Abstract
Rationale
Exercise training confers sustainable protection against ischemia-reperfusion injury in animal models and has been associated with improved survival following a heart attack in humans. It is still unclear how exercise protects the heart, but it is apparent that endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) play a role.
Objective
To determine the role of β3-adrenergic receptors (β3-ARs), eNOS activation, and NO metabolites (nitrite and nitrosothiols) in the sustained cardioprotective effects of exercise
Methods and Results
Here we show that voluntary exercise reduces myocardial injury in mice following a 4-week training period and that these protective effects can be sustained for at least 1 week following the cessation of the training. The sustained cardioprotective effects of exercise are mediated by alterations in the phosphorylation status of eNOS (increase in serine 1177 and decrease in threonine 495) leading to an increase in NO generation and storage of NO metabolites (nitrite and nitrosothiols) in the heart. Further evidence revealed that the alterations in eNOS phosphorylation status and NO generation were mediated by β3-AR stimulation and that in response to exercise a deficiency of β3-ARs leads to an exacerbation of myocardial infarction following ischemia-reperfusion injury.
Conclusions
Our findings clearly demonstrate that exercise protects the heart against myocardial ischemia-reperfusion injury by stimulation of β3-ARs and increased cardiac storage of nitric oxide metabolites (i.e., nitrite and nitrosothiols).
Keywords: β3-adrenergic receptor, nitric oxide, cardioprotection, exercise, nitrite, nitrosothiol
Exercise training reduces many risk factors related to cardiovascular disease1. Exercise also consistently provides sustainable protection against myocardial infarction in animal models2,3 and is associated with improved survival following ischemic insults in humans.4 The acute cardioprotective effects of exercise have been attributed to an increase in a number of classical preconditioning molecules, such as catalase, heat shock proteins (HSPs), and ATP-sensitive potassium channels.3 Interestingly, the cardioprotective effects of exercise are not confined to the period of exercise, as it has been reported that protection is sustained against experimental myocardial ischemia-reperfusion (MI/R) injury for as long as 9 days after the cessation of exercise training.2 The mechanism(s) responsible for this sustained protection are currently not known, but it is known that HSPs and catalase do not play a role since their upregulation wanes by 7–9 days, suggesting that some other cardioprotective molecule(s) are responsible for this sustained protection.2
Previous studies have suggested a role for endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) in exercise-mediated cardioprotection, as it has been reported that plasma levels of the NO metabolites, nitrite and nitrosothiols, increase during exercise in both rodents and humans.3,5 Nitrite represents a critically important storage reservoir of NO in blood and tissues that can readily be reduced to NO and nitrosothiols during ischemia or hypoxia.6 The circulating levels of both metabolites directly regulate their tissue storage7 and increasing the cardiac levels of both nitrite and nitrosothiols is an effective cardioprotective strategy.8,9 Based on this evidence, one can speculate that increasing the levels of NO metabolites during exercise may contribute to its cardioprotective effects. However, it is currently not known if exercise can augment the cardiac levels of NO metabolites.
An additional question that remains to be fully answered relates to the molecular mechanisms that lead to the activation of eNOS during exercise. Recently, the β3-adrenergic receptor (β3-AR) has emerged as a potential target for the treatment of cardiovascular diseases including hypertension, acute MI, and heart failure.10 This is partially related to the evidence indicating that its stimulation increases eNOS activity and NO bioavailability.11 During exercise, there is an increase in β3-AR stimulus (i.e. catecholamines)12, making the β3-AR a possible source for eNOS activation. However, the role that β3-ARs play in mediating the cardioprotective effects of exercise is currently unknown.
To address these issues, we examined the protective effects of voluntary exericise (VE) training in an established in vivo mouse model of MI/R injury. Specifically, we investigated if NO metabolites formed during exercise were stored in the heart and if they contributed to the sustained cardioprotective effects of exercise. Additionally, we investigated the role that β3-ARs play in mediating the cardioprotective effects of exercise.
Materials and Methods
Animals
Male C57BL6/J mice (Jackson Labs, Bar Harbor, ME; 8–10 weeks of age) were utilized. eNOS deficient mice (eNOS−/−; 8–10 weeks of age) on a C57BL6/J background13 and β3-adrenergic receptor deficient (β3-AR−/−; 8–10 weeks of age) mice14, as well as littermate controls were also utilized. The β3-AR−/− mice were developed on a FVB background and backcrossed 9 generations to a C57BL6/J background. All experimental procedures were approved by the Institute for Animal Care and Use Committee at Emory University School of Medicine and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86-23, Revised 1996) and with federal and state regulations.
Subjects and study procedures
All procedures with human subjects were approved by the Institutional Review Board (Human Research Committee) of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation. A total of 23 healthy young (aged 18–31 years) men were studied: 16 non-exercise trained individuals and 7 endurance athletes. The non-exercise trained subjects had performed no regular exercise for ≥2 years, whereas the trained endurance athletes performed >3 sessions/week of vigorous aerobic-endurance exercise for ≥2 years.
Voluntary Exercise Protocol
Mice were placed in custom designed cages fitted with running wheels (Mini Mitter, Bend, OR) for a period up to 4 weeks. Running distances were monitored daily. After the exercise-training period, the running wheel was removed from the cage and the mice were allowed to rest for a 24-hour, 1-week, or 4-week period before further experimentation was conducted.
Myocardial Ischemia-Reperfusion (I/R) Protocol and Myocardial Injury Assessment
Surgical ligation of the left coronary artery (LCA), myocardial infarct size determination, and Troponin-I measurements were performed similar to methods described previously.15
Western blot analysis
Western blot analysis was performed as described previously.15
Analysis of Nitrite, Nitrate, and Nitrosothiols
Nitrite and nitrate concentrations were quantified by ion chromatography (ENO20 Analyzer, Eicom). Tissue nitrosothiol compounds were quantified using group specific reductive denitrosation by iodine-iodide with subsequent detection of the NO liberated by gas-phase chemiluminescence. All NO analysis procedures have been previously described in detail.8
Analysis of catecholamine levels
Catecholamines were measured in blood samples taken from mice using the Bi-CAT Elisa (ALPCO, Salem, NH) according the manufactor’s instructions.
Statistical Analysis
All the data in this study are expressed as mean ± standard error (SEM). Differences in data between the groups were compared using Prism 4 (GraphPad Software, Inc) with Student’s paired 2-tailed t-test, one-way analysis of variance (ANOVA), or two-way ANOVA (comparison of results from experiments using eNOS−/− and β3-AR−/− mice). For the one-way and two-way ANOVA, if a significant variance was found, the Tukey or Bonferroni test was used as the post hoc analysis. A p value less than 0.05 was considered statistically significant.
Results
VE training reduces infarct size following MI/R injury
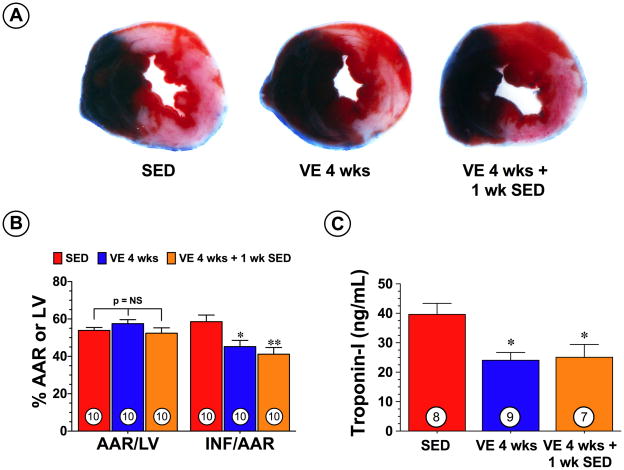
To determine if VE training attenuated myocardial injury following myocardial I/R, groups of mice were housed in cages fitted with running wheels and allowed to exercise voluntarily for 4 weeks (VE 4 wks). The intensity of the exercise training remained constant throughout the 4-week training period with an average of 7.4±0.2 km/day (Online Figure I). Control mice (sedentary, SED) were housed in cages without running wheels for the same durations as the VE mice. At the end of the 4-week training period, the mice were subjected to 45 min of left coronary artery occlusion followed by 24 hr of reperfusion at which time myocardial injury was assessed by determining infarct size (INF) and measuring circulating levels of troponin-I. The VE mice displayed a 23% reduction (p<0.05 vs. SED) in INF relative to the area-at-risk (AAR) (58.6±3.6% for SED vs. 45.2±3.3% for VE 4 wks) and a 39% (39.6±3.7 ng/mL for SED vs. 24.0±2.7 ng/mL for VE 4 wks) reduction in circulating troponin-I levels (Figure 1B–C).
Figure 1.
Voluntary exercise (VE) training reduced the extent of injury in mice following myocardial ischemia and reperfusion. Mice were housed in cages fitted with running wheels and allowed to exercise voluntarily for 4 weeks. Control mice (sedentary, SED) were housed in cages without running wheels for the same durations as the VE mice. Groups of mice were subjected to 45 min of left coronary artery occlusion followed by 24 hr of reperfusion either immediately after the training period (VE 4 wks) or 1 week after the training period (VE 4 wks + 1 wk SED). (A) Representative mid-ventricular photomicrographs of hearts from each of the three groups demonstrate infarct size reduction in VE groups of mice. (B) Myocardial area-at-risk (AAR) as a percentage (%) of total left ventricle (LV) and infarct size (INF) as a percentage of area-at-risk (AAR) and (C) circulating Troponin-I levels (ng/mL) were evaluated at 24 hours of reperfusion. Values are means ± S.E.M. Numbers inside the bars are the number of animals investigated. *p<0.05 and **p<0.01 vs. SED.
We next evaluated if the cardioprotective effects of VE training could be maintained after the mice stopped exercising. For these experiments, mice were allowed to exercise for 4 weeks (average running distance of 7.3±0.2 km/day, Online Figure I). At the end of the training period, the mice were removed from the cages for a period of 1 week (VE 4 wks + 1 wk SED) and then subjected to 45 min of left coronary artery occlusion followed by 24 hr of reperfusion. The VE 4 wks + 1 wk SED mice displayed a 30% reduction (p<0.01 vs. SED; Figure 1B) in INF relative to the AAR and a 37% reduction (p<0.05 vs. SED; Figure 1C) in circulating troponin-I levels. This demonstrates that VE training provides significant cardioprotection against I/R injury for at least 1 week following the cessation of the training.
VE training increases CuZnSOD expression and alters the phosphorylation status of AMPK
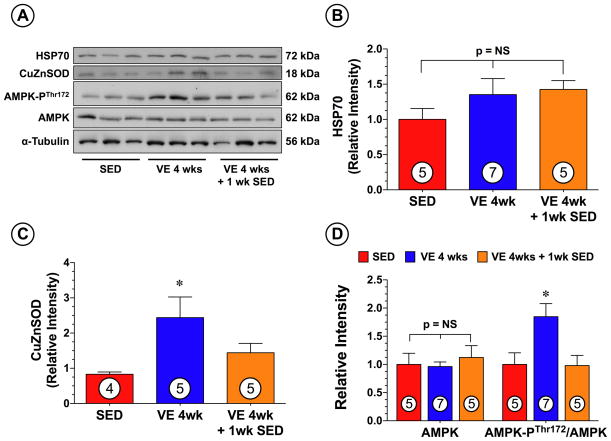
We next investigated if VE training altered the expression of several cardioprotective signaling molecules purported to play a role in mediating exercise-induced cardioprotection. Western blot analysis (Figure 2) revealed that the expression of HSP70 remained unchanged in the hearts of mice following VE training when compared to SED mice. However, a significant increase in CuZnSOD was observed in the hearts of the VE 4 wk group (p<0.05 vs. SED), but not in the hearts of the VE 4 wk + 1 wk SED group. AMP-activated protein kinase (AMPK) is another cardioprotective signaling molecule that is activated by exercise.16 Western blot analysis revealed (Figure 2) that VE significantly increased the phosphorylation of AMPK at Thr172 in the hearts of the VE 4 wk group (p<0.05 vs. SED), but not in the hearts of the VE 4 wk + 1 wk SED group.
Figure 2.
VE training increased the cardiac expression of CuZnSOD and altered the phosphorylation status of AMPK as compared to sedentary (SED) controls. (A) Representative immunoblots and densitometric analysis of cardiac (B) HSP70, (C) CuZnSOD, (D) total AMPK and phosphorylated AMPK at threonine residue 172 (AMPK-PThr172) following 4 weeks of VE training. Values are means ± S.E.M. *p<0.05 vs. SED.
VE training alters the phosphorylation status of eNOS and increases circulating and cardiac NO metabolite Levels
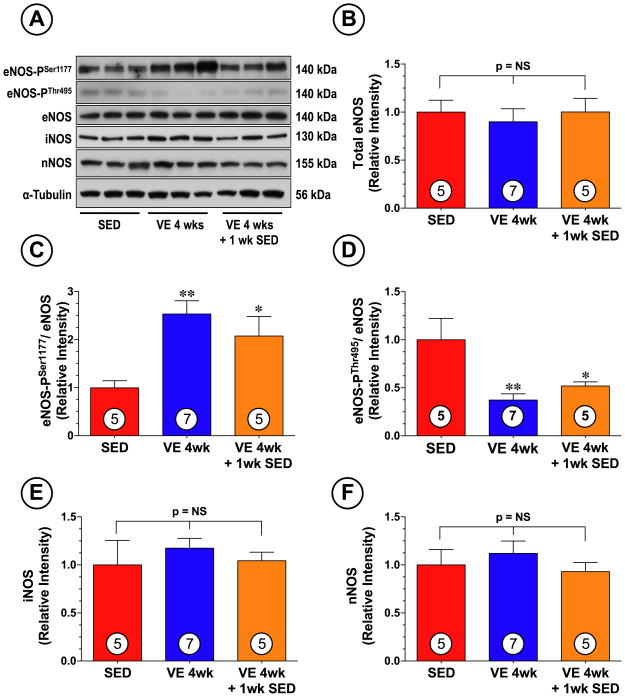
We next studied the effects of VE on eNOS expression and phosphorylation status. For these experiments, we exploited phosphorylation site-specific antibodies to probe immunoblots prepared from collected heart tissue. VE training promoted a significant increase (Figure 3) in the phosphorylation of eNOS at serine residue 1177 (eNOS-PSer1177; phosphorylation here increases enzyme activity; p<0.01 vs. SED). VE training also promoted the dephosphorylation of eNOS at threonine residue 497 (eNOS-PThr495; phosphorylation here inhibits the enzyme activity, p<0.05 vs. SED). Importantly, the alterations in the phosphorylation status of eNOS (especially eNOS-PSer1177) were still present 1 week after the end of the 4 week VE training period (p<0.05 vs. SED). No changes in the expression of total eNOS were noted in either of the VE groups. Additionally, the expression of iNOS and nNOS remained unchanged in response to VE training (Figure 3A and 3E–F).
Figure 3.
VE training altered the phosphorylation status of cardiac eNOS. (A) Representative immunoblots and densitometric analysis of (B) total eNOS, (C) phosphorylated eNOS at serine residue 1177 (eNOS-PSer1177), (D) phosphorylated eNOS at threonine residue 495 (eNOS-PThr495), (E) iNOS, and (D) nNOS following 4 weeks of VE training. Values are means ± S.E.M. *p<0.05 and **p<0.01 vs. SED.
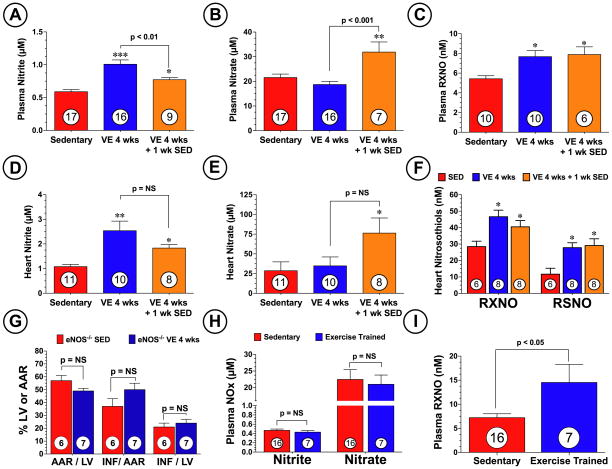
Exercise has been associated with increasing plasma nitrite levels5,16,17, but it is not known if exercise increases nitrite levels in the heart. Since we have previously demonstrated that increasing nitrite levels in the heart results in cardioprotection8, we evaluated the effects of exercise training on the levels of nitrite, nitrate, and nitrosothiols (RXNO and RSNO) in the plasma and heart. As shown in Figure 4A–F, a significant increase in the levels of nitrite and nitrosothiols were observed in the plasma and hearts of the VE 4 wks group when compared to the SED group. Importantly, these elevations were still present 1 week after the end of the training period. Interestingly, plasma and heart nitrate levels were only significantly increased 1 week after the end of the training period (p<0.05 vs. SED). These results suggest that NO metabolites may be responsible for the sustained cardioprotective effects of VE training, given that nitrite, nitrate, and nitrosothiols have all been shown to provide cardioprotection during myocardial ischemia by increasing NO levels and signaling.8,9,18
Figure 4.
VE training increased the circulating and myocardial levels of NO metabolites. Levels of nitrite, nitrate, and nitrosothiols (RXNO and RSNO) were measured in the (A–C) plasma and (D–F) heart following 4 weeks of VE training. (G) Mice deficient in eNOS (eNOS−/−) were subjected to 4 weeks of VE training and then to 45 min of left coronary artery occlusion followed by reperfusion. Myocardial AAR/LV, INF/AAR, and INF/LV were then evaluated at 24 hours of reperfusion. Steady-state levels of plasma (H) nitrite, nitrate, and (I) nitrosothiol levels in individuals who exercised for at least 45 minutes a day, 3 times a week for at least 2 years (trained endurance athletes) and from non-trained individuals. Values are means ± S.E.M. *p<0.05, **p<0.01, and ***p<0.001 vs. SED.
We next investigated if eNOS was critical for the cardioprotection afforded by VE training. Mice deficient in eNOS (eNOS−/−) were subjected to 4 weeks of VE training followed by MI/R (Figure 4G). Analysis revealed that VE training did not abrogate myocardial infarct size in eNOS−/− mice (p=N.S. vs. SED), suggesting that eNOS plays an important a role in the cardioprotective actions of VE. Additionally, the eNOS−/− mice did not exercise to the same extent as the C57BL/6J wild-type mice exercised (Online Figure I, 4.0±0.9 vs. 7.4±0.2 km/day, p<0.001), suggesting that eNOS is important for exercise training.
VE increases the expression of eNOS and increases the levels of NO metabolites in the Skeletal Muscle
Since VE involves changes in blood flow in active muscles, it is conceivable that the circulating levels of NO metabolites are derived from additional sources other than the heart. Therefore, we evaluated if VE could alter the expression of eNOS in another working muscle, the gastrocnemius. As shown in Online Figure II, the expression of eNOS was found to be significantly increased at the end of the 4-week training period (VE 4 wks; p<0.01 vs. SED), but had returned to control levels 1 week after the cessation of exercise (VE 4 wks + 1 wk SED). We also found that there was no difference in the expression of eNOS-PSer1177 in either group of exercised mice when the expression was compared to the expression of total eNOS. However, there was a significant increase in eNOS-PSer1177 in the VE 4 wk group when the expression was compared to the expression of α-tubulin (p<0.05 vs. SED), suggesting that there was more eNOS-PSer1177 after VE. Additionally, a significant decrease in the expression of eNOS-PThr495 in the gastrocnemius of the VE 4 wk group (p<0.05 vs. SED), but not the VE 4 wk + 1 wk SED group was observed. VE also significantly increased the levels of nitrite and nitrosothiols in the skeletal muscle of the VE 4 wks group (Online Figure III; p<0.01 vs. SED). However, unlike the trend observed in the heart, the levels of nitrite and nitrosothiols in the skeletal muscle declined back to near baseline levels at 1 week after the end of the VE period. In contrast, nitrate levels were significantly higher 1 week after the end of VE training (p<0.05 vs. SED).
Exercise increases circulating levels of nitrosothiols in trained endurance athletes
Additional studies were also performed to determine if VE training increased the steady-state levels of NO metabolites in human athletes. Blood samples were taken from individuals who exercised for at least 45 minutes a day, >3 times a week for at ≥2 years (trained endurance athletes) and from non-trained individuals. Subject characteristics are shown in Online Table I. Analysis revealed that trained endurance athletes (age 24±2, n=7) and non-trained individuals (age 27±1, n=16) had similar plasma levels of nitrite and nitrate (Figure 4H). In contrast, trained endurance athletes were found to have significantly higher (p=0.014) plasma nitrosothiol levels (15±4 vs. 7±1 nM vs. non-trained individuals, Figure 4I).
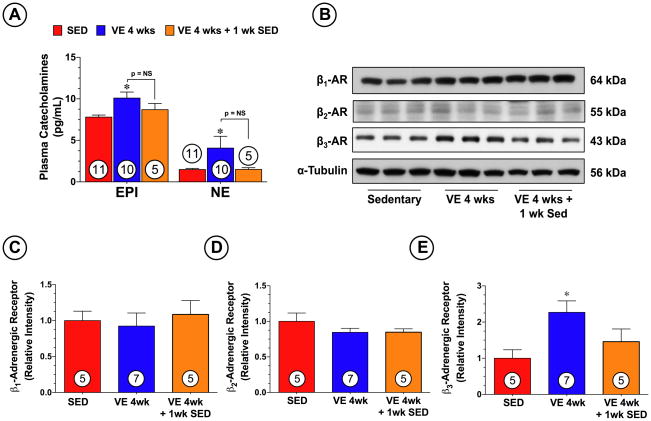
VE increases the levels of circulating catecholamines and increases the expression of the β3-AR
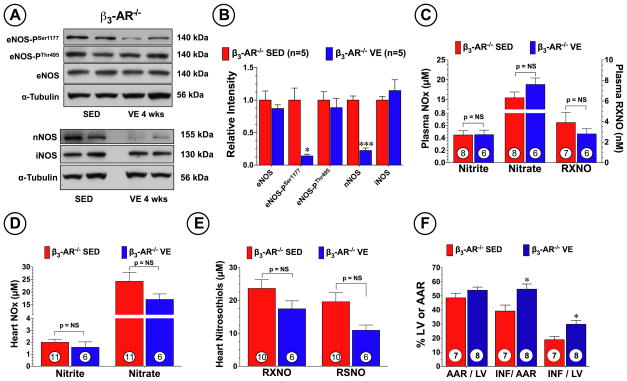
Recent studies have indicated an upregulation in the density of β3-ARs19 and an increase in β3-AR stimulants (i.e. catecholamines)12 in response to exercise training. As shown in Figure 5A, we found that VE significantly (p<0.05 vs. SED) increased the circulating levels of epinephrine and norepinphrine at the end of the training period. However, both had returned to normal levels 1 week after the end of the training. Furthermore, Western blot analysis revealed (Figure 5B–C) a significant increase in the protein expression of cardiac β3-AR in the hearts of the VE 4 wk group (p<0.05 vs. SED), but not in the hearts of the VE 4 wk + 1 wk SED group. No changes in the expression of β1-AR and β2-AR were noted in response to VE training. Given that β3-AR stimulation results in the production of NO from eNOS20, we next evaluated the role that β3-AR play in mediating the generation of NO metabolites during VE, as well as the role that they play in mediating the cardioprotective effects of VE. First we examined the effects of VE on eNOS expression and phosphorylation status in mice deficient in β3-AR (β3-AR−/−). VE training promoted a significant decrease (Figure 6A–B) in the expression of eNOS-PSer1177 (p<0.05 vs. SED), as well as a significant decrease in the expression of nNOS (p<0.001 vs. SED). No changes in the expression of eNOS, eNOS-PThr495, or iNOS were noted. As a result of these alterations, VE failed to increase the plasma or heart levels of nitrite, nitrate, and nitrosothiols in the β3-AR−/− mice (Figure 6C–E). We then evaluated how these alterations would affect VE-mediated cardioprotection. β3-AR−/− mice were subjected to 4 weeks of VE training followed by MI/R (Figure 6F). We found that the deficiency of β3-AR resulted in a significant increase in myocardial injury in response to VE training, as seen by a 40% increase in INF/AAR (39.2±4.2 vs. 54.8±3.7, p<0.05) and a 58% increase in INF/LV (18.9±2.2 vs. 29.9±2.8, p<0.05). Additionally, the β3-AR −/− mice did not exercise to the same extent as the C57BL/6J wild-type mice exercised (Online Figure I, p<0.001), suggesting that a deficiency in β3-ARs dampens the ability of mice to exercise.
Figure 5.
VE training increased circulating catecholamines and increased the expression of myocardial β3-adrenergic receptors. (A) Levels of epinephrine and norepinephrine were measured in the plasma of mice following 4 weeks of VE training. Representative (B) immunoblots and densitometric analysis of cardiac (C) β1-adrenergic receptors (D) β2-adrenergic receptors and (E) β3-adrenergic receptors following 4 weeks of VE training. Values are means ± S.E.M. *p<0.05 vs. SED.
Figure 6.
VE training failed to increase the expression of eNOS-PSer1177 and failed to provide cardioprotection in β3-Adrenergic Receptor deficient mice. (A) Representative immunoblots and (B) densitometric analysis of total eNOS, eNOS-PSer1177, eNOS-PThr495, iNOS, and nNOS from the hearts of β3-Adrenergic Receptor deficient mice (β3-AR−/−) following 4 weeks of VE training. Levels of nitrite, nitrate, and nitrosothiols (RXNO and RSNO) were measured in the (C) plasma and (D–E) heart following 4 weeks of VE training. β3-AR−/− mice subjected to 4 weeks of VE training were also subjected to 45 min of left coronary artery occlusion followed by reperfusion. (C) Myocardial AAR/LV, INF/AAR, and INF/LV were then evaluated at 24 hours of reperfusion. Values are means ± S.E.M. *p<0.05 and ***p<0.001 vs. SED.
Additional experiments were performed to further demonstrate a relationship between catecholamines and eNOS/NO. For these experiments, we studied the effects of a single injection of epinephrine on eNOS expression and phosphorylation status, as well as the levels of plasma and cardiac NO. Epinephrine dose dependently increased the heart rate and cardiac contractility of mice at concentrations ranging from 0.050 μg/kg to 10 μg/kg (Online Figure IVA–B). Using the higher dose (10 μg/kg), we found that epinephrine increased the expression of eNOS-PSer1177 in a time dependent manner (Online Figure VA–B). No changes in the expression of total eNOS, iNOS, or nNOS were observed. Epinephrine also increased the levels of both nitrite and nitrosothiols in the plasma and heart in a time dependent manner (Online Figure VI). This data suggests that β-AR stimulation rapidly induces changes in eNOS phosphorylation resulting in an increase in NO bioavailability.
The sustained cardioprotective effects of VE are lost when NO metabolites return to normal levels
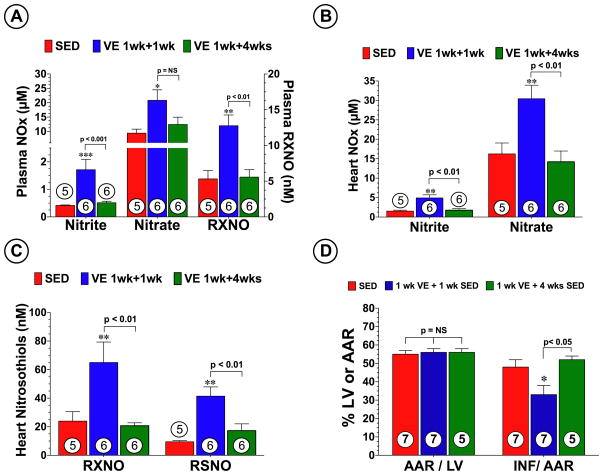
Additional experiments were performed to determine if the sustained cardioprotective effects of VE were present at a time when NO metabolites had returned to normal levels. For these experiments, mice were allowed to exercise for 1 week and then removed them from the cages for a period of either 1 week (VE 1 wk + 1 wk SED) or 4 weeks (VE 1 wk + 4 wks SED). Mice in the VE 1 wk + 1 wk SED group displayed a significant increase the levels of nitrite, nitrate, and nitrosothiols in both the plasma and heart (p<0.05 vs. SED; Figure 7A–C). In contrast, mice in the VE 1 wk + 4 wk SED group displayed no changes in plasma or heart NO metabolite levels when compared to SED mice, suggesting that the VE-induced alterations in eNOS/NO had returned to baseline levels 4 weeks after the end of the training period. Additionally, following MI/R, the VE 1 wk + 1 wk SED mice displayed a 31% reduction (p<0.05 vs. SED; Figure 7D) in INF relative to the AAR when compared to the SED control mice. In contrast, the VE 1 wk + 4 wks SED mice were not protected against MI/R injury, suggesting that the sustained cardioprotective effects of exercise are lost when NO metabolite levels are not elevated.
Figure 7.
The sustained cardioprotective effects of VE are lost when NO metabolites return to normal levels. Levels of nitrite, nitrate, and nitrosothiols (RXNO and RSNO) were measured in the (A) plasma and (B–C) heart from each group. Mice in each group were also subjected to 45 min of left coronary artery occlusion followed by reperfusion. SED mice and mice subjected to 1 week of VE and a period of either 1 week (VE 1 wk + 1 wk SED) or 4 weeks (VE 1 wk + 4 wks SED) of rest. (D) Myocardial AAR/LV and INF/AAR were then evaluated at 24 hours of reperfusion. Values are means ± S.E.M. *p<0.05, **p<0.01 vs. SED, and ***p<0.001 vs. SED.
Discussion
The endothelium plays a critical role in mediating the protective effects associated with exercise. Specifically, increased vascular wall shear stress induced by exercise increases the expression and activity of vascular eNOS, which subsequently increases the production and bioavailability of NO throughout the body.21,22 In the current study, we not only confirmed this finding but also found that exercise altered the expression and phosphorylation status of eNOS in a very tissue-specific manner. In the heart, we found that exercise increased the expression of eNOS-PSer1177 and decreased the expression of eNOS-PThr495 without altering the expression of total eNOS, whereas in the skeletal muscle, exercise increased the expression of total eNOS and decreased the expression of eNOS-PThr495. Furthermore, the degree to which exercise mediated these changes was also found to be tissue-specific, since the alterations observed in the heart persisted for at least 1 week after the end of the training period, while those changes observed in the skeletal muscle returned to baseline levels at this time point. This suggests that the mechanism(s) responsible for the alterations in eNOS may also be tissue-specific.
Importantly, the observed alterations in eNOS resulted in an increase in NO bioavailability, as evidenced by an increase in the plasma, skeletal muscle, and cardiac levels of nitrite and nitrosothiols. Circulating nitrite and nitrosothiol levels have traditionally been considered an acute marker of NO production and surrogate for endothelial function and cardiovascular health status.5 As such, their role in mediating the cardioprotective effects of exercise has not been investigated. Given the recent paradigm shift in NO biology, the role of these NO metabolites in exercise should be reconsidered. In the current study, we for the first time provide strong evidence that the generation and storage of nitrite and nitrosothiols plays a role in mediating the acute and sustained cardioprotective effects of exercise. First, we report that 4 weeks of VE training increased the levels of nitrite and nitrosothiols in the plasma, skeletal muscle, and heart in mice. Given that nitrite is reduced to NO during myocardial I/R8 and that nitrosothiols act as a redox sensitive NO donor23, it can be suggested that some of the acute cardioprotective effects of VE can be attributed to both nitrite and nitrosothiols. Second, we found that nitrite, nitrate, and nitrosothiols were stored in the heart for one week after the cessation of VE. Since nitrate can also be reduced to nitrite, which can then subsequently be reduced to NO18, it is probable that all three of these NO metabolites play a role in mediating the sustained cardioprotective effects of VE. This is further supported by the observation that the cardiac expression of CuZnSOD and AMPK were unchanged when compared to the SED control mice at this same time point. Third, we found that the cardioprotective effects of exercise did not extend to 4 weeks after the cessation of the training period when the levels of nitrite and nitrosothiols in both the plasma and heart had returned to baseline levels. Fourth, we found that nitrosothiol levels were increased in the plasma of trained endurance athletes. While this data does not offer any insights into storage levels of NO metabolites in the heart, it does provide evidence that VE increases the circulating levels of nitrosothiols in humans and provides a clinical basis to support the experimental findings of the current study.
Accumulating evidence indicates that nitrosothiols (S-nitrosothiols in particular) play an important role both in normal physiological processes and in a broad spectrum of human diseases. Nitrosothiols, which are formed by the ubiquitous redox-related modification of cysteine thiols in a process known as nitrosylation, have emerged as the most important mechanisms by which NO imparts its cellular effects.24 In regards to cardiovascular physiology, protein S-nitrosylation can influence cardiac contractility through the regulation of β-AR signaling and calcium cycling.25–27 Additionally, increasing the S-nitrosylation of proteins in the heart can reduce I/R injury8 by preventing the irreversible oxidation of proteins during early reperfusion9, by inhibiting apoptosis28 and inflammation29, by influencing blood flow and oxygen delivery30, and by modulating angiogenesis.31 Based on this evidence, the findings of the current study suggest that nitrosothiols may be a more significant indicator of NO-mediated protection during exercise when compared to the other NO metabolites given that both NO and nitrite can covalently modify a reactive cysteine to form a nitrosothiol.7,32
Another major finding of the current study relates to the role of β3-ARs in exercise. In the heart, three populations of β-ARs potentially modulate cardiac function (β1-, β2-, and β3-AR).20 These different subtypes belong to the G protein-coupled receptor superfamily and modulate cardiac function after stimulation by catecholamines.33 The effects of β1 and β2-AR are well established both in human and other mammals, as their stimulation produces positive chronotropic and inotropic effects. Although the precise physiological and pathophysiological roles of β3-ARs remain uncertain, recent observations suggests that β3-AR stimulation produces a negative inotropic effect via the production of NO from eNOS.20 The activation of eNOS during exercise can be caused by shear stress inducing a signaling cascade involving Akt (protein kinase B), protein kinase A (PKA) and/or AMPK.34,35 A recent study reported that inhibition of Akt signaling with wortmannin blunts the increase in the vascular expression of eNOS-PSer1177 in mice subjected to treadmill running without altering the expression of phosphorylated CREB (PKA signaling) or AMPK.34 Since wortmannin did not completely attenuate the increase in the expression of eNOS-PSer1177, this data simply suggests that Akt plays a major role in regulating this phosphorylation. This also suggests that other signaling molecules regulate the expression of eNOS-PSer1177 during exercise.
Here, we provide strong evidence that β3-ARs are involved in this process. First, in agreement with other studies12, we found that 4 weeks of VE significantly increased the circulating levels of epinephrine and norepinephrine, as well as the protein expression of cardiac β3-ARs. Previously in cultured cells, it has been reported that epinephrine can rapidly increase the expression of eNOS-PSer1177 in a β3-AR-dependent manner.36 We also found that acute injections of epinephrine rapidly increased the expression of eNOS-PSer1177 and the levels of NO metabolites in both the plasma and heart. While these studies do not provide direct evidence that β3-AR stimulation can increase the expression of eNOS-PSer1177, it does suggest that epinephrine can modulate eNOS/NO levels in vivo. However, a direct relationship was demonstrated with our studies using β3-ARs deficient mice. Based on the existing literature, we predicted that a deficiency in β3-ARs would dampen the increase in the expression of eNOS-PSer1177 in response to exercise in a similar manner as that reported for wortmannin. Interestingly, we found that in response to exercise the increase in the expression of eNOS-PSer1177 was not simply blunted in the hearts of β3-AR deficient mice, but rather its expression was significantly decreased when compared to sedentary control levels. Additionally, exercise induced a significant decline in the expression of nNOS in the hearts of β3-AR deficient mice. This was also a rather surprising finding given that nNOS levels did not change in the hearts of wild-type mice that exercised but supportive of the recent evidence indicating that β3-AR stimulation can also regulate nNOS.11 As a result of this downregulation, exercise failed to increase the plasma or heart levels of nitrite, nitrate, and nitrosothiols in the β3-AR−/− mice. Importantly, in response to exercise a deficiency of β3-ARs leads to an exacerbation of myocardial infarction following ischemia-reperfusion injury. Taken together, these results suggest that β3-ARs not only play a major role in regulating the phosphorylation of eNOS at serine 1177, as well as maintaining the basal expression of myocardial nNOS during exercise, but also are necessary for regulating the production of NO during exercise and for mediating the cardioprotective effects of exercise.
While it is not fully known how β3-ARs regulate the expression of eNOS, it is known that interfering with this regulation can lead to detrimental effects. Previously, it has been reported that lack of β3-AR signaling exacerbates cardiac pressure-overload-induced remodeling by enhancing eNOS uncoupling and increasing superoxide production.37 It is currently unknown if exercise causes eNOS to become uncoupled in the hearts of β3-AR−/−. However, the uncoupling of eNOS would certainly provide an explanation as to why the β3-AR−/− mice in the current study displayed exacerbated myocardial injury following exercise training. This would also, in part, explain the different response to MI/R injury between the eNOS−/− and β3-AR−/− mice, given that the eNOS−/− could not experience eNOS uncoupling and subsequent superoxide production.
In summary, the current study demonstrates that 4 weeks of VE training provides acute and sustained cardioprotection against MI/R injury. Additionally, the current study provides evidence that NO metabolites play a major role in the observed sustained cardioprotective effects and provides novel evidence suggesting a role for β3-ARs in exercise-mediated cardioprotection. Based on these results, we propose that during exercise sympathetic stimulation via the β3-AR leads to the activation of eNOS resulting in an increase in the production of NO. The NO generated would then have two fates. It can either be used to induce vasodilatation38 to match blood flow to metabolic demands39 or be metabolized into nitrite and nitrosothiols, which can then be stored in the heart and circulation. This can continue with each passing exercise period until the steady-state levels of the metabolites in the heart are elevated above normal baseline levels. This would be analogous to the effects of oral nitrite supplementation on the heart that was observed in a previous study.8 Increasing these stores in the heart prior to myocardial ischemia is important because the bioavailability of NO is decreased during ischemia. The cause of this decrease is still not completely understood but it has been suggest that NO levels are reduced during myocardial ischemia due to a decrease in production from eNOS because of low oxygen and diminished substrate delivery and/or an increase in ROS production.6,40 In any event, the stored nitrite can be reduced to NO during myocardial ischemia by any of the identified nitrite reductases found in the heart, thereby providing an increase in the bioavailability of NO. The nitrosothiols can act as a reversible protective shield to prevent the irreversible oxidation of proteins during the early oxidative burst of reperfusion9 and act as a redox sensitive NO donor. The increase in NO can then serve as a signaling molecule to protect the heart against MI/R injury.
Supplementary Material
Novelty and Significance.
What is Known?
Exercise training reduces many risk factors associated with cardiovascular disease. It confers sustainable protection against myocardial infarction in animal models and improves survival following myocardial ischemia in humans.
The cardioprotective effects of exercise are not confined to the period of exercise.
The mechanism(s) by which exercise protects the heart against myocardial ischemia-reperfusion injury are not completely understood, but it is apparent that endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) play a role.
What New Information Does This Article Contribute?
Mice that engaged in 4 weeks of voluntary exercise training were protected against myocardial ischemia-reperfusion injury at the end of the training period and displayed sustained protection for 1 week following the cessation of the training period.
Voluntary exercise increased the circulating levels of epinephrine and norepinephrine, as well as the abundance of cardiac β3-adrengeric receptors (β3-ARs).
β3-AR deficient mice were not protected against MI/R injury following exercise.
The cardioprotective effects of exercise were in part mediated by alterations in the phosphorylation status of eNOS leading to an increase in cardiac NO metabolite (nitrite and nitrosothiols) levels.
The alterations in eNOS phosphorylation status and NO generation were mediated in part by β3-AR stimulation.
The cardioprotective effects of exercise did not extend to 4 weeks after the cessation of the training period when the levels of nitrite and nitrosothiols in the heart had returned to baseline levels.
The NO metabolites nitrite and nitrosothiols are potent signaling molecules and potential therapies for ischemic disease. The present investigation clearly demonstrates that when mice exercise there is an activation of cardiac eNOS and NO generation resulting in increased cardiac nitrite and nitrosothiol levels. Additionally, we report a novel role for cardiac β3-ARs in exercise-mediated cardioprotection. Specifically, we found that β3-ARs play a critical role in regulating the phosphorylation of eNOS and the generation of NO in response to exercise. Collectively, our results provide novel insights into the cardioprotective effects of exercise and may aid in the design of treatment modalities to treat patients who suffer from ischemic heart disease.
Acknowledgments
Sources of Funding
Supported by grants from the American Diabetes Association (7-09-BS-26) to J.W.C. and the National Institutes of Health National Heart Lung and Blood Institute (NHLBI) 5R01HL-092141-02, and 1R01HL093579-01 to D.J.L. and 1R01HL098481-01 to J.W.C. This work was also supported by funding from the Carlyle Fraser Heart Center (CFHC) of Emory University Hospital Midtown.
Non-standard Abbreviations and Acronyms
- AMPK
AMP-activated protein kinase
- AAR
Area-at-risk
- β-AR
β-adrenergic receptor
- β1-AR
β1-adrenergic receptor
- β2-AR
β2-adrenergic receptor
- β3-AR
β3-adrenergic receptor
- eNOS
endothelial nitric oxide synthase
- eNOS-PSer1177
eNOS phosphorylated at serine residue 1177
- eNOS-PThr495
eNOS phosphorylated at threonine residue 495
- HSPs
heat shock proteins
- iNOS
inducible nitric oxide synthase
- INF
infarct size
- LCA
left coronary artery
- MI/R
myocardial ischemia-reperfusion
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- RSNO
s-nitrosothiol
- RXNO
nitrosothiol
- SED
sedentary
- SOD
superoxide dismutase
- VE
voluntary exercise
Footnotes
Disclosures
None
References
- 1.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–2518. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- 2.Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, Powers SK. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol. 2004;96:1299–1305. doi: 10.1152/japplphysiol.00920.2003. [DOI] [PubMed] [Google Scholar]
- 3.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the katp channel in the rat. J Physiol. 2005;569:913–924. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull SS, Jr, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation. 1994;89:548–552. doi: 10.1161/01.cir.89.2.548. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY. Amp-activated protein kinase is involved in endothelial no synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 6.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 8.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogg N, Broniowska KA, Novalija J, Kettenhofen NJ, Novalija E. Role of s-nitrosothiol transport in the cardioprotective effects of s-nitrosocysteine in rat hearts. Free Radic Biol Med. 2007;43:1086–1094. doi: 10.1016/j.freeradbiomed.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Rozec B, Gauthier C. Beta3-adrenoceptors in the cardiovascular system: Putative roles in human pathologies. Pharmacol Ther. 2006;111:652–673. doi: 10.1016/j.pharmthera.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Moens AL, Yang R, Watts VL, Barouch LA. Beta 3-adrenoreceptor regulation of nitric oxide in the cardiovascular system. J Mol Cell Cardiol. 48:1088–1095. doi: 10.1016/j.yjmcc.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoo EY, Wallis J, Tsintzas K, Macdonald IA, Mansell P. Effects of exenatide on circulating glucose, insulin, glucagon, cortisol and catecholamines in healthy volunteers during exercise. Diabetologia. 2010;53:139–143. doi: 10.1007/s00125-009-1579-1. [DOI] [PubMed] [Google Scholar]
- 13.Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd’Heuil D, Huang PL, Lefer DJ. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol. 1999;276:H1567–1573. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- 14.Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB. Targeted disruption of the beta 3-adrenergic receptor gene. J Biol Chem. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 15.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg GR, Kemp BE. Ampk in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 17.Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol. 1997;272:H1070–1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- 18.Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 19.Barbier J, Rannou-Bekono F, Marchais J, Tanguy S, Carre F. Alterations of beta3-adrenoceptors expression and their myocardial functional effects in physiological model of chronic exercise-induced cardiac hypertrophy. Mol Cell Biochem. 2007;300:69–75. doi: 10.1007/s11010-006-9370-9. [DOI] [PubMed] [Google Scholar]
- 20.Tavernier G, Toumaniantz G, Erfanian M, Heymann MF, Laurent K, Langin D, Gauthier C. Beta3-adrenergic stimulation produces a decrease of cardiac contractility ex vivo in mice overexpressing the human beta3-adrenergic receptor. Cardiovasc Res. 2003;59:288–296. doi: 10.1016/s0008-6363(03)00359-6. [DOI] [PubMed] [Google Scholar]
- 21.Napoli C, Williams-Ignarro S, De Nigris F, Lerman LO, Rossi L, Guarino C, Mansueto G, Di Tuoro F, Pignalosa O, De Rosa G, Sica V, Ignarro LJ. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2004;101:8797–8802. doi: 10.1073/pnas.0402734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Hogg N. Biological chemistry and clinical potential of s-nitrosothiols. Free Radic Biol Med. 2000;28:1478–1486. doi: 10.1016/s0891-5849(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 24.Foster MW, Hess DT, Stamler JS. Protein s-nitrosylation in health and disease: A current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of beta-adrenergic receptor signaling by s-nitrosylation of g-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 26.Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ, Stamler JS. S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez DR, Treuer A, Sun QA, Stamler JS, Hare JM. S-nitrosylation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:188–195. doi: 10.1097/FJC.0b013e3181b72c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, Stamler JS. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 29.Calvert JW, Gundewar S, Yamakuchi M, Park PC, Baldwin WM, 3rd, Lefer DJ, Lowenstein CJ. Inhibition of n-ethylmaleimide-sensitive factor protects against myocardial ischemia/reperfusion injury. Circ Res. 2007;101:1247–1254. doi: 10.1161/CIRCRESAHA.107.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singel DJ, Stamler JS. Blood traffic control. Nature. 2004;430:297. doi: 10.1038/430297a. [DOI] [PubMed] [Google Scholar]
- 31.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous s-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seth D, Stamler JS. The sno-proteome: Causation and classifications. Curr Opin Chem Biol. doi: 10.1016/j.cbpa.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Germack R, Dickenson JM. Induction of beta3-adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. J Pharmacol Exp Ther. 2006;316:392–402. doi: 10.1124/jpet.105.090597. [DOI] [PubMed] [Google Scholar]
- 34.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: Role of vascular signalling kinases. J Physiol. 2009;587:3911–3920. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of akt in human endothelial cells: Involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 36.Kou R, Michel T. Epinephrine regulation of the endothelial nitric-oxide synthase: Roles of rac1 and beta3-adrenergic receptors in endothelial no signaling. J Biol Chem. 2007;282:32719–32729. doi: 10.1074/jbc.M706815200. [DOI] [PubMed] [Google Scholar]
- 37.Moens AL, Leyton-Mange JS, Niu X, Yang R, Cingolani O, Arkenbout EK, Champion HC, Bedja D, Gabrielson KL, Chen J, Xia Y, Hale AB, Channon KM, Halushka MK, Barker N, Wuyts FL, Kaminski PM, Wolin MS, Kass DA, Barouch LA. Adverse ventricular remodeling and exacerbated nos uncoupling from pressure-overload in mice lacking the beta3-adrenoreceptor. J Mol Cell Cardiol. 2009;47:576–585. doi: 10.1016/j.yjmcc.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5:211–224. [PubMed] [Google Scholar]
- 39.Kingwell BA. Nitric oxide as a metabolic regulator during exercise: Effects of training in health and disease. Clin Exp Pharmacol Physiol. 2000;27:239–250. doi: 10.1046/j.1440-1681.2000.03232.x. [DOI] [PubMed] [Google Scholar]
- 40.Giraldez RR, Panda A, Xia Y, Sanders SP, Zweier JL. Decreased nitric-oxide synthase activity causes impaired endothelium-dependent relaxation in the postischemic heart. J Biol Chem. 1997;272:21420–21426. doi: 10.1074/jbc.272.34.21420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.