Abstract
Purpose
To investigate the mechanisms that mediate the release of ATP induced by cyclic mechanical stress (CMS) and the role of extracellular ATP in the mobilization of arachidonic acid (AA) and prostaglandin secretion.
Methods
Porcine TM cells (pTM) were subjected to CMS. Extracellular ATP was detected using a luciferin-luciferase assay in presence or absence of transport inhibitors and a lipid raft disrupter. ATP vesicles were visualized using quinacrine. The release of AA (AA 1–14C) was measured with and without ATP, ATP inhibitors, and phospholipase A and C inhibitors. Prostaglandin E2 (PGE2) and viability were measured using ELISA and lactate dehydrogenase assays, respectively.
Results
CMS induced ATP release that was inhibited by the vesicle inhibitors NEthylmaleimide (NEM) and monensin. Lipid rafts disruption increased significantly the extracellular ATP induced by CMS. CMS induced AA release (1–4 fold increase) and its metabolic product PGE2 (3.9 fold increase). The AA mobilization induced by CMS could be mimicked by addition of extracellular ATP and was partially inhibited by a P2 antagonist, by an ATP inhibitor, and by inhibitors of phospholipases A2 and C. Addition of PGE2 (10μM) to the media exerted cytoprotective effects against long-term CMS.
Conclusions
Extracellular release of ATP induced by CMS leads to the mobilization of AA from the plasma membrane. The IOP lowering effects of some AA derivatives, through the uveo-scleral but also through the conventional pathway, together with the increased production of PGE2 might contribute to the prevention of cell loss that may result from exposure to chronic CMS.
Keywords: trabecular meshwork, arachidonic acid, prostaglandins, glaucoma anterior segment
Introduction
The best characterized risk factor for Primary Open Angle Glaucoma (POAG) is elevated intraocular pressure (IOP)1, 2 that results from an increase in aqueous humor outflow resistance at the level of the conventional outflow pathway (trabecular meshwork [TM] and Schlemm's canal [SC])3. The mechanisms involved in homeostasis of normal outflow resistance, as well as those leading to abnormal levels of resistance in POAG, are still poorly understood.
The TM is constantly subjected to mechanical forces due to transient spikes of IOP associated with systole of the cardiac cycle, blinking and eye movement.4, 5 These changes in IOP result in cyclic stretching and relaxation of TM cells, and the resulting cyclic mechanical stress (CMS) has been hypothesized to induce cellular responses that may have a significant role in both the maintenance of normal levels of outflow resistance and the pathological alterations in glaucoma.6–9
One response to mechanical stress frequently observed in different cell types is a regulated release of ATP into the extracellular space. The specific mechanisms involved in this release of ATP have not been fully elucidated and appear to be cell-type dependent.10–13 The extracellular release of ATP in response to mechanical stress has been previously reported in TM cells.14 Similarly, increased hydrostatic pressure in bovine eye cups has been shown to induce an increase in extracellular ATP content of the vitreal compartment adjacent to the retina. The ATP levels correlated with the pressure and were transient at lower pressures but sustained at higher pressures.11 Increased concentrations of extracellular ATP have also been observed in the vitreous and anterior chamber in acute glaucoma.15
Extracellular ATP and the products generated by its digestion by ecto-ATPases are now recognized as important autocrine/paracrine signaling mediators that participate in the regulation of a broad range of cellular functions.16–19 Specific targets of extracellular ATP and other nucleotides include P2Y (G-coupled) and P2X (ion-channel) receptors. In addition, extracellular ATP can also generate adenosine, which is an agonist of the P1 receptor family.20, 21
A potentially important response elicited by extracellular ATP signaling in several cell types is the mobilization of arachidonic acid (AA) from the plasma membrane through the activation of phospholypases.22–24 The regulation of AA mobilization in TM cells could be particularly important in the physiology of the outflow pathway because AA can be metabolized by cyclooxygenases, lipoxygenases, and cytochrome P450 monooxygenase enzymes to an extensive array of biologically active products, including leukotrienes, thromboxanes, prostaglandins (PG) and endocannabinoids, 25–27 some of which have demonstrated IOP lowering effects.28–32 Importantly, AA is also the rate-limiting substrate for prostaglandin H synthetase-2 (PGHS-2), also known as cyclooxygenase 2 (COX-2), for the production of PGs.33 TM cells have been demonstrated to be capable of converting AA in a variety of biologically active products including leukotrienes, hydroxyeicosatetraenoic acids, and PGE2. In addition, the biosynthesis of these products has been shown to be partially inhibited by dexamethasone.34, 35 Prostaglandins have been recently shown to exert their IOP lowering effects by increasing aqueous humor outflow not only through the uveo-scleral pathway, but through the conventional pathway as well.36
Currently there is little information about the specific mechanisms by which CMS mediates the extracellular release of ATP in TM cells and any possible relationship to the metabolism of AA and its derivatives. Therefore, we investigated the routes for extracellular release of ATP mediated by CMS in TM cells, the potential role of extracellular ATP signaling in the modulation of AA mobilization from the plasma membrane, and whether the mobilization of AA results in increased production of PGE2. In addition, we evaluated the effects of PGE2 on cell viability.
Methods
1.Reagents
Monensin, N-Ethylmaleimide (NEM), Orthovanadate, Piceatannol, Methyl-β-cyclodextrin (MβCD), Quinacrine, Suramin, Adenosine 5' triphosphate disodium salt (ATP), Apyrase, bromoenol lactose (BEL), O-Tricyclo[5.2.1.02,6]dec-9-yl dithiocarbonate potassium salt (D609), Methyl arachidonyl fluorophosphonate (MAFP), Bromophenacyl bromide (BPB), and Prostaglandin E2 (PGE2) were purchased from Sigma (St Louis, MO). Arachidonic acid radioactive labeled (AA 1–14C) was purchased from Moraveck Biochemicals, (Brea, CA).
2. Cell culture
Porcine TM (pTM) cells were obtained from fresh pig eyes.37 Cell cultures were maintained at 37°C in 5% CO2 in media (low glucose Dulbecco's Modified Eagle Medium with L-glutamine, 110mg/ml sodium pyruvate, 10% fetal bovine serum, 100μM non-essential amino-acids, 100 units/ml penicillin, 100μg/ml streptomicyn sulfate and 0.25μg/ml amphotericin B). All the reagents were obtained from Invitrogen Corporation (Carlsbad, CA).
3. Cyclic Mechanical Stress (CMS)
pTM cells on passage 3 or 4 were cultured on type I collagen-coated flexible silicone bottom plates (Flexcell, Hillsborough, NC). Culture medium was switched to serum-free DMEM 2 hours before stretching. Cells were subjected to cyclic mechanical stress (20% stretching, 1 cycle per second) for different periods of time (15 min to 18 hours), using the computer-controlled, vacuum-operated FX-3000 Flexercell Strain Unit (Flexcell, Hillsborough, NC). Control cells were cultured under the same conditions, but no mechanical force was applied.
4. Extracellular ATP measurements
For ATP measurements inhibitors were added 30 minutes before stretching (piceatannol 20μM; monensin 100μM; NEM 100μm; MβCD 10mM and orthovanadate 5mM). Extracellular ATP concentration was detected using a luciferin-luciferase bioluminescent assay (ATP Determination kit, Invitrogen, Eugene, OR). Bioluminescence was measured by a luminometer (Turner Designs, TD-20/20, Sunnyvalley, CA).
5. Intracellular ATP staining
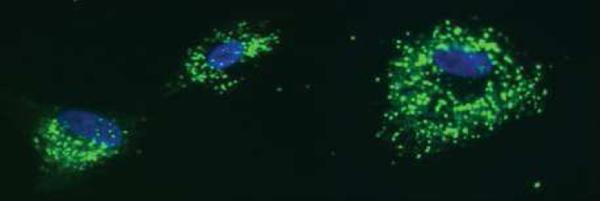
For visualization of ATP vesicles pTM cells were culture on cover slides and incubated for 20 minutes with the fluorescent dye quinacrine (25μM). ATP vesicles were visualized using a fluorescent microscope (Zeiss X-cite series 120).
7. Arachidonic Acid (AA) measurements
Confluent pTM cultures were labeled with radioactive AA (0.05μC/ml or 5.7μg) for 18 to 20 hours in serum free media. Cells were then washed 3 times (0.2% BSA in PBS) and kept in serum free DMEM for 2 hours before any treatment. Inhibitors were added 30 minutes before CMS (BEL 10μM; D609 20μM; MAFP 5μM; BPB 20μM; Suramin 10μM; and Apyrase 5U/ml). ATP (100μM) was added to complete media. Supernatants were collected at different time points after treatments, centrifugated, and loaded on filter paper. Radioactivity was estimated after 48 hours exposure in a Phosphor screen cassette (Molecular Dynamics, Sunnyvale, CA). Measurements were made using the Personal Molecular Imager FX (Bio-Rad, Hercules, CA) and analyzed using Quantity One software (Bio-Rad, Hercules, CA).
8. Prostaglandin E2 (PGE2) Assay
Prostaglandin E2 was measured using Parameter™ PGE2, a competitive binding ELISA assay (R&D systems, Minneapolis, MN) following manufacturer's instructions.
9. Cell viability assay
To evaluate the effects of PGE2 on changes in cell viability induced by CMS, cells were treated with 10μM PGE2 or vehicle 30 minutes before stretching. Cell viability was assayed after 18 hours of CMS by measuring the lactate dehydrogenase released to the culture media as a result of plasma membrane damage using the Cito Tox 96® Non-Radioactive Cytotoxicity assay (Promega, Madison, WI) following manufacturer's instructions.
10. Statistical analysis
Significance was analyzed using non-paired Student's t- test, a probability less than 5% was considered statistically significant.
Results
CMS induced ATP release through vesicles
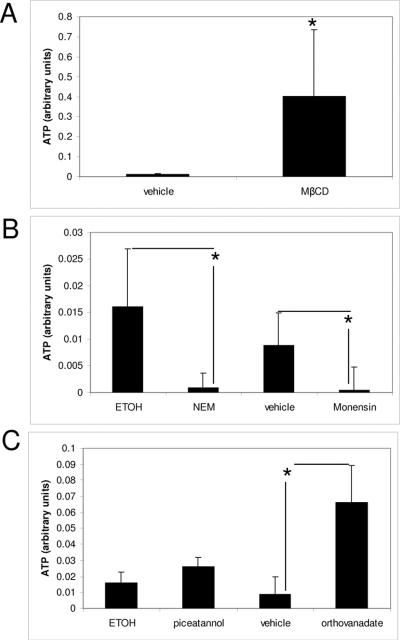
In order to elucidate the mechanisms of ATP transport and release during CMS, several inhibitors were used. MβCD, a disrupter of lipid raft, increased significantly the amount of extracellular ATP. Comparison of extracellular ATP levels between cells subjected to CMS with and without MβCD showed a 98% increase in cells treated with MβCD (Fig 1A). The vesicle inhibitors NEM and monensin clearly reduce the ATP induced by cyclic mechanical stress (Fig 1B). The ABC cassette inhibitor, orthovanadate and the ATP synthase inhibitor, piceatannol, did not reduce the amount of extracellular ATP; furthermore they increased it (Fig 1C). We also evaluated extracellular ATP synthesis (ADP + inorganic P) after incubation with and without Piceatannol (cell surface ATP synthase inhibitor). Extracellular ATP highly increased after 10 minutes incubation and then gradually declined. Cells treated with piceatannol showed 85% reduction in ATP synthesis (data not showed). ATP vesicles were visualized with quinacrine, a fluorescent dye used for detecting releasable ATP stores (Fig. 2).
Figure 1.
Effects of transport inhibitors in the release of extracellular ATP induced by CMS.
Lipid rafts disruption increased the extracellular ATP induced by CMS (1A). Vesicle inhibitors NEM (vehicle ethanol) and monensin (vehicle water) significantly reduced ATP induced by CMS (1B). The ABC cassette inhibitor, orthovanadate (vehicle water) and the ATP synthase inhibitor, piceatannol, (vehicle ethanol) increased extracellular ATP (Fig 1C). N=4 (*) p-value ≤ 0.05
Figure 2.
ATP vesicles visualized with the fluorescent dye quinacrine
CMS induced release of AA is mediated by ATP
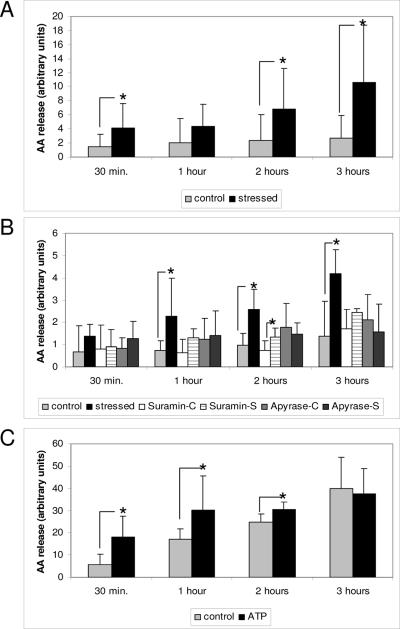
To elucidate the role of ATP as a signal molecule in CMS, we used arachidonic acid label with C14. We first showed that CMS induced AA released into the extracellular medium compared to controls (Fig 3A) and was partially inhibited by the P2 antagonist suramin and by the ATP inhibitor apyrase (Fig. 3B), To confirm the ATP role in the release of AA, ATP was added to labeled cells and this induced a rapid and significant release of AA, mainly in the first 2 hours (Fig 3C).
Figure 3.
CMS induced release of AA is mediated by ATP CMS induced AA released in samples subjected to CMS (S) compared to non stressed controls (C) (3A). P2 antagonist (suramin), and ATP inhibitor (apyrase) partially inhibited the AA release induced by CMS (3B), and ATP added to the media mimicked the release of AA (Fig 3C). N= 4 (*) p-value ≤ 0.05
Phospholipase A2 (PLA2) and Phospholipase C (PLC) mediated in the mobilization of AA by CMS
To clarify if PLA2 and/or PLC were involved in the CMS induced release of AA from the membrane; we used the following inhibitors of phospholipases: BPB for secretory PLA2; BEL for calcium independent PLA2; MAFP for calcium independent and dependent but no secretory PLA2, and D609 for PLC. Both phospholipases (A and C) participated in the CMS induced release of AA from the membrane. AA mobilization by CMS was partially prevented by the inhibition of the three PLA2 and by the PLC (Table 1).
Table 1.
AA mobilization by CMS was partially prevented by the inhibition of phospolipases A2 and C. Table 1 shows mean, standard deviation (s.d), and p-value of non stressed controls (C) and samples subjected to CMS (S), with and without inhibitors for PLA2 (BB, MAFF and BEL) and PLC (D609). N=3
| 30 min. | 1 hour | 2 hours | 3 hours | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | s.d | p-value | mean | s.d | p-value | mean | s.d | p-value | mean | s.d | p-value | |
| BB-C | 3.233 | 6.849 | 4.567 | 9.602 | 3.800 | 8.728 | 6.100 | 0.141 | ||||
| BB-S | 7.767 | 9.421 | 0.052 | 13.067 | 11.347 | 0.047 | 4.300 | 12.155 | 0.065 | 3.000 | 8.415 | 0.393 |
| MAFF-C | 3.033 | 5.036 | 7.433 | 6.529 | 7.333 | 3.765 | 6.050 | 9.018 | ||||
| MAFF-S | 0.433 | 3.020 | 0.362 | 4.033 | 4.136 | 0.282 | 1.800 | 10.482 | 0.316 | 11.200 | 11.902 | 0.120 |
| BEL-C | 4.567 | 4.821 | 5.833 | 11.632 | 8.867 | 3.818 | 8.667 | 5.196 | ||||
| BEL-S | 1.033 | 3.092 | 0.217 | 4.433 | 2.797 | 0.362 | 6.000 | 14.425 | 0.129 | 8.625 | 14.076 | 0.168 |
| D609-C | 0.400 | 2.281 | 2.500 | 4.842 | 8.775 | 0.010 | 10.500 | 7.202 | ||||
| D609-S | 0.475 | 0.950 | 0.431 | 6.025 | 4.465 | 0.081 | 12.325 | 7.670 | 0.057 | 14.033 | 1.050 | 0.061 |
| control | 1.275 | 6.485 | 0.933 | 6.357 | 9.450 | 11.255 | 7.633 | 12.632 | ||||
| stressed | 8.475 | 10.157 | 0.038 | 11.175 | 8.895 | 0.151 | 23.750 | 15.830 | 0.008 | 30.750 | 18.458 | 0.030 |
CMS increased Prostaglandin E2
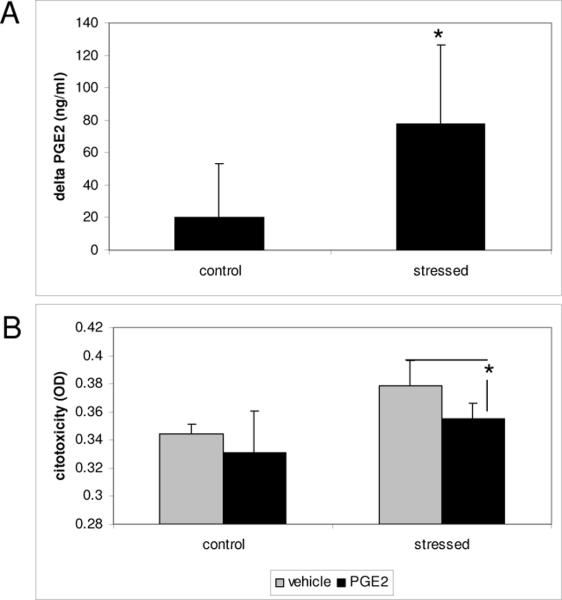
We investigated if the induction of AA by CMS also resulted in an increase of metabolic products, such as prostaglandins. PGE2 was measured by ELISA assay in cells subjected to CMS or no-stressed controls. Stressed cells showed an increase in PGE2 of 78 ± 48pg/ml, whereas in non-stressed cells this increase was 20.5 ± 33pg/ml after 3 hours (Fig 4A).
Figure 4.
PGE2 increased with CMS and conferred protection against mechanical stress. Stressed cells increase PGE2 after 3 hours of CMS when compare to non-stressed cells (4A). Cells treated with PGE2 showed a small but significantly increase in viability after 18 hours of CMS (4B). N=3 (*) p-value ≤ 0.05
Prostaglandin E2 protected TM cells against CMS
To examine whether PGE2 confers protection to CMS; we subjected pTM cells to cyclic mechanical stress for 18 hours with and without PGE2 and analyzed viability. Cells treated with PGE2 showed a small, but significant increase in viability (Fig 4B).
Discussion
The specific mechanisms that mediate ATP release in different cell types are not completely clear and may be cell-type dependent.10–12 Our results showed that inhibition of vesicle transport and exocytosis prevented most of the increase in extracellular ATP induced by CMS in TM cells, pointing to exocytosis of ATP-storing vesicles as the main mechanism involved in this response. Consistent with this concept, staining revealed the presence of a distinct pool of vesicles enriched in ATP in cultured pTM cells. A similar exocytic release of ATP is known to occur at synapses and in activated platelets, chromaffin cells, and mast cells.38 Exocytosis of ATP-storing vesicles are also known to contribute to the release of ATP induced by hypotonic stress in retinal pigment epithelium cells, osteoblasts, and hepatocytes.39–41 Inhibition of ABC cassette (orthovanadate) and ATP synthase (piceatannol), the other two mechanisms of ATP transport tested, did not contribute to the release of ATP induced by CMS. The increase in extracellular ATP induced by these inhibitors, in our model, are probably due to its other properties; orthovanadate is a strong inhibitor of some ecto-ATPases42 and ATP synthases participate in both synthesis and hydrolysis of ATP.43 Although potential contributions of other mechanisms to the ATP release induced by mechanical stress cannot be ruled out, our results strongly support the exocytic pathway as the main route for such ATP release.
Our results also showed that when lipid rafts were disrupted, the increase of detectable ATP in the cell culture media was much larger than that observed in cells with intact lipid rafts. This observation suggests that the ATP liberated in response to CMS may be released to lipid rafts. One important property attributed to lipid rafts is their ability to segregate receptors and other signaling components among different domains of the cell membrane. Many components of the machinery associated with G protein-coupled receptors have been found to localize in lipid rafts, which include P2Y and P1 receptors. Although P2X receptors are not coupled to G-proteins, there is evidence that at least some of them may also be present in lipid rafts.44
In addition, the enzymes that convert ATP into ADP, AMP, and adenosine (ecto-alkaline phosphatases, and ecto-5'-nucleotidases) are glycosylphosphatidylinositol-anchored proteins which are typically localized in lipid rafts.44, 45 Therefore, the preferential release of ATP into lipid rafts observed during CMS could serve to activate the purinergic and P1 receptors by concentrating ATP and its derivatives. In our model, ATP induced by CMS should reach concentrations large enough to activate some of these receptors, since the use of ATP and purinoreceptors inhibitors (apyrase and suramin) inhibited the release of AA induced by CMS. The effects of apirase and suramin on the release of AA strongly support the role of ATP release and subsequent activation of purinergic receptors in the mobilization of AA during CMS. In addition, administration of ATP in absence of CMS also resulted in increased AA mobilization. It is generally accepted that the mobilization of AA from the plasma membrane after purinergic receptor activation is regulated by PLA2. PLC is also known to be involved in this process either through activation of PLA2 or through PLA2 independent mechanisms.22, 46 Our results showed that inhibition of both PLC and PLA2 clearly prevented AA mobilization after CMS suggesting that, similar to what has been observed in other cell types, this response was mediated by these two phospholipases. Given the strong effects on AA release associated with inhibition of PLA2, our results also suggest that PLC may induce AA release through activation of PLA2 rather then through other mechanisms.
Trabecular meshwork cells have been demonstrated to transform AA in a variety of products including leukotrienes, hydroxyeicosatetraenoic acids, and PGE2.34, 35 Consistent with this observation, our results showed that the increased mobilization of AA induced by CMS in TM cells was indeed associated with a significant increase in the concentration of PGE2 in the culture medium. Several of the metabolic derivatives of AA, including prostaglandins and endocannabinoids, have been demonstrated to increase outflow facility.47 Although the IOP lowering effects of some of these molecules may be mediated through an increase in uveo-scleral outflow facility, PGs have also been observed to increase the outflow facility at the levels of the TM/SC pathway.48 Therefore, one expected effect of the mobilization of AA induced by CMS would be the increase in outflow facility mediated by AA derivatives such as PGE2. This possibility is consistent with the effects of purinergic agonists on trabecular outflow facility in perfused bovine ocular anterior segments.49
However, the effects of mechanical stress are likely to be complex and might also contribute to pathologic changes in the TM similar to those observed in other tissues such as blood vessels.50 In this regard, it is important to notice that some end-products of the AA metabolism are also known to have profibrotic properties and induce the synthesis of several collagen types, and fibronectin.51 The profibrotic and inflammatory actions of some of these AA derivatives is believed to contribute to the progression of several pathologic conditions including cardiac fibrosis, Alzheimer's disease, and Parkinson's disease.26, 52, 53 In contrast, a potential mechanism that could compensate for some of the potentially pathologic effects of CMS in the TM could involve cytoprotective effects of PGs.54 For example, it has recently been reported that, in addition to their effects on aqueous humor outflow, PGs protect TM cells against oxidative stress damage55 and our own data indicates that PGs could help prevent cell loss induced by chronic exposure to CMS.
In conclusion, our results demonstrated that the extracellular release of ATP induced by CMS in TM cells is mediated by exocytosis of ATP-enriched vesicles into lipid rafts. The resulting activation of purinergic receptors leads to mobilization of AA from the plasma membrane. The known IOP lowering effects of AA derivatives together with the release of PGE could exert protective effects by preventing TM cell loss that may result from chronic exposure to CMS.
Acknowledgments
This work was supported by NEI EY01894, NEI EY016228, NEI EY05722, and Research to Prevent Blindness.
References
- 1.Coleman AL. Glaucoma. Lancet. 1999;354:1803–1810. doi: 10.1016/S0140-6736(99)04240-3. [DOI] [PubMed] [Google Scholar]
- 2.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Survey of ophthalmology. 2008;53(Suppl1):S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Johnson M. What controls aqueous humour outflow resistance? Experimental eye research. 2006;82:545–557. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. Journal of glaucoma. 2004;13:421–438. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- 5.Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Archives of ophthalmology. 1969;82:637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez P, Epstein DL, Borras T. Genes upregulated in the human trabecular meshwork in response to elevated intraocular pressure. Investigative ophthalmology & visual science. 2000;41:352–361. [PubMed] [Google Scholar]
- 7.Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Investigative ophthalmology & visual science. 1998;39:1361–1371. [PubMed] [Google Scholar]
- 8.Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Investigative ophthalmology & visual science. 2005;46:2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- 9.Ramos RF, Stamer WD. Effects of cyclic intraocular pressure on conventional outflow facility. Investigative ophthalmology & visual science. 2008;49:275–281. doi: 10.1167/iovs.07-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto K, Shimizu N, Obi S, et al. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 11.Reigada D, Lu W, Zhang M, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience. 2008;157:396–404. doi: 10.1016/j.neuroscience.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. Journal of cardiovascular pharmacology. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Katnik C, Adams DJ. An ATP-sensitive potassium conductance in rabbit arterial endothelial cells. The Journal of physiology. 1995;485(Pt 3):595–606. doi: 10.1113/jphysiol.1995.sp020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow J, Liton PB, Luna C, Wong F, Gonzalez P. Effect of cellular senescence on the P2Y-receptor mediated calcium response in trabecular meshwork cells. Molecular vision. 2007;13:1926–1933. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Li A, Ge J, Reigada D, Laties AM, Mitchell CH. Acute increase of intraocular pressure releases ATP into the anterior chamber. Experimental eye research. 2007;85:637–643. doi: 10.1016/j.exer.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Rieg T, Vallon V. ATP and adenosine in the local regulation of water transport and homeostasis by the kidney. American journal of physiology. 2009;296:R419–427. doi: 10.1152/ajpregu.90784.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hovater MB, Olteanu D, Welty EA, Schwiebert EM. Purinergic signaling in the lumen of a normal nephron and in remodeled PKD encapsulated cysts. Purinergic signalling. 2008;4:109–124. doi: 10.1007/s11302-008-9102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Marcus DC. Purinergic signaling in the inner ear. Hearing research. 2008;235:1–7. doi: 10.1016/j.heares.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oury C, Toth-Zsamboki E, Vermylen J, Hoylaerts MF. The platelet ATP and ADP receptors. Current pharmaceutical design. 2006;12:859–875. doi: 10.2174/138161206776056029. [DOI] [PubMed] [Google Scholar]
- 20.Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G401–410. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 21.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balboa MA, Balsinde J. Oxidative stress and arachidonic acid mobilization. Biochimica et biophysica acta. 2006;1761:385–391. doi: 10.1016/j.bbalip.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe-Tomita Y, Suzuki A, Shinoda J, Oiso Y, Kozawa O. Arachidonic acid release induced by extracellular ATP in osteoblasts: role of phospholipase D. Prostaglandins, leukotrienes, and essential fatty acids. 1997;57:335–339. doi: 10.1016/s0952-3278(97)90553-6. [DOI] [PubMed] [Google Scholar]
- 24.Chen WC, Chen CC. ATP-induced arachidonic acid release in cultured astrocytes is mediated by Gi protein coupled P2Y1 and P2Y2 receptors. Glia. 1998;22:360–370. doi: 10.1002/(sici)1098-1136(199804)22:4<360::aid-glia5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Meves H. Arachidonic acid and ion channels: an update. British journal of pharmacology. 2008;155:4–16. doi: 10.1038/bjp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levick SP, Loch DC, Taylor SM, Janicki JS. Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. J Immunol. 2007;178:641–646. doi: 10.4049/jimmunol.178.2.641. [DOI] [PubMed] [Google Scholar]
- 27.Malcher-Lopes R, Franco A, Tasker JG. Glucocorticoids shift arachidonic acid metabolism toward endocannabinoid synthesis: a non-genomic anti-inflammatory switch. European journal of pharmacology. 2008;583:322–339. doi: 10.1016/j.ejphar.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njie YF, Qiao Z, Xiao Z, Wang W, Song ZH. N-arachidonylethanolamide-induced increase in aqueous humor outflow facility. Investigative ophthalmology & visual science. 2008;49:4528–4534. doi: 10.1167/iovs.07-1537. [DOI] [PubMed] [Google Scholar]
- 29.Denis P, Lafuma A, Khoshnood B, Mimaud V, Berdeaux G. A meta-analysis of topical prostaglandin analogues intra-ocular pressure lowering in glaucoma therapy. Current medical research and opinion. 2007;23:601–608. doi: 10.1185/030079907X178720. [DOI] [PubMed] [Google Scholar]
- 30.Juntunen J, Huuskonen J, Laine K, et al. Anandamide prodrugs. 1. Water-soluble phosphate esters of arachidonylethanolamide and R-methanandamide. Eur J Pharm Sci. 2003;19:37–43. doi: 10.1016/s0928-0987(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 31.Stumpff F, Boxberger M, Krauss A, et al. Stimulation of cannabinoid (CB1) and prostanoid (EP2) receptors opens BKCa channels and relaxes ocular trabecular meshwork. Experimental eye research. 2005;80:697–708. doi: 10.1016/j.exer.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Njie YF, He F, Qiao Z, Song ZH. Aqueous humor outflow effects of 2-arachidonylglycerol. Experimental eye research. 2008;87:106–114. doi: 10.1016/j.exer.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Vogt W, Schmidt G. Formation of anaphylatoxin in rat plasma, a specific enzymic process. Biochemical pharmacology. 1966;15:905–914. doi: 10.1016/0006-2952(66)90167-5. [DOI] [PubMed] [Google Scholar]
- 34.Weinreb RN, Polansky JR, Alvarado JA, Mitchell MD. Arachidonic acid metabolism in human trabecular meshwork cells. Investigative ophthalmology & visual science. 1988;29:1708–1712. [PubMed] [Google Scholar]
- 35.Polansky JR, Kurtz RM, Alvarado JA, Weinreb RN, Mitchell MD. Eicosanoid production and glucocorticoid regulatory mechanisms in cultured human trabecular meshwork cells. Progress in clinical and biological research. 1989;312:113–138. [PubMed] [Google Scholar]
- 36.Bahler CK, Howell KG, Hann CR, Fautsch MP, Johnson DH. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. American journal of ophthalmology. 2008;145:114–119. doi: 10.1016/j.ajo.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamer WD, Seftor RE, Snyder RW, Regan JW. Cultured human trabecular meshwork cells express aquaporin-1 water channels. Current eye research. 1995;14:1095–1100. doi: 10.3109/02713689508995815. [DOI] [PubMed] [Google Scholar]
- 38.Burnstock G. Historical review: ATP as a neurotransmitter. Trends in pharmacological sciences. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Romanello M, Codognotto A, Bicego M, Pines A, Tell G, D'Andrea P. Autocrine/paracrine stimulation of purinergic receptors in osteoblasts: contribution of vesicular ATP release. Biochemical and biophysical research communications. 2005;331:1429–1438. doi: 10.1016/j.bbrc.2005.03.246. [DOI] [PubMed] [Google Scholar]
- 40.Reigada D, Mitchell CH. Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol. 2005;288:C132–140. doi: 10.1152/ajpcell.00201.2004. [DOI] [PubMed] [Google Scholar]
- 41.Fitz JG. Regulation of cellular ATP release. Transactions of the American Clinical and Climatological Association. 2007;118:199–208. [PMC free article] [PubMed] [Google Scholar]
- 42.Del Carmen Camberos M, Cresto JC. Insulin-degrading enzyme hydrolyzes ATP. Experimental biology and medicine (Maywood, NJ. 2007;232:281–292. [PubMed] [Google Scholar]
- 43.Pedersen PL, Ko YH, Hong S. ATP synthases in the year 2000: defining the different levels of mechanism and getting a grip on each. Journal of bioenergetics and biomembranes. 2000;32:423–432. doi: 10.1023/a:1005652605340. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Marcos M, Dehaye JP, Marino A. Membrane compartments and purinergic signalling: the role of plasma membrane microdomains in the modulation of P2XR-mediated signalling. The FEBS journal. 2009;276:330–340. doi: 10.1111/j.1742-4658.2008.06794.x. [DOI] [PubMed] [Google Scholar]
- 45.Bannas P, Adriouch S, Kahl S, Braasch F, Haag F, Koch-Nolte F. Activity and specificity of toxin-related mouse T cell ecto-ADP-ribosyltransferase ART2.2 depends on its association with lipid rafts. Blood. 2005;105:3663–3670. doi: 10.1182/blood-2004-08-3325. [DOI] [PubMed] [Google Scholar]
- 46.Boarder MR, Weisman GA, Turner JT, Wilkinson GF. G protein-coupled P2 purinoceptors: from molecular biology to functional responses. Trends in pharmacological sciences. 1995;16:133–139. doi: 10.1016/s0165-6147(00)89001-x. [DOI] [PubMed] [Google Scholar]
- 47.Green K, Kim K. Interaction of adrenergic antagonists with prostaglandin E2 and tetrahydrocannabinol in the eye. Investigative ophthalmology. 1976;15:102–111. [PubMed] [Google Scholar]
- 48.Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Survey of ophthalmology. 2008;53(Suppl1):S107–120. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soto D, Pintor J, Peral A, Gual A, Gasull X. Effects of dinucleoside polyphosphates on trabecular meshwork cells and aqueous humor outflow facility. The Journal of pharmacology and experimental therapeutics. 2005;314:1042–1051. doi: 10.1124/jpet.105.085274. [DOI] [PubMed] [Google Scholar]
- 50.Hayoz D, Mazzolai L. Endothelial function, mechanical stress and atherosclerosis. Advances in cardiology. 2007;44:62–75. doi: 10.1159/000096721. [DOI] [PubMed] [Google Scholar]
- 51.Priante G, Musacchio E, Valvason C, Baggio B. EPA and DHA suppress AngII-and arachidonic acid-induced expression of profibrotic genes in human mesangial cells. Journal of nephrology. 2009;22:137–143. [PubMed] [Google Scholar]
- 52.Yagami T. Cerebral arachidonate cascade in dementia: Alzheimer's disease and vascular dementia. Current neuropharmacology. 2006;4:87–100. doi: 10.2174/157015906775203011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Yamada N, Maruyama W, Osawa T. Formation of dopamine adducts derived from brain polyunsaturated fatty acids: mechanism for Parkinson disease. The Journal of biological chemistry. 2008;283:34887–34895. doi: 10.1074/jbc.M805682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scher JU, Pillinger MH. The Anti-Inflammatory Effects of Prostaglandins. J Investig Med. 2009 doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 55.Yu AL, Fuchshofer R, Kampik A, Welge-Lussen U. Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Investigative ophthalmology & visual science. 2008;49:4872–4880. doi: 10.1167/iovs.07-0984. [DOI] [PubMed] [Google Scholar]