Abstract
Human embryonic stem cells provide a useful source of material for studying basic human development and various disease states. However, ethical issues concerning their procurement limit their acceptance and possible clinical applicability. Recent advances in stem cell technology have provided an alternative source of pluripotent stem cells that do not require the use of an embryo. This review addresses the generation of induced pluripotent stem cells from skin fibroblasts taken from various patient populations, with a specific focus on the pediatric disorder spinal muscular atrophy. These patient-derived cells may help devise more appropriate therapies through a greater understanding of the molecular mechanisms underlying neuron dysfunction and death in a number of diseases. Furthermore, they provide an ideal platform for small molecule screening and subsequent drug development.
Pluripotent stem cells
Since their isolation in 19981, human embryonic stem cells (hESCs) have garnered much attention for a wide range of experimental and therapeutic applications. hESCs are pluripotent because following injection into immunodeficient mice they will form teratomas consisting of cell types from all three primitive germ layers (endoderm, mesoderm, and ectoderm) including muscle, heart, liver, and central nervous system. Importantly for neurodegenerative diseases, hESCs can be further lineage restricted to generate very specific neural subtypes, including dopaminergic neurons, motor neurons, oligodendrocytes, and astrocytes that display many of the neurochemical and electrical attributes of mature neurons (e.g. neurotransmitters, transporters, and evoked action potentials).2 Because of these attributes, hESCs offer a tremendous advantage to model diseases. However, ethical concerns surround their use because the embryo is destroyed in the process of their procurement.
The groundbreaking discovery that mouse and human fibroblasts have the capacity to be reverted to an embryonic stem cell fate through reprogramming has significantly advanced the field of stem cell and neurodegenerative disease research. Although done independently and with some variation, these cells were all modified using DNA technology to over-express important pluripotent stem cell genes in human neonatal or adult fibroblasts. These exogenously expressed genes reprogrammed the DNA of the fibroblast cells to make them display embryonic stem cell-like morphology, exponential growth properties, and gene expression profiles. Similar to hESCs, the newly reprogrammed cells, termed induced pluripotent stem cells (iPSCs), can form teratomas and can be lineage restricted into the various cell types in the body.3 Since these cells are derived from adult skin, two very important implications arise. First, a fertilized embryo is not needed for the production of iPSCs, thus reducing some of the ethical concerns with their generation and use. Second, iPSCs can be derived from any patient population,4–7 which allows for the analysis and modeling of the disease in the cell types particularly affected. Importantly, this technique also provides the generation of a human model system for diseases with no known genetic component or for which few appropriate experimental models are available.
Spinal muscular atrophy
Spinal muscular atrophy (SMA) is an autosomal recessive disease that causes specific loss of alpha motor neurons in the spinal cord and is one of the leading genetic causes of infant mortality. SMA is caused by a mutation in the survival motor neuron (SMN) gene leading to a loss of SMN1 protein8 and concomitant loss of motor neurons. SMA can be divided into four categories based on disease severity with Type I being the most severe and Type IV being the least severe (Table). The SMN gene is located on chromosome 5, and approximately 95% of all SMA patients are missing this gene. Humans are unique in that they have two versions of SMN: SMN1 and SMN2.9 SMN1 produces a full-length protein found in both the cytoplasm and the nucleus and is part of a large complex involved in a variety of RNA processes.10–12 In contrast, a single C to T nucleotide transition in SMN2 causes exon 7 to be excluded generating low levels (~10–15%) of full-length SMN protein and high levels of an essentially non-functional, truncated SMN2 protein (SMNΔ7).13,14 Because SMN2 can produce some full-length SMN protein, studies have shown that the disease severity is mitigated by how much full-length protein is produced.15 Therefore, many of the current experimental approaches are using small molecule induction or RNA manipulation to increase SMN2 protein production to compensate for the loss of SMN1 protein.16
| Classification | Age of onset | Symptoms | Prognosis |
|---|---|---|---|
| Type I (Werdnig-Hoffman disease) |
Birth to 6mo | Severe proximal muscle weakness Reduced muscle tone Inability to hold head up Possible skeletal deformities | Short life expectancy Death generally by 2yrs |
| Type II | 6 to 18mo | Moderate proximal muscle weakness Developmental motor delay May be able to sit unaided | Reduced lifespan (<30yrs) |
| Type III (Kugelberg-Welander syndrome) |
>18mo | Slow and mild proximal muscle weakness May need assistance with standing and walking | Normal lifespan |
| Type IV (adult onset) |
>18yrs | Slow and mild proximal muscle weakness May need assistance with standing and walking | Normal lifespan |
SMA model systems
Several experimental models using single and multiple cellular organisms have been employed to study the molecular processes involved in SMA. Mice have become the most often used vertebrate model for mammalian genetic research because of the ability to manipulate the genome. Using homologous recombination technology and mating crosses, Schrank and colleagues17 found that embryos lacking SMN die before uterine implantation, which underscores the importance of SMN during development in all cell types, not just motor neurons. Other mice have been developed harboring the entire human SMN2 locus or the SMNΔ7 mutation on the mouse SMN knockout background to better represent the human condition.18–20 Despite providing invaluable protein and disease information, these animal models may not adequately represent the human condition as mouse physiology and anatomy are radically different from humans, especially with regard to the central nervous system.
Possibly some of the most useful cells studied in culture have been fibroblasts taken from SMA patients. Fibroblasts are relatively easy to obtain from skin biopsies, are easy to grow and maintain in the culture dish, and have the added benefit that they naturally lack SMN1. One important weakness is that fibroblasts do not make motor neurons, astrocytes, or muscle. Due to the selective loss of motor neurons and the importance of astrocytes and muscle on motor neuron health,21,22 having a source of human cells harboring the genetic mutation and capable of making these specific tissues would be highly beneficial. Patient derived iPSCs can fulfill this need.
We recently generated iPSCs from commercially available fibroblast samples taken from a three year old boy with Type I SMA and his unaffected (WT) mother.7 To do this, we used lentiviral vectors to stably express reprogramming genes in the fibroblast samples. After a few weeks in culture, iPSCs formed, which are visually and functionally distinct from fibroblast cells, and expressed a range of pluripotency markers found in hESCs. We further showed that these cells were indeed pluripotent as, unlike the fibroblasts, they could generate teratomas. Importantly, the iPS-SMA cells retained a lack of SMN1 expression compared to the iPS-WT cells.7 Finally, given the appropriate culture conditions, both the iPS-SMA and iPS-WT cells were able to produce neural cells that expressed some of the molecular markers typical of motor neurons (Fig 1). Taken together, these characteristics represent a full conversion from the fibroblast state to a pluripotent stem cell.
Figure 1. iPSCs can be lineage restricted to form motor neurons.
Motor neurons were differentiated from iPSCs produced from an unaffected fibroblast sample and stained for the neurofilament protein SMI-32 (red). Cell nuclei are labeled in blue. Magnification = 100x
Although SMN is present in all cell types, it is curious that such an essential and ubiquitously expressed cellular protein should cause such specific degeneration of motor neurons. In this regard, we analyzed motor neuron production in the iPS-SMA and iPS-WT cells. Early in the differentiation protocol, both the affected and non-affected iPSCs were able to form motor neurons of approximately the same number and size, which we used as an indication of neuron health. However, following a few more weeks of motor neuron development, we observed a selective reduction in the number and size of motor neurons derived from the iPS-SMA cells compared to non-diseased iPS-WT derived motor neurons.7 Additional data are needed to determine the mechanism inducing the motor neuron changes, but our data suggest that an intrinsic property of the SMA derived motor neuron was leading to damaged (or dying) neurons rather than a lack of motor neuron development (Fig 2). Interestingly, SMN1 protein has been shown to have anti-oxidant properties, and it is possible that the metabolism and energy requirements within the SMA motor neurons generate more reactive oxygen species compared to other cells types. Oxidative stress can be initiated by a number of processes including mitochondrial dysfunction that may lead to motor neuron dysfunction through activation of programmed cell death pathways. Answering these mechanistic questions hopefully will provide insight into the best method of therapeutic intervention.
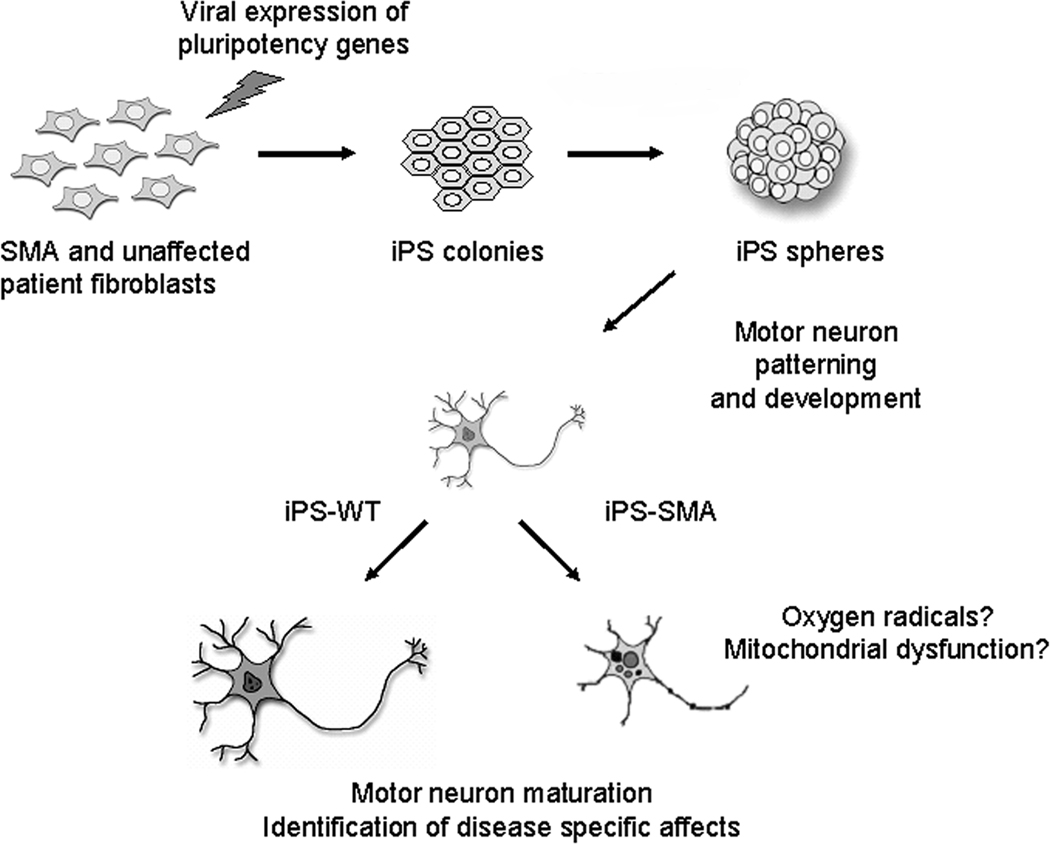
Figure 2. Fibroblast cells can be converted to iPSCs to model SMA.
Fibroblast cells were infected with viruses to express reprogramming genes. The iPSCs were cultured as flat colonies then lifted to form free-floating aggregate spheres in a nutrient medium. Next, using a series of chemical induction signals, iPSCs were instructed to form motor neurons, which importantly, both the iPS-SMA and iPS-WT cells were able to do. Only after additional time in culture did we observe a specific deficit in the iPS-SMA derived motor neurons. We speculate that this effect could be due to increased reactive oxygen species production or damaged mitochondria within the iPS-SMA cells.
Developing therapies for SMA using iPSCs
Despite the fact that gene therapy for SMA (e.g. using RNA technology or oligonucleotides to modify SMN2 splicing16 is only recently becoming more widely used in research, both experimental and clinical applications for gene therapy have been developed for other neurodegenerative diseases.23 Interestingly, using viral vectors to replace SMN1 was shown to decrease motor neuron death and increase lifespan in SMA mice.24 While this technique holds great clinical promise, the future of gene therapy may one day combine iPSC technology and cellular replacement with gene modification. In this regard, Hanna and colleagues reported an elegant study in which sickle cell anemia was corrected in a mouse by generating iPSCs from this mouse, using gene specific targeting to repair the hemoglobin allele, generating hematopoietic progenitor cells, and transplanting the repaired blood cells back into the same mouse.25 It is intriguing to consider the possibility of generating iPSCs from a patient with SMA, genetically elevating SMN1 expression, deriving motor neurons and astrocytes that now express SMN1, and then transplanting these cells back to repopulate and repair the patient’s spinal cord. While there are major challenges associated with this approach, some reports have shown the growth of axons from transplanted motor neurons can re-innervate the muscle and have functional effects.26
A more near-term use of iPS-SMA cells may be in the area of small molecule drug discovery. SMN protein is found in aggregate nuclear structures called gems, and the number of gems present is inversely correlated to disease severity;15,27 therefore, therapeutic agents that increase protein output would be clinically valuable. Various compounds have been tested on SMA patient fibroblast cells and shown to increase SMN protein through stabilization or prevention of exon 7 skipping,28–31 and one such compound, valproic acid, is currently being used in clinical trials for SMA.32 Using two previously tested compounds (valproic acid and tobramycin), we found that the number of gems significantly increased in the iPS-SMA cells following treatment with each compound compared to untreated iPS-SMA cells7 suggesting that SMN protein production and/or stabilization was enhanced. Our results confirmed that patient-derived iPSCs have similar molecular responses to drug treatment as the routinely used fibroblast cells have. We went on to identify gems in motor neurons, so the next step will be to derive high throughput screening assays assessing gem number specifically in SMA patient derived motor neurons. Further drug discovery investigation is crucial, and by using iPSCs, novel compounds can be tested in a human system on the particularly vulnerable cell types thereby increasing efficacy and the discovery of highly clinically relevant compounds.
Conclusions
SMA is a devastating and oftentimes fatal disease without an effective treatment. Much effort has been focused on determining the role of SMN in cellular function, identifying the molecular processes involved in neuronal death, and on developing effective therapies. The ability to generate patient specific iPSCs has opened new avenues of study for both cell therapy and disease modeling. SMA may serve as a “proof of concept” that a specific neurological pathology can be mirrored in the culture dish. Armed with this model system, the hope is that new discoveries will be made allowing cell replacement and new drugs to be found to treat this disease.
Acknowledgements
This work was supported by the ALS Association and the National Institutes of Health.
Footnotes
Conflict of interest:
The authors declare no competing financial interests.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Muller LU, Daley GQ, Williams DA. Upping the Ante: Recent Advances in Direct Reprogramming. Mol Ther. 2009 doi: 10.1038/mt.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 5.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soldner F, Hockemeyer D, Beard C, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebert AD, Yu JY, Rose FF, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 9.Rochette CF, Gilbert N, Simard LR. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum Genet. 2001;108:255–266. doi: 10.1007/s004390100473. [DOI] [PubMed] [Google Scholar]
- 10.Pellizzoni L, Yong J, Dreyfuss G. Essential role for the SMN complex in the specificity of snRNP assembly. Science. 2002;298:1775–1779. doi: 10.1126/science.1074962. [DOI] [PubMed] [Google Scholar]
- 11.Fischer U, Liu Q, Dreyfuss G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell. 1997;90:1023–1029. doi: 10.1016/s0092-8674(00)80368-2. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Fischer U, Wang F, et al. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell. 1997;90:1013–1021. doi: 10.1016/s0092-8674(00)80367-0. [DOI] [PubMed] [Google Scholar]
- 13.Monani UR, Lorson CL, Parsons DW, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- 14.Lorson CL, Hahnen E, Androphy EJ, et al. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci. U.S.A. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre S, Burlet P, Liu Q, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- 16.Baughan T, Shababi M, Coady TH, et al. Stimulating full-length SMN2 expression by delivering bifunctional RNAs via a viral vector. Mol Ther. 2006;14:54–62. doi: 10.1016/j.ymthe.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Schrank B, Gotz R, Gunnersen JM, et al. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci. U. S. A. 1997;94:9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh-Li HM, Chang JG, Jong YJ, et al. A mouse model for spinal muscular atrophy. Nat Genet. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- 19.Monani UR, Sendtner M, Coovert DD, et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(−/−) mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- 20.Le TT, Pham LT, Butchbach MER, et al. SMN Delta 7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 21.Guettier-Sigrist S, Coupin G, Braun S, et al. Muscle could be the therapeutic target in SMA treatment. J Neurosci Res. 1998;53:663–669. doi: 10.1002/(SICI)1097-4547(19980915)53:6<663::AID-JNR4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Van Damme P, Bogaert E, Dewil M, et al. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc Natl Acad Sci. U.S.A. 2007;104:14825–14830. doi: 10.1073/pnas.0705046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjorklund A, Kirik D, Rosenblad C, et al. Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- 24.Azzouz M, Le T, Ralph GS, et al. Lentivector-mediated SMN replacement in a mouse model of spinal muscular atrophy. J Clin Invest. 2004;114:1726–1731. doi: 10.1172/JCI22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 26.Deshpande DM, Kim YS, Martinez T, et al. Recovery from paralysis in adult rats using embryonic stem cells. Ann Neurol. 2006;60:32–44. doi: 10.1002/ana.20901. [DOI] [PubMed] [Google Scholar]
- 27.Coovert DD, Le TT, McAndrew PE, et al. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6:1205–1214. doi: 10.1093/hmg/6.8.1205. [DOI] [PubMed] [Google Scholar]
- 28.Brichta L, Hofmann Y, Hahnen E, et al. Valproic acid increases the SMN2 protein level: a well-known drug as a potential therapy for spinal muscular atrophy. Hum Mol Genet. 2003;12:2481–2489. doi: 10.1093/hmg/ddg256. [DOI] [PubMed] [Google Scholar]
- 29.Mattis VB, Rai R, Wang JH, et al. Novel aminoglycosides increase SMN levels in spinal muscular atrophy fibroblasts. Hum Genet. 2006;120:589–601. doi: 10.1007/s00439-006-0245-7. [DOI] [PubMed] [Google Scholar]
- 30.Sumner CJ, Huynh TN, Markowitz JA, et al. Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol. 2003;54:647–654. doi: 10.1002/ana.10743. [DOI] [PubMed] [Google Scholar]
- 31.Wolstencroft EC, Mattis V, Bajer AA, et al. A non-sequence-specific requirement for SMN protein activity: the role of aminoglycosides in inducing elevated SMN protein levels. Hum Mol Genet. 2005;14:1199–1210. doi: 10.1093/hmg/ddi131. [DOI] [PubMed] [Google Scholar]
- 32.Tsai LK, Yang CC, Hwu WL, et al. Valproic acid treatment in six patients with spinal muscular atrophy. Eur J Neurol. 2007;14:E8–E9. doi: 10.1111/j.1468-1331.2007.01992.x. [DOI] [PubMed] [Google Scholar]