages.
FIGURE 8.
Ratio of US/Europe screening rates for four major cancers8
In these surveys, screening rates were based on respondent self-report. Medicare claims data were used to assess the quality of the US data. The authors found that while the mammography and colorectal screening rates from Medicare claims data were below the survey-based rates from HRS and MEPS, they were still well above the survey based rates from SHARE. This result suggests that the large observed differences in screening rates are not due to differences in the propensity to misreport screening between the US and Europe. The authors hypothesize that differences in screening rates may originate from differing institutional approaches to screening. Most European countries have organized screening programs with upper age limits; thus, screening “invitations” may only be mailed to women in the target age group. In contrast, screening in the US is relatively decentralized and without age-based limits. US residents are more likely to be screened at both the older and younger ages than are European residents (Ibid).
A number of studies have examined international variations in relative survival rates from cancer (relative, that is, to persons of the same age and sex at diagnosis). Gatta et al. (2000) conducted a thorough and careful examination of survival rates using SEER and EUROCARE data, making great efforts to ensure comparability. They found that although survival rates declined with age at diagnosis for all cancers (except for breast and prostate cancer in the US and Europe and for colon cancer in the US), the decline was more marked in European patients. Other studies concur with these findings; for example, Coleman et al. (1999) found that five-year survival for patients diagnosed at age 75 years or older during the 1990s was nearly 20% higher in the US than in Europe.
Colorectal cancer provides a good illustration of these trends. In Europe, 5-year relative survival rates for colorectal cancer were lower among elderly patients than among younger patients. This decline has been attributed to declining health at older ages and more advanced stage at diagnosis. In the US, relative survival for colorectal cancer did not decline with age, possibly because patterns of surgery for colon and rectum cancer patients did not vary substantially with age. In contrast, the proportion of surgically-treated patients declined with age in Europe (Gatta et al. 2000).
Breast cancer was one of the diseases included in the OECD’s Aging-Related Diseases Study. In European countries, older women generally have lower 5-year relative survival rates from breast cancer than their younger counterparts. The decline with age is particularly dramatic in England and Wales, where the survival rate is 80% for women aged 50-59 and only 53% for women over the age of 80. Survival rates also declined with age in Japan, Norway, and Ontario. In the remaining countries, including the US, however, older women experienced fairly similar outcomes compared to their younger counterparts in 1989-95. The survival rate was approximately 82% for both age groups in the US. The international differences in age patterns of survival were hypothesized to be attributable to differences in stage at diagnosis and screening and treatment patterns (Hughes 2003).
Early stage disease was more frequent among younger European patients (aged 45-49) than older European patients (aged 70-99) diagnosed with breast cancer between 1990 and 1992. In contrast, early-stage disease was more frequent among older US breast cancer patients than among younger breast cancer patients diagnosed in the same time period (Sant et al. 2004). Stage at diagnosis is an important prognostic variable for almost every cancer and for patients at any age; however, it may be even more important for the elderly. Vercelli et al. (1998) examined relative survival among elderly cancer patients in Europe and found that for many cancers, including breast cancer, the survival variation between younger and older European patients is greater at 1 than at 5 years after diagnosis. This pattern is related to the fact that the elderly generally presented with a more advanced stage. However, when breast cancer is detected early and treated with curative intent, the elderly achieve relatively good survival (Ibid).
High Cholesterol
High cholesterol is a risk factor for coronary heart disease and stroke. Crimmins, Garcia, and Kim (2009) examined the use of lipid-lowering drugs in the US, Japan, the Netherlands, and Italy. For the age groups 40-49, 50-59, 60-69, and 70+, the US had the highest percent of adults who were taking lipid-lowering drugs. The ratio of the US to the average of the other three countries was highest in age group 40-49 and 50-59 for females and males, respectively. For both sexes, the ratio of the percent being treated in the US to the average of the comparison countries was higher in the age group 70+ than in the age group 60-69. The US advantage was present for both men and women, although the differences in the percent of the population taking lipid-lowering drugs between the US and the other countries were particularly large for men (Ibid).
Ischaemic Heart Disease (IHD) and Acute Myocardial Infarction (AMI)
Ischaemic heart disease (IHD) is the world’s leading cause of death. A review of cross-national differences in the treatment and outcomes of IHD was conducted as part of the OECD Ageing-Related Diseases Study (Moise 2003). In order to facilitate comparisons across countries, the analysis of treatment patterns was based on data on AMI admissions from hospital inpatient databases.9 AMI (acute myocardial infarction, or heart attack) is a well-defined clinical condition around the world and inpatient data, which are the most reliable data in most countries, are relatively complete sources of information on acute care for heart attacks. AMI is an important medical condition to consider because of its burden of mortality in developed countries and because knowledge of effective treatment has changed in recent years. Clinical trials and other data suggest that changes in medical practices may account for a large part of the improvements observed in AMI outcomes (Technological Change in Health Care Research Network 2001).
In the OECD study, the US had the highest rates of use of both angioplasty and coronary artery bypass graft (CABG). It achieved lower case fatality rates for older persons than other countries but not for the youngest age group (40-64 years) (Moise and Jacobzone 2002). Compared to Australia, Belgium, and Ontario, Canada, the US had the highest use of catheterizations; approximately half of male and female AMI patients aged 40-64 were given a catheterization in 1996. In contrast, less than 10% of AMI patients in Italy, Norway, and the UK received catheterization. Use of catheterization decreases with age, but the steepness of the gradient differs across countries. Among female AMI patients in the US, 50% aged 40-64 received catheterization compared to 10% of those aged 85-90. The corresponding figures for the Oxford region of the UK were 4% and 1%, respectively (Ibid).
Percutaneous transluminal coronary angioplasty (PTCA) is a minimally invasive surgical procedure similar to cardiac catheterization that is increasingly being used to treat AMI. It has also been shown to reduce angina, a significant sub-category of IHD. AMI patients in the US underwent PTCA more often than patients in the comparison countries (Australia, Canada, Finland, Spain, Sweden, and the UK) for all age groups, but the largest differences were observed for the elderly (Moise and Jacobzone 2002). In 1997, the US had the highest proportion of male AMI patients undergoing PTCA in the age groups 40-64 and 80-84. Utilization of PTCA among males aged 40-64 was 38.7% in the US and 26.9% in Australia, the country with the next highest proportion of AMI patients undergoing PTCA. The corresponding figures for males aged 80-84 were 16.0% and 4.9%. Among female patients aged 40-64, a slightly higher proportion underwent PTCA in Australia than the US. Among women aged 80-84, however, 13.4% of US patients underwent PTCA compared to 2.4% in Canada, the country with the second highest use of PTCA in this age group (Moise 2003). Moise (2003) suggests that a greater reliance on PTCA in the US may result in the US’s lower readmission rates relative to the comparison countries. The US experienced particularly large increases in the use of PTCA among older patients between 1990 and 1996; use of PTCA for treating AMI patients aged 85-90 increased 2.7 times for males and 3.9 times for females (Ibid).
CABG is an intensive and complicated cardiac procedure. It is rarely used to treat AMI except in an emergency or as a follow up procedure; instead, it is used far more extensively to treat less acute forms of IHD, such as angina. CABG can be seen as a secondary prevention measure for reducing the risk of later heart attacks. Proponents of CABG (versus PTCA) argue that CABG is more effective in reducing the likelihood of readmissions for IHD (Moise and Jacobzone 2002). AMI patients in the US in both age groups (40-64 and 80-84) were more likely to receive CABG than AMI patients in Australia, Canada, Finland, Spain, Sweden, and the UK in 1997. The proportion of male AMI patients aged 80-84 undergoing CABG was 12.4% in the US; the figure for the country with the next highest proportion, Australia, was only 3.3%. For female patients aged 80-84, these figures were 8.5% in the US and 1.2% in Canada, which had the next highest proportion of female AMI patients aged 80-84 undergoing CABG (Moise 2003).
Similar patterns have been observed in the Technological Change in Health Care Research Network (2001) study, which evaluated cross-country differences in AMI treatment from 1989 to 1998. This study found that intensive treatment rates for elderly heart attack patients (age 65+) in the US were only moderately different from treatment rates for nonelderly patients. In Canada, the catheterization rates for the elderly were two-thirds to three-fourths as large as for the nonelderly. In Finland and Scotland, the catheterization rate in elderly heart attack patients was only half as high as in the overall population. Similar patterns were observed in the use of CABG and PTCA. Compared to the comparison countries, intensive procedures diffuse more rapidly among the US elderly population (Ibid).10
Data on one-year case fatality rates for patients admitted for AMI in 1996 were available for Australia, Canada, Denmark, Finland, Sweden, the UK, and the US. Patients aged 40-64 in the US had the third-lowest one-year case fatality rates for males and females. For the oldest age group,85-90, case fatality rates in the US were generally lower than in other countries. In this age group, males had the second-lowest and females had the lowest case fatality rates among the comparison countries (Moise 2003). The US survival advantage may be attributable to patient case mix (US patients may present with less severe cases of AMI) or greater capacity constraints in Europe (Moise and Jacobzone 2002).
Pilote et al. (2003) conducted a cohort study of cardiac procedure use and outcomes among elderly patients in the US and Canada (Quebec). They were able to include nearly the entire population of elderly AMI patients in the US and Quebec by using Medicare claims data and provincial claims data. Patients 65 years and older who were hospitalized with a principal diagnosis of a new AMI between 1988 and 1994 were included in the study. Overall, the authors conclude that cardiac procedures were used much more intensively among the oldest patients in the US relative to Canadian patients in this age group (Ibid).
Hypertension
Hypertension is universally recognized as a risk factor for cardiovascular disease (CVD) and stroke. In an analysis of primary care in five English-speaking countries, the proportion of adults having their blood pressure checked in the last year was highest in the US (Schoen et al. 2004: Exhibit 6). Wolf-Maier et al. (2004) evaluated hypertension treatment strategies in the 1990s in the following countries: Canada, England, Germany, Italy, Spain, Sweden, and the United States. They found that hypertension treatment has been pursued more aggressively in North America than Europe, and most aggressively in the US. Based on the current standard of 140/99 mm Hg, treatment of hypertension was highest in the US (53%) and Canada (36%), and lowest in England (24%), Sweden (26%), and Germany (26%). Cross-country variation in treatment guidelines did not entirely account for the varying success in control efforts. Treatment rates increased with age in Europe, particularly for women, but remained fairly constant across age in the US. The percentage of hypertensives who had their blood pressure controlled at the 140/90 mm Hg level was the highest in the US for each age group (35-45, 45-55, 55-65, and 65-75). The proportion of hypertensive men whose blood pressure was controlled at the 140/99 mm Hg threshold increased markedly with age. This was especially true in the US, where it rose from 9% for men aged 35 to 30-44% for men over the age of 65 (Ibid).
O’Neill and O’Neill (2007) used the Joint Canada/US Survey of Health (JCUSH), a telephone survey designed to maximize cross-country comparability of the responses, to determine the percent of patients with chronic conditions who received treatment in the US and Canada. 88.3% of US adults aged 18-64 with high blood pressure reported receiving treatment, compared to 84.1% of Canadian adults in the same age group. At older ages (65+), these percentages increase to 97.7% and 95.1% for the US and Canada, respectively (Ibid).
Stroke
Proper management of hypertension has been shown to be highly effective in reducing the risk of stroke for all age groups. The burden of mortality from stroke is sizeable: in 1997, stroke deaths accounted for 10% of all deaths in OECD countries (Moon 2003). An in-depth review of stroke treatment and care was included in the OECD Aging-Related Diseases project. This report focused on ischaemic stroke, the subtype of stroke which accounts for the largest proportion of strokes and which is more amenable to treatment.11 Ischaemic stroke is related to aging, with mortality increasing sharply with age (Moon, Moise, and Jacobzone 2003). 7-day and 30-day hospital fatality rates were calculated for patients hospitalized with ischaemic stroke in 1998. Three age groups were compared, 40-64, 65-74, and 75+.12 For the 7-day hospital fatality rates, US women went from ranking 1st out of 9 countries in the age group 40-64 to ranking 3rd and 2nd out of 9 in the older age groups. Men started out in 5th place at ages 40-64 and improved to 2nd place in the age group 75+. Patterns were similar for the 30-day hospital fatality rates, with men showing improvement with age while women’s ranking fell slightly in age groups 65-74 and 75+ relative to 40-64. For the 30-day and one-year case fatality rates, US Medicare data are used, so comparisons for the 40-64 age group cannot be performed. Counting all deaths and not simply deaths in the hospital, and limiting comparison to six regions including two in Canada, the US 30-day survival rate ranked 1st for men aged 65-74 and 75+ and 2nd for women in these ages. The US one-year survival rate among this set of populations was considerably poorer, ranking 5th of 6 for men aged 65-74 and 4th of 6 for men aged 75+. For women at these two ages, the rankings were 4th and 3rd. In these rankings, the US position was consistently better at 75+ than at 65-74 (Ibid).
To summarize this section (see also Table 1), the US has a clear-cut advantage relative to other OECD countries in frequency of cancer screening, and the US screening advantage increases with age. According to Gatta et al. (2000), the US survival advantage also increases with age, but they do not supply the corresponding data. Since cancer is an important cause of death at older ages, the superior performance of the US health care system with respect to cancer identification and survival is a plausible contributor to the age-pattern of US rankings that we have identified.
Data on and analyses of the relative performance of the US health care system at older ages are less abundant with respect to heart disease and stroke. The US has higher proportions receiving anti-hypertensive medication and medication to control cholesterol levels than the median of other countries, but the relative proportions do not grow systematically with age. (Of course, mortality at any particular age can be affected by treatments received at younger ages, and the advantages of superior treatment may cumulate.) Surgery following a heart attack is more common in the US than in other countries and the international disparity grows with age. US one-year survival rates following a heart attack are better than average among seven countries and the US advantage grows with age. Seven day survival rates from stroke are better in the US than in the median of comparison countries but one-year survival rates are worse. There is no clear pattern of change in US rankings with age.
Selection
It is possible that the unusual age pattern in the US is produced by selection, a process whereby weaker individuals die disproportionally at younger ages, leaving behind a hardier group of survivors at older ages. It is clear that some sort of selection mechanisms must be operating in all populations. The question is whether those processes are more powerful in the US than elsewhere.
This question cannot be answered definitively. The correlations among rankings across age intervals in our data are not negative, as the US pattern might imply, but are uniformly positive, whether we use Spearman’s rank correlation coefficient or the Kendall tau rank correlation coefficient and whether the US is included or excluded from the data set. This result is consistent with observations over time and space. A large collection of life tables assembled from different countries and eras found correlations of +0.8 or higher between death rates at any two ages (Coale and Demeny 1983). Janssen et al. (2005) used a cohort approach to examine the correlation between mortality at ages 55-69 and ages 80-89 for cohorts born between 1895 and 1910 in northwestern Europe. The authors consistently found positive correlations between mortality rates at these ages for the same cohort and concluded that trends in old age mortality were predominantly determined by accumulated exposures over the life course rather than by mechanisms of selection (Ibid). Finch and Crimmins (2005) reached a similar conclusion based on 250 years of cohort data from Sweden.
However, Vaupel (2010) formally describes a situation in which selection could plausibly account for the pattern observed. Suppose that the age-trajectory of mortality is identical for all individuals in a population but that the initial value of mortality (or frailty) varies from person to person. Then the age-slope of mortality for the population will depend not only on the mean level of frailty in the population but also on its distribution. At a particular mean level, the slope will be lower – mortality will rise less rapidly with age – the greater the degree of heterogeneity in a population.
As the largest country in our data set, the US has the greatest degree of geographic and ethnic diversity. Using various measures of geographic variance in mortality, Wilmoth et al. (2010) find that the US is more diverse than any other OECD country examined, although the degree of diversity is roughly the same as that of western Europe as a whole. Thus, an unusually high degree of heterogeneity in the US may be related to the pattern we observe.
Some elements of heterogeneity are observable and others are not. One element that can be directly observed in the US is racial heterogeneity. Oddly enough, the observed age pattern of mortality for African Americans is more exaggerated than that of the population as a whole, although the data are of relatively poor quality. They show higher death rates than national averages at younger ages but rates that approach, or may even fall below, white death rates at the oldest ages (Preston et al. 2003). Such a pattern is not consistent with a heterogeneity explanation since the black population is presumably more homogeneous than the US population as a whole.
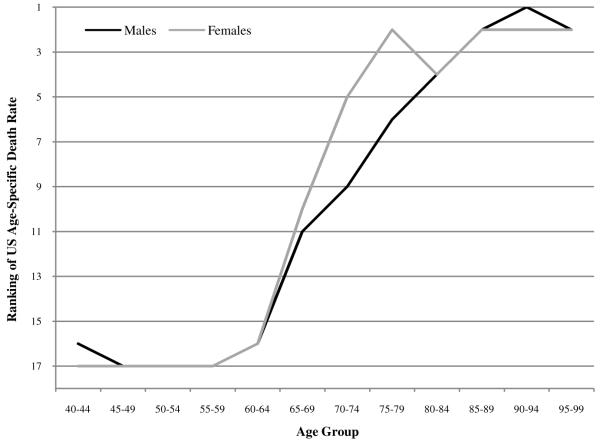
Figure 9 shows what happens to the pattern when only whites in the US are considered. The pattern is similar to that for the total population, although males improve by several ranks at younger ages. The basic pattern of rapid improvement in US rankings with age is maintained.
FIGURE 9.
Ranking of US age-specific death rates (whites only) among 18 OECD countries in 2005
Racial heterogeneity is only one source of heterogeneity and others are more difficult to observe. We cannot dismiss selection – in particular, that which would be produced by greater heterogeneity in the US – as a factor in the pattern we observe. However, we note that Japan, a country with relatively little genetic diversity on several measures (Jorde et al. 2000), does not have an age-pattern of rankings that deteriorates with age, as might be anticipated under a selection argument.
Other Explanations
We have investigated three other potential sources of the age-pattern of US rankings. First, we removed all deaths from violent causes – accidents, suicides, homicides (excluding complications of medical procedures) – from death rates in all countries. Excluding violent deaths has an effect on ratios at ages 40-49 but a trivial effect thereafter. At ages 40-44, excluding violent deaths improves the US ratio by .061 for men and .082 for women. At ages 45-49, the equivalent improvements are .025 and .048. Beyond age 50, the change in the ratio for men never reaches .01 and it reaches that value only at age 50-54 for women, where it is .014. From age 55 onwards, the changes in the ratios are actually slightly negative, indicating that the US has slightly below-average violent mortality. So violent mortality contributes to the high US mortality at ages 40-49, but not to the broader age pattern that we have described.
Another possibility is that the educational attainment of the oldest old in the US may be high relative to the comparison countries. We use figures from Lutz et al. (2007) obtained through back-projection methods and compare age groups 50-54 and 65+ in 1970, 1980, 1990, and 2000. In every year, the US ranked 7th out of 18 countries for both age groups in terms of the population that has attained at least a secondary education. US adults at older ages (65+) were above the median of comparison countries in terms of educational attainment, but so were US adults at younger ages (50-54). There does not appear to be sufficient differentiation by age in US educational rankings to account for the age-profile of mortality rankings.
The third added factor that we investigated is obesity. According to World Health Organization estimates, men and women in the United States had a higher prevalence of obesity in 2005 (defined as a Body Mass Index, or BMI, of 30.00 or higher) than any other country in Europe, North America, or East Asia (World Health Organization 2005). It is not possible to identify very precisely the effect of this risk factor on international comparisons of mortality because there is no settled understanding about the impact of obesity on mortality (e.g., Alley et al. 2010). Most consequentially for our analysis, the mortality risk associated with obesity appears to have been declining sharply in the US (Mehta 2010; Flegal et al. 2007).
To investigate the potential impact of obesity on our comparisons, we utilize certain results presented in Preston and Stokes (2010). They estimate the proportion of deaths attributable to obesity, by age and sex, in various countries. They used the mortality risks associated with various detailed BMI categories that were developed in a large international review that synthesized data from 57 prospective studies, primarily from North America and Western Europe (Prospective Studies Collaboration 2009). They applied these risk factors to the distribution of population in detailed BMI categories. These distributions were drawn from the US National Health and Nutrition Examination Survey, from the Survey of Health and Retirement in Europe, and from several other nationally representative surveys. The question they asked was how much mortality would be reduced if people with above-optimal BMI were redistributed to the optimal category. The data on BMI are primarily from self-reports but measured BMI was used in several comparisons.
The study presented results for 14 of the 18 countries considered here (Preston and Stokes 2010).13 In Table 2, we have summarized the results of comparisons between the US and the other 13 countries. The table presents the average, across 13 countries, of the difference between the fraction of deaths attributable to obesity in a particular country and the attributable fraction in the US. Relative to the comparison countries, 10-12% of deaths at ages 50-54 in the US can be attributed to its higher proportion obese. This fraction declines to 2% by ages 80-89. So the prevalence of obesity in the US, combined with an estimate of the relative risk from obesity, may in fact be related to the age-pattern of US ratios and rankings that we have described. In particular, it may help explain the high US mortality in the age interval 50-59, which we have otherwise been unable to account for. Since the ratios are declining with age, it is reasonable to suppose that the consequences of obesity may be even higher at ages 40-49.
TABLE 2.
Average Difference in Obesity Attributable Fraction Between the US and 13 Comparison Countries
| Age Group | Males | Females |
|---|---|---|
| 50-59 | 0.10341 | 0.11800 |
| 60-69 | 0.08991 | 0.09422 |
| 70-79 | 0.06343 | 0.05147 |
| 80-89 | 0.01858 | 0.01962 |
Compiled from Preston and Stokes (2010).
However, we are inclined to view the estimates in Table 2 as an upper bound on the effect of obesity on the relative position of US mortality rates. The median year of death in the combined set of studies producing the risk factors employed in this table was 1986 (Prospective Studies Collaboration 2009). As noted earlier, the risks seem to be declining over time, at least in the US. One explanation offered for the decline is innovation in the treatment of cardiovascular disease, the main disease entity through which obesity operates (Mehta 2010, Flegal et al. 2007). A second explanation is that the very rapid increase in obesity in the US produces many newcomers to the obese category, so that the physical hazards associated with obesity (e.g., high blood pressure, diabetes) have not had long to operate. In either case, risk estimates drawn from earlier international studies would be too high for application to the contemporary US. Use of Mehta’s risk factors (estimated on NHANES data for 1988-2006 and not differentiated by age or sex) gives comparative attributable risk values that are roughly half of those in Table 2 (compiled from Preston and Stokes 2010). Even in this case, the prevalence of obesity and the associated risk factors contribute moderately to the age pattern of mortality rates.
Conclusion
This paper identifies an unusual age-pattern of mortality rankings in the United States. Mortality rankings below age 75 are among the worst in the set of 18 countries we consider but rise rapidly with age thereafter. We have considered four principal explanations of the unusual age pattern. Two of the explanations – health care access and smoking patterns – do not appear promising. The sharp upward slope in US age-specific mortality rankings was already present in 1960, suggesting that the advent of Medicare and its associated health care entitlements is not a decisive factor in the pattern. The age-pattern was also present in 1965, 1975, 1985, and 1995. The poorest rankings at older ages in the US for both men and women at all ages above 70 are observed in 2005. While the removal of smoking-attributable deaths has an important effect on the relative level of mortality, especially for women at younger ages, the removal of smoking-attributable deaths does not erase the upward slope of US rankings and in fact produces the largest improvements in rankings at the oldest ages.
We cannot dismiss the possibility that selection mechanisms have contributed to the pattern observed. Such an outcome could be produced by a greater degree of heterogeneity in the US population than in comparison countries. One direct test indicates that racial heterogeneity is not a major factor in the pattern, which is apparent among both whites and blacks. And Japan, which is likely to have an unusually low degree of heterogeneity, does not show a pattern that is inverse to that of the US, as might be expected if selection were a dominant factor.
High mortality from violence contributes to poor US rankings in the age interval 40-49 but is not a factor thereafter. The very high prevalence of obesity in the US is probably contributing to the pattern but uncertainty about the size of associated risks prevents a firm conclusion about its importance.
The hypothesis that is most strongly supported by our analysis is that the US health care system is performing especially well for older patients. Earlier, we documented US advantages in identification and treatment of cancer and in the treatment of heart disease (Preston and Ho 2009). Here we have supplemented that analysis by examining age patterns of identification and treatment of diseases within the older population. Such evidence is necessarily more limited. The clearest pattern emerges for cancer screening, where the ratio of US screening rates to those of other OECD countries increases with age for four major cancers. US one-year survival rates following a heart attack are also better than average among seven countries and the US advantage grows with age. Survival rates following a stroke are exceptions to this general pattern. US survival rates are superior at seven days but below average at one year, and the age patterns are not clear cut.
The possibility that health care advantages in the US grow with age is partially supported by medical expenditure data. Hagist and Kotlikoff (2005) examine per capita public expenditure on health care by age in 10 OECD countries. They find that, above age 65, the age-slope of expenditure is steeper than average in the US although several other countries are comparable. Between ages 65-69 and 80+, the increase in the public expenditure per capita in the US is third fastest among the 10 countries, after Canada and Australia (Ibid).
Future research on this topic would fruitfully explore international differences in the deployment of medical technology during earlier periods. If the health care system is currently responsible for the improved ranking of US death rates with age, and if persistent phenomena are believed to be responsible for the pattern of improvement that has been observed since 1960, then it would be useful to demonstrate that life-saving medical technologies (e.g., antibiotics for pneumonia) were also deployed more frequently in the US than elsewhere in earlier years. It would also be useful to investigate whether the age patterns of mortality that we have documented reflect enduring features of American society that may be manifest in attitudes and behavior towards the very old.
Acknowledgments
This research was supported by the U.S. Social Security Administration through the National Bureau of Economic Research and by the National Institute of Aging. The findings and conclusions expressed are solely those of the authors and do not represent the views of SSA, any agency of the Federal Government, or the NBER. We are grateful to Douglas Ewbank and James Smith for comments and suggestions.
Footnotes
We use the following formula for decomposing life expectancy at birth: e(0) = 50L0 + p(50)[e(50)], where e(0), e(50) are life expectancy at birth and at age 50, respectively, 50L0 is the number of years lived per newborn between ages 0 and 50, and p(50) is the probability of surviving from birth to age 50. To estimate the proportion of a difference in life expectancy at birth between two countries to that is attributable to differences in life expectancy at age 50, we weight the difference in e(50) by the mean of the two countries’ p(50) values. When applied to the 4.55 year difference in life expectancy at birth in 2006 between the US and Japan (both sexes combined), this formula indicates that 69.3% of the difference in life expectancy at birth is attributable to differences in life expectancy at age 50. When applied to the 2.66-year difference in life expectancy at birth between the US and France in 2006, the formula indicates that 67.5% of the difference in life expectancy at birth is attributable to life expectancy differences at age 50.
We further investigated the ratio of US cause-specific death rates to the average of the comparison countries for each age-sex group in 2005. We use WHO data to calculate the proportion of all deaths attributable to five categories: violent deaths (ICD-10 codes V01-Y98, excluding Y40-84 and Y88), deaths due to lung cancer (ICD-10 codes C33-C34, deaths due to all other cancers (ICD-10 codes C00-D48, excluding C33-C34), deaths due to circulatory disease (ICD-10 codes I00-I99), and all remaining causes of death (all remaining codes). These proportions were applied to the HMD death rates to obtain cause-specific death rates. For four countries, data by cause of death were not available for the year 2005, so the closest and most recent years available were used. These were Australia (2006), Belgium (2004), Italy (2006), and Portugal (2003). Some results of this effort are referred to below in sections on violence and smoking. Full figures are available on request from the authors.
We examine the patterns in 1960 using the HMD data set for the comparison countries’ death rates and the 1959-1961 NCHS life table and the 1960 Social Security Administration (SSA) life table for the US death rate (Bell and Miller 2007). The NCHS and SSA series were very similar, with the exception of the death rates for women between ages 80-99. In those age groups, the NCHS estimates slightly higher death rates for women in the oldest age groups compared to SSA. Figure 3 depicts the rankings in 1960 using the SSA series for the US.
Data for Germany before 1995 is for the Federal Republic of Germany. All data are drawn from the Human Mortality Database except the US series in 2005, which is taken from the newest NCHS life tables.
See Peto et al (1994) and Preston and Wang (2006), among others.
The procedure used provides estimates for smoking-attributable mortality only for ages 50+. For four countries, data by cause of death were not available for the year 2005, so the closest and most recent years available were used. These were Australia (2006), Belgium (2004), Italy (2006), and Portugal (2003). The coefficients, which were estimated for 2003, were adjusted for the time trend.
According to the authors, this was necessary due to differences in the wording of survey questions and may result in an understatement of the differences in screening rates between the US and Europe.
Source data are Exhibits 1 and 2 in Howard, Richardson, and Thorpe (2009).
AMI cases are less diverse in terms of severity than IHD, so using outcomes data based on AMI rather than IHD admissions increases the homogeneity of the patient population.
Depending on the procedure, comparison countries include Canada, Finland, Sweden, Denmark, France, Japan, Italy, and Norway, among others.
Ischaemic stroke accounts for 80% of strokes in the countries under consideration, with the exception of Japan, where haemorrhagic stroke contributes a higher proportion of stroke incidence.
For 7-day hospital fatality rates, the 9 countries compared were: Australia, Canada (Ontario), Denmark, Italy, Japan, Sweden, Switzerland, Great Britain (Oxford), and the United States. For 30-day hospital fatality rates, the same set of countries were compared, excluding Italy.
These countries are Austria, Belgium, Canada, Denmark, England, France, Germany, Italy, Japan, the Netherlands, Spain, Sweden, Switzerland, and the United States.
References
- Alley Dawn E., Lloyd Jennifer, Shardell Michelle. Can obesity account for cross-national differences in life expectancy? In: Cohen Barney, Crimmins Eileen, Preston Samuel., editors. Divergent Trends in Life Expectancy at Older Ages. National Academy Press; Washington, D.C.: 2010. Forthcoming. [Google Scholar]
- Arias Elizabeth, Rostron Brian, Tjada-Vera Betzaida. United States Life Tables 2005. National Vital Statistics Reports. 10. Vol. 58. US Department of Health and Human Services; 2010. [PubMed] [Google Scholar]
- Bell Felicitie C., Miller Michael L. [accessed January 2010];Life Tables for the United States Social Security Area 1900-2100. 2007 Actuarial Study No. 116. from http://www.ssa.gov/OACT/NOTES/as116/as116LOT.html.
- Card David, Dobkin Carlos, Maestas Nicole. The impact of nearly universal insurance coverage on health care utilization: evidence from Medicare. American Economic Review. 2008;98(5):2242–58. doi: 10.1257/aer.98.5.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coale Ansley J., Demeny Paul. Regional Model Life Tables and Stable Populations. Princeton University Press; 1983. [Google Scholar]
- Coleman MP, Babb P, Damiecki P, et al. The Stationery Office; London: 1999. Cancer survival trends in England and Wales 1971–1995: deprivation and NHS Region. Studies on Medical and Population Subjects; pp. 1–695. No. 61. [Google Scholar]
- Commonwealth Fund . 2007 International Symposium on Health Care Policy. Descriptions of Health Care Systems: Australia, Canada, Germany, the Netherlands, New Zealand, the United Kingdom, and the United States. Commonwealth Fund; New York: 2007. [Google Scholar]
- Crimmins Eileen, Garcia K, Kim JK. Are international differences and trends in life expectancy in sync with differences and trends in health and/or disability? In: Cohen Barney, Crimmins Eileen, Preston Samuel., editors. Divergent Trends in Life Expectancy at Older Ages. National Academy Press; Washington, D.C.: 2009. Forthcoming. [Google Scholar]
- DeNavas-Walt Carmen, Proctor Bernadette D., Smith Jessica C. Income, Poverty, and Health Insurance Coverage in the United States: 2008. U.S. Census Bureau; U.S. Government Printing Office; Washington, DC: 2009. Current Population Reports, P60-236. [Google Scholar]
- Elo Irma, Preston S. Educational Differentials in Mortality: United States, 1979-85. Social Science and Medicine. 1996;42(1):47–57. doi: 10.1016/0277-9536(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Finch Caleb, Crimmins Eileen. Inflammatory exposure and historical changes in Human Life Span. Science. 2005;305:1736–39. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Flegal Katherine M., Graubard Barry I., Williamson David F., Gail Mitchell H. Cause-specific excess deaths associated with underweight, overweight, and obesity. Journal of the American Medical Association. 2007;298(17):2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Forey Barbara, Hamling J, Lee P, Wald N., editors. International Smoking Statistics: A Collection of Historical Data from 30 Economically Developed Countries. Oxford University Press; London: 2002. [Google Scholar]
- Gatta Gemma, Capocaccia Ricardo, Coleman Michel P., Ries Lynn A. Gloeckler, et al. Toward a comparison of survival in American and European cancer patients. Cancer. 2000;89(4):893–900. [PubMed] [Google Scholar]
- Hagist Christian, Kotlikoff Laurence J. National Bureau of Economic Research Working Paper. Dec, 2005. Who’s going broke? Comparing healthcare costs in ten OECD countries. Number 11833. [Google Scholar]
- Howard David, Richardson Lisa C., Thorpe Kenneth E. Cancer screening and age in the United States and Europe. Health Affairs. 2009;28(6):1838–1847. doi: 10.1377/hlthaff.28.6.1838. [DOI] [PubMed] [Google Scholar]
- Hughes Melissa. A Disease Based Comparison of Health Systems: What is Best and at What Cost? Organisation for Economic Co-operation and Development (OECD); Paris: 2003. Summary of results from breast cancer disease study; pp. 77–94. [Google Scholar]
- Human Mortality Database (HMD) [Retrieved September 2009];University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany) 2008 from www.mortality.org.
- Janssen F, Peeters A, Mackenbach JP, Kunst AE. Relation between trends in late middle age mortality and trends in old age mortality – Is there evidence for mortality selection? Journal of Epidemiology and Community Health. 2005;59(9):775–781. doi: 10.1136/jech.2004.028407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde LB, Watkins WS, Bamshad MJ, Dixon ME, Ricker CE, Seielstad MT, Batzer MA. The distribution of human genetic diversity: A comparison of mitochondrial, autosomal, and Y-chromosome data. American Journal of Human Genetics. 2000;66:979–988. doi: 10.1086/302825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Evelyn, Hauser Philip. Differential mortality in the United States: a study in socioeconomic epidemiology. Harvard University Press; Cambridge, MA: 1973. [Google Scholar]
- Lutz W, Goujon A, K.C. S, Sanderson W. [Retrieved December 2009];Reconstruction of population by age, sex and level of educational attainment of 120 countries for 1970-2000. Vienna Yearbook of Population Research. 2007 :193–235. from http://www.iiasa.ac.at/Research/POP/edu07/index.html?sb=12.
- Manton Kenneth G., Vaupel James W. Survival after the age of 80 in the United States, Sweden, France, England, and Japan. New England Journal of Medicine. 1995;333(18):1232–5. doi: 10.1056/NEJM199511023331824. [DOI] [PubMed] [Google Scholar]
- Mehta Neil. University of Michigan; 2010. Secular declines in the association between obesity and mortality among middle-aged U.S. adults: 1948-2006. Manuscript. [Google Scholar]
- Moise P, Jacobzone S. OECD Health Working Papers. OECD Publishing; 2002. OECD study of cross-national differences in the treatment, costs and outcomes of ischaemic heart disease. No. 3. [Google Scholar]
- Moise Pierre. A Disease-Based Comparison of Health Systems: What is Best and at What Cost? Organisation for Economic Co-operation and Development (OECD); Paris: 2003. The heart of the health care system: Summary of the ischaemic heart disease part of the OECD Ageing-Related Diseases Study; pp. 27–52. [Google Scholar]
- Moon L, Moïse P, Jacobzone S. OECD Health Working Papers. OECD Publishing; 2003. Stroke care in OECD countries: A comparison of treatment, costs and outcomes in 17 countries. No. 5. [Google Scholar]
- Moon Lynelle. A Disease-Based Comparison of Health Systems: What is Best and at What Cost? Organisation for Economic Co-operation and Development (OECD); Paris: 2003. Stroke treatment and care: A comparison of approaches in OECD countries; pp. 53–76. [Google Scholar]
- Nolte Ellen, McKee C. Martin. Measuring the health of nations: Updating an earlier analysis. Health Affairs. 2008;27(1):58–71. doi: 10.1377/hlthaff.27.1.58. [DOI] [PubMed] [Google Scholar]
- O’Neill June E., O’Neill Dave M. Health status, health care and inequality: Canada vs. the US. Forum for Health Economics and Policy. 2007;10(1):1–43. [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, et al. Mortality from Smoking in Developed Countries, 1950-2000: Indirect Estimates from National Vital Statistics. Oxford University Press; New York: 1994. [Google Scholar]
- Pilote Louise, Saynina Olga, Lavoie Frederic, McClellan Mark. Cardiac procedure use and outcomes in elderly patients with acute myocardial infarction in the United States and Quebec, Canada, 1988 to 1994. Medical Care. 2003;41(7):813–822. doi: 10.1097/00005650-200307000-00005. [DOI] [PubMed] [Google Scholar]
- Preston SH, Elo I, Hill M, Rosenwaike I. The Demography of African Americans, 1930-1990. Kluwer Academic Publishers; Dordrecht, Netherlands: 2003. [Google Scholar]
- Preston Samuel H., Wang Haidong. Sex mortality differences in the United States: The role of cohort smoking patterns. Demography. 2006;43(4):631–646. doi: 10.1353/dem.2006.0037. [DOI] [PubMed] [Google Scholar]
- Preston Samuel H., Ho Jessica. National Bureau of Economic Research Working Paper. Jul, 2009. Low life expectancy in the United States: Is the health care system at fault? Number 15213. 2009. [Google Scholar]; Cohen Barney, Crimmins Eileen, Preston Samuel., editors. Divergent Trends in Life Expectancy at Older Ages. National Academy Press; Washington, D.C.: Forthcoming in. [Google Scholar]
- Preston Samuel H., Glei Dana, Wilmoth John. Contribution of smoking to international differences in life expectancy. In: Cohen Barney, Crimmins Eileen, Preston Samuel., editors. Divergent Trends in Life Expectancy at Older Ages. National Academy Press; Washington, D.C.: 2010. Forthcoming. [Google Scholar]
- Preston Samuel, Stokes Andrew. Is the high level of obesity in the United States related to its low life expectancy? Population Studies Center, University of Pennsylvania; 2010. Manuscript. [Google Scholar]
- Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant Milena, Allemani Claudia, Berrino Franco, Coleman Michel P. Breast carcinoma survival in Europe and the United States: A population-based study. Cancer. 2004;100(4):715–22. doi: 10.1002/cncr.20038. [DOI] [PubMed] [Google Scholar]
- Schoen Cathy, Osborn Robin, Huynh Phuong Trang, Doty Michelle, et al. Primary care and health system performance: Adults’ experiences in five countries. Health Affairs. 2004;23:w487–503. doi: 10.1377/hlthaff.w4.487. [DOI] [PubMed] [Google Scholar]
- Technological Change in Health Care (TECH) Research Network Technological change around the world: Evidence from heart attack care. Health Affairs. 2001;20(3):25–42. doi: 10.1377/hlthaff.20.3.25. [DOI] [PubMed] [Google Scholar]
- United States National Center for Health Statistics . Deaths: Final Data for 2006. National Vital Statistics Reports. 14. Vol. 57. National Center for Health Statistics; Hyattsville, MD: 2009. [PubMed] [Google Scholar]
- Vaupel James W. The remarkable improvements in survival at older ages. Philosophical Transactions of the Royal Society of London: Series B – Biological Sciences. 1997;352(1363):1799–1804. doi: 10.1098/rstb.1997.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel James W. Max Planck Institute for Demographic Research; Rostock, Germany: 2010. The theory of heterogeneity: A concise primer. Manuscript. [Google Scholar]
- Vercelli M, Quaglia A, Casella C, Parodi S, et al. Relative survival in elderly cancer patients in Europe. European Journal of Cancer. 1998;34(14):2264–2270. doi: 10.1016/s0959-8049(98)00325-6. [DOI] [PubMed] [Google Scholar]
- Wilmoth J, Boe C, Barbieri M. Geographic differences in life expectancy at age 50 in the United States compared with other high-income countries. In: Cohen Barney, Crimmins Eileen, Preston Samuel., editors. Divergent Trends in Life Expectancy at Older Ages. National Academy Press; Washington, D.C.: 2010. Forthcoming. [Google Scholar]
- Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- World Health Organization [accessed June 16, 2010];The SuRF Report 2: Surveillance of chronic disease Risk Factors. 2005 from https://apps.who.int/infobase/surf2.start.html.
- World Health Organization [retrieved September 2009];Detailed data files of the WHO mortality database. 2008 from www.who.int/whosis/mort/download/en/index.htm.
- World Health Organization [retrieved July 2009];World Health Organization Statistical Information System. 2009