Abstract
Objective
The goal of this study was to examine the effectiveness and safety of low-frequency rTMS to the temporoparietal junction in a cohort of patients with bothersome tinnitus.
Design
Cross-over, double-blind, randomized clinical trial.
Setting
Outpatient, academic medical center
Participants
14 adults between the ages of 42 and 59 with subjective, unilateral or bilateral, non-pulsatile tinnitus of 6 months duration or greater, and a score of 38 or greater on the Tinnitus Handicap Inventory (THI).
Interventions
Low-frequency (1 Hz) 110% motor threshold rTMS or sham to left temporoparietal junction for 2 weeks.
Main Outcome Measure
The difference in the change of the Tinnitus Handicap Inventory score between active and sham rTMS.
Results
Active treatment was associated with a median (95% CI) reduction in THI of 5 points (0 to 14) and sham treatment was associated with a median reduction in THI of 6 points (−2 to 12). The difference in THI between the change associated with active and sham rTMS ranged from 34 points reduction in THI score after active treatment when compared to THI score after sham, to an increase of 22 points, with a median difference change of only 1 point (−6 to 4).
Conclusions
Daily low-frequency rTMS to the left temporoparietal junction area for 2 weeks was no more effective than placebo for patients with chronic bothersome tinnitus. Possible explanations for the negative findings are short duration of treatment, failure of rTMS stimulation over the temporoparietal area to affect auditory cortex buried within the Sylvian fissure, or more widespread cortical network changes associated with severe bothersome tinnitus not amenable to localized rTMS effects.
INTRODUCTION
The physiological process(es) responsible for subjective tinnitus remain unknown. The available evidence1–3 suggests that tinnitus is associated with disturbances in spontaneous neural activity in the auditory system. These abnormalities, as measured in animal models of tinnitus, include increased spontaneous activity (hyperactivity4), changed timing of neural discharges (i.e., the temporal firing properties of neurons5), and increased bursting activity of neurons.6
Multiple neuroimaging studies7–13 indicate that changes in auditory association areas and the primary auditory cortex are associated with tinnitus. Observations based on PET imaging, however, indicate additional activation of temporoparietal cortex located immediately posterior to auditory regions.11,14–16 However, it is difficult to evaluate the findings from these imaging studies because of methodological weaknesses17, including the tinnitus patients in each study were not matched against a non-tinnitus control group with comparable hearing.
Melcher et al18 used sound-evoked activation of the auditory pathway of individuals with normal hearing and tinnitus lateralized to one ear and found abnormal functional MRI activation asymmetry in the inferior colliculi of tinnitus patients. In a subsequent paper, Melcher et al19 selected tinnitus patients and controls matched on age, emotional state, and hearing loss and found abnormal gain within the auditory pathway. The studies by Melcher et al18,19 and Lanting et al20 both suggest increased sound-evoked activation within the auditory mid brain of patients with tinnitus.
Rauschecker et al21 proposed a model for tinnitus based on neuroimaging findings, which focuses on the cortex, thalamus, and ventral striatum. In this model, the perception of tinnitus is viewed as an interaction between the limbic, auditory, and thalamic areas wherein feedback connections from limbic regions provide a “noise-cancellation” mechanism. Llinas et al22 used magnetoencephalography in patients with tinnitus, neurogenic pain, Parkinson’s disease, or depression and controls described the presence of a thalamocortical dysrhythmia, whereby symptoms are a result of inhibitory asymmetry between high- and low-frequency thalamocortical modules at the cortical level. The identification of alterations in non-auditory cortical areas is consistent with the clinical observation that tinnitus does not habituate23, alters attention and memory24–26, and can affect emotions.27–31
Repetitive transcranial magnetic stimulation (rTMS) is a novel diagnostic and therapeutic tool for a variety of psychological and neurological conditions. rTMS temporarily produces disruptions in a circumspect area of the brain that interrupts normal functioning and has acute and chronic effects. Depending on the stimulus parameters, rTMS can alter different excitatory and inhibitory circuits in the brain.32 The temporary effects can be followed by more enduring changes in neuronal excitability with low-frequency (1 Hz) stimulation inhibiting responses in a persistent fashion.33 Although the mechanism(s) by which rTMS reduces cortical excitability remains unclear, GABAergic involvement seems plausible given the possible involvement of GABAergic interneurons in intracortical inhibition.34
Several studies35–44 evaluated the effect of rTMS on tinnitus. Findings from slightly over 200 patients suggested that low-frequency (1 Hz) rTMS applied to the scalp overlying the lateral temporal cortex in the region of the primary and secondary auditory cortices reduced tinnitus severity. In their recent review of clinical results of rTMS for tinnitus, Londero and colleagues45 concluded that “TMS appears to be a very promising tool for the diagnosis and the treatment of tinnitus patients”. Despite the promise offered by these findings, many of the studies suffered from various methodological weaknesses, most prominently failure to include adequate controls and valid sham.
The goal of this study was to examine the effectiveness and safety of applying low-frequency rTMS in a cohort of patients with bothersome tinnitus. The target site was the temporoparietal junction (TPJ) (secondary and integrative auditory areas). We selected the TPJ because prior anatomical and PET imaging studies12 suggested TPJ involvement in tinnitus and success with rTMS applied to this area.35 We used a valid sham magnet, a cross-over double-blind design treatment protocol, and matched for hearing loss by having each patient serve as their own control.
METHODS
Design
This was a cross-over, double-blind, randomized clinical trial. Eligible patients were adults between the ages of 18 and 60 with subjective, unilateral or bilateral, non-pulsatile tinnitus of 6 month’s duration or greater, a score of 38 or greater on the Tinnitus Handicap Inventory (THI)46, and less than 14 on the Beck Depression Inventory.47 Patients who remained eligible at the end of the screening period were randomized to receive as first treatment either active or sham rTMS. The project biostatistician was responsible for generating the randomization code. Block randomization was used with blocks of size 4, 6, and 8, such that for any block half of the patients were randomly assigned to active treatment before placebo and the other half to placebo before active treatment. The research nurse coordinator enrolled the patients. Patients were randomized to receive either active rTMS for 2 weeks and then switch to sham rTMS for 2 weeks or start with sham rTMS for 2 weeks and then active rTMS for 2 weeks. The washout period between the two interventions was a minimum of 2 weeks. Prior to starting the next intervention after the washout period, the participant’s tinnitus severity was re-assessed. To ensure no carryover effect, the washout period was extended for those patients whose tinnitus severity, as defined by the THI, was more than 20 points different than their baseline THI score.
Measurements
All patients completed the following Oregon Hearing Research Center [http://www.tinnitusarchive.org/forms] forms at enrollment: Tinnitus Description and History, Medical and Health Information, and Hearing History and Occupational Exposure. Patients also completed the Brief Symptom Inventory – 18 (BSI-18)48, Beck Depression Inventory – II (BDI – II)47, and the Mini-Mental Status Exam (30-item version).49 Neurocognitive assessment included: California Verbal Learning Test (CVLT)50, Controlled Oral Word Association Test (COWAT)51, Semantic Association Test (SAT)52, Trails A & B53, Grooved Pegboard54, and the Digit Symbol Substitution Task.55
All patients underwent audiometric and neurocognitive assessment, PET, and MRI scans upon completion of each intervention arm. The hearing level was tested at the following 4 frequencies: 500, 1000, 2000, and 3000 hertz.
rTMS Procedure
The Neuronetics Model 2100 Therapy System investigational device (Neuronetics Inc., Malvern, Pennsylvania) and Gharieni Patient Chair was used in this study. Three separate magnetic coils (two active and one sham), similar in weight, external appearance, and acoustic properties when actively pulsed, were used. One active coil was unblinded and used as the coil to determine motor thresholds. The Neuronetics Model 2100 CRS sham coil is created by modifying an active coil by internally interposing an aluminum plate between the magnet pole faces and the external surface that touches the patient’s head. The aluminum acts to redirect the magnetic flux away from the patient and back into the coil assembly. In addition, the sham coil is driven at a fixed level of 45% of the stimulator output, which acoustically matches the sound created when driving an active coil at an average treatment setting. The resulting magnetic field in the center of the stimulation volume (i.e. 2 cm from the scalp surface into the cortex) is reduced to less that 10% of that produced by an active coil driven at 100% stimulator output, which is well below levels required for cortical neuron stimulation. The sham coil is designed to be identical in appearance and external design as the active treatment coil, with the only distinguishing visible characteristic being a label identifying it as an “A”, “B” or “C”- type coil. The resulting sound and percussive effect are virtually indistinguishable to the patient or operator.
The Gharieni Patient Chair has the capability to be adjusted to allow for various patient positions from sitting to lying supine. The chair has a removable head support system which allows for additional patient comfort. The custom boom-arm system attaches to a wheeled cart on top of which sits the power-delivery console. The arm and its coil-holding attachment are custom designed to allow positioning of the coil over the temporoparietal cortex region. Without the arm, the rTMS machine is limited in motion and does not allow enough axial rotation to enable treatment on the temporoparietal cortex region of the brain. The system was designed especially for this clinical trial. The motor threshold was determined by stimulating the motor cortex with a dedicated motor threshold magnet to elicit a reproducible response (1/2 of the time or more) in the right abductor brevis pollicis (thumb abductor).
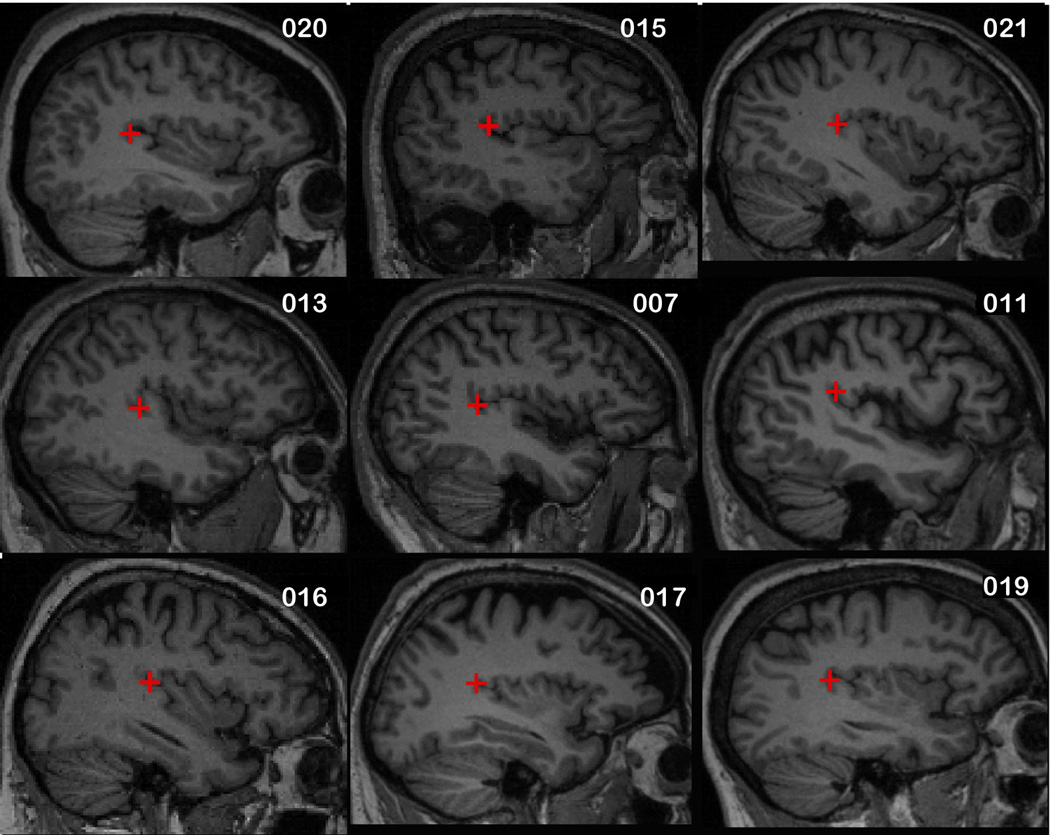
The stimulation site was over the left temporoparietal junction (TPJ), regardless of side of tinnitus laterality or baseline PET scan results. The magnet placement for the first five subjects was determined using surface EEG coordinates, a location midway between C3 and T5 based on 10–20 system of EEG electrode placement. For the remaining 9 subjects, the TPJ was identified by one of the co-authors (MM) using the Analyze software (BIR, Mayo Clinic, Rochester, MN, USA) from the structural MRI. (Figure 1). The coordinates of the TPJ were then determined by the software program using a standard coordinate system. A surface image of the face was then created using the Volume Render feature of Analyze. The location of the stimulation site in relation to the surface of the scalp was then determined by the software. The Angle Measure feature was used to describe the angle created by the junction of a line from the surface landmark for the stimulation site to the center of the external auditory canal (EAC) and a second line from the lateral canthus of the left eye to the center of the EAC. The length, in mm, of these lines was determined by a trained neuroimaging research coordinator and used to calculate the height and the length of the placement template. The placement of the magnet was at the top corner of the template representing the stimulation site.
Figure 1.
Sagital MRI View With Marker Overlying Left Temporoparietal Junction.
Patients received 5 stimuli (active rTMS or sham) sessions per week (one per day) for 2 weeks. The stimulation settings were 1Hz at 110% of motor threshold for a total of 42½ minutes (330 seconds per train for the first 5 trains with the last train 350 seconds, with 90 seconds off between trains). The volume of stimulated cortex is determined by coil position and drive level. When the coil is placed firmly against the scalp, at a drive level of 120% of the average motor threshold, the cross sectional area of the stimulation volume at a depth of 2 cm from the scalp surface into the cortex is approximately 3 cm × 6 cm.
All patients and research personnel were blinded to magnet status (active versus sham). To assess the success of blinding, patients were asked after completion of each intervention arm to guess which magnet, active or sham, they believe they had just received.
Analysis
The main analysis of this study was to evaluate the impact of rTMS on the perception of tinnitus. The use of a sham and cross-over design allowed for each patient to serve as their own control. The null hypothesis was that active rTMS is no more effective than sham rTMS in the treatment of tinnitus.
The primary efficacy measure was defined as the change in the Tinnitus Handicap Inventory score between active rTMS and sham rTMS and was defined as follows:
Change in Tinnitus Handicap Inventory score after active rTMS ΔTHIactive = THIactive before − THIactive after
Change in Tinnitus Handicap Inventory score after sham rTMS ΔTHIsham = THIsham before − THIsham after
Primary Efficacy Parameter Δ(ΔTHI) = ΔTHIactive − ΔTHIsham
To assess whether the observed difference in the change in THI was statistically significant, we used a Wilcoxon Signed Ranks Test with p-value adjusted for number of comparisons. Statistical significance was defined as a p –value ≤ 0.05. Clinical significance was defined as a change in the THI of 20 points or greater(JFP personal communication with S. Jacobson, one of the developers of the Tinnitus Handicap Inventory).
The secondary efficacy measures included the Patient Global Impression of Change (PGIC) assessment performed at the end of each intervention arm. In addition, patients were asked if they would continue treatment and if they would recommend this treatment to a friend. And finally, the change in BSI-18 and BDI-II scores after each intervention arm was assessed.
The data was captured on paper-based forms and transferred to a specially created SPSS dataset using double data entry to ensure data accuracy. Standard descriptive statistics were used to describe the study population and outcomes of interest. Where appropriate, 95% confidence intervals were calculated.
Sample Size and Power
All sample size and power computations were based on a cross-over design and two-sided tests at the α = 0.05 level of significance. Anticipated characteristics of this population and study design, including standard deviation in the change in THI, were estimated from data of 115 patients enrolled in the randomized placebo-controlled clinical trial of gabapentin conducted previously at Washington University.56 Since we desired to not only reject the null hypothesis but to also obtain convincing confidence bounds for the size of the true effect, an expected confidence interval length based on a proposed sample size of 50 was computed. The confidence interval, based on a sample size of 50, is expected to be of the form Δ(ΔTHI) ± 4.8. Even allowing for natural variation in the point estimate of the effect, this interval will be well bounded away from the null value of 0.
RESULTS
Patient’s characteristics
Potential patients were identified through the Washington University School of Medicine Research Participant Registry or responded to an on-line web site (tinnitus.wustl.edu). Of the 285 people interested in participating in the study, 22 passed the initial phone screening process and were consented to participate in the study. The flow of patients through the study is shown in Figure 2. Of the 22 patients who were screened, 14 of them passed the screening process and were enrolled and randomized between August, 2008 and July, 2009. All 14 patients completed both arms of treatment and the 4-week follow-up assessment. Only one participant missed one day of treatment due to an unrelated household accident. No participant was lost to follow-up or refused to answer questions. Of the 8 who failed screening, 5 had scores on the Beck Depression Inventory greater than 14, which is suggestive of depression. A motor threshold could not be elicited for one subject, one subject became claustrophobic in the MRI, and one subject changed his mind mid point in screening and chose not to continue with the screening process.
Figure 2.
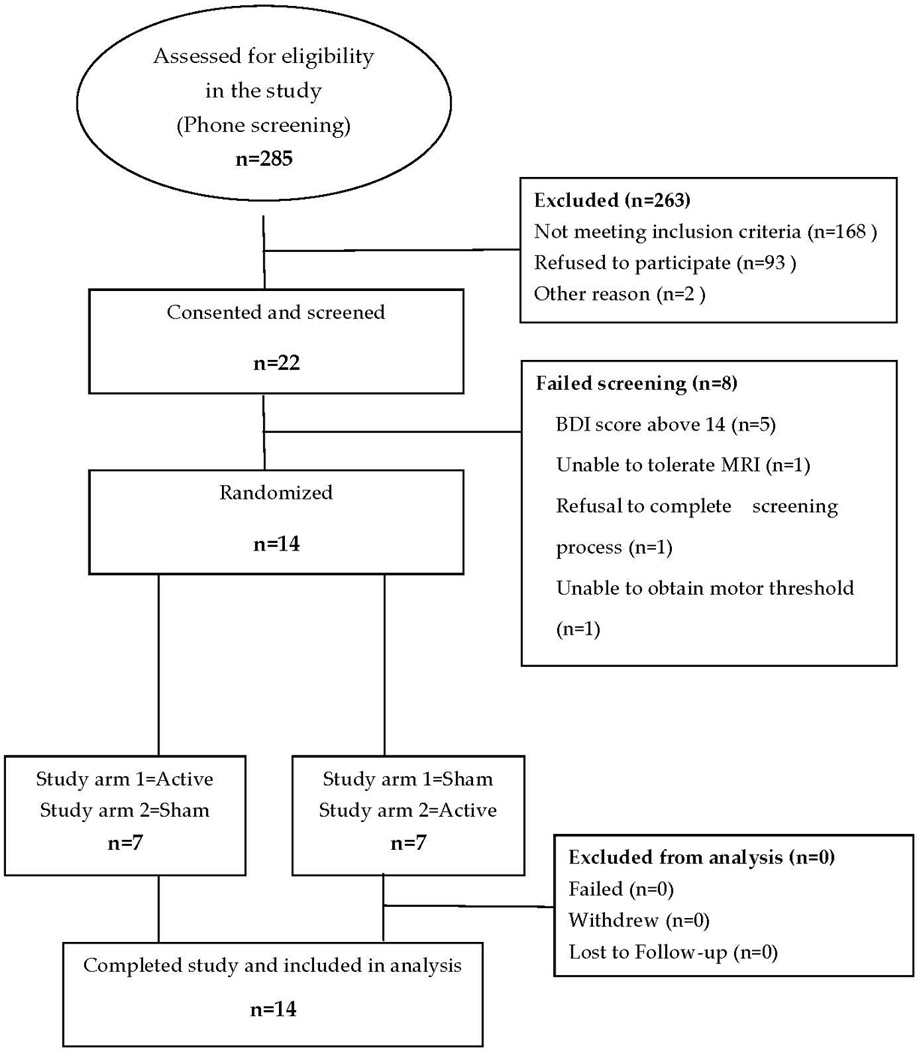
Flow Diagram of Participation.
The 14 enrolled patients are described in Table 1. The patients (median age 52 ±5.1 years, 4 female) had tinnitus for at least 6 months (median 7.0 ± 5.1 years, range 0.5–18 years) with an average loudness of 7.5 ± 1.2 (on a 1–10 visual analog scale). Tinnitus interfered with sleep for 13 of 14 patients. Tinnitus was bilateral in 9 and unilateral in 5 patients (3 right, 2 left). None had hyperacusis. Pure tone thresholds (PTT) and speech reception thresholds (SRT) were binaurally similar (PTT right: mean 23.5 ± 22 dB, range 0–70 dB; left: mean 17.9 ± 14.9 dB, range 0–75 dB; SRT right: mean 18.2 ± 19.9 dB, range 0–80 dB; left: mean 12.4 ± 8.5 dB, range 0–25 dB). The audiometric examination of the 14 patients revealed normal hearing in 4 (29%) and hearing loss in 10 patients. The hearing loss was slight in 5 (36%), moderate in 3 (21%), and severe in 2(14%) patients. Patients were excluded who had clinical depression (a score of 14 or greater on the Beck Depression Inventory-II)47 or other psychiatric or neurologic disorders.
Table 1.
Description of Study Population at Baseline
| Characteristics | Value |
|---|---|
| Demographics | |
| Age, Median (Min–Max) | 52 (42–59) |
| Gender | |
| Male | 10 (71%) |
| Female | 4 (29%) |
| Race | |
| White | 13 (93%) |
| Other (Native-American +White) | 1 (7%) |
| Tinnitus Characteristics at Baseline | |
| Duration, years Median(Min–Max) | 7.0 (0.5–17.9) |
| Loudness, Median(Min–Max) | 7.5 (5.0–9.0) |
| Interference with sleep | |
| Yes, Often | 7 (50%) |
| Yes, Sometimes | 6 (43%) |
| No | 1 (7%) |
| Effort to ignore | |
| Can never ignore | 4 (29%) |
| Considerable effort | 6 (43%) |
| Some effort | 3 (21%) |
| Slight effort | 1 (7%) |
| Discomfort | |
| Great deal of discomfort | 4 (29%) |
| Moderate discomfort | 6 (43%) |
| Mild discomfort | 1 (7%) |
| Very mild discomfort | 2 (14%) |
| No discomfort | 1 (7%) |
| Previous sought medical help | |
| Yes | 10 (71%) |
| No | 4 (29%) |
| Audiometric parameters | |
| Type of Hearing Loss | |
| None | 4 (29%) |
| Sensorineural | 10 (71%) |
| Severity of Hearing Loss | |
| None (−10 dB to 15 dB) | 4 (29%) |
| Slight (16 dB to 25 dB) | 5 (36%) |
| Moderate (41 dB to 55 dB) | 3 (21%) |
| Severe (71 dB to 90dB) | 2 (14%) |
| Configuration of Audiogram | |
| Flat | 3 (21%) |
| Gradually Sloping | 4 (29%) |
| Sharply Sloping | 3 (21%) |
| Precipitously Sloping | 3 (21%) |
| Notch | 1 (8%) |
The distribution of scores on the various self-assessment instruments is shown in Table 2. The median (95% CI) baseline THI score was 51 (40 to 70). The median (95% CI) for THI scores post treatment and post sham were 43 (28 to 66) and 47 (30 to 62), respectively. The median (95% CI) THI score at 4-week follow-up was 45 (34 to 66).
Table 2.
Comparison of THI, BSI-18, BDI-II Assessed at Different Time Points in the Study
| Baseline Median(95% CI) |
Post Treatment Median(95% CI) |
Post Sham Median(95% CI) |
4-week follow up Median(95% CI) |
|
|---|---|---|---|---|
| THI | 51 (40 to 70) | 43 (28 to 66) | 47 (30 to 62) | 45 (34 to 66) |
| BSI-18 | 3.0 (2 to 5) | 1.5 (0 to 4) | 1.5 (0 to 5) | 2.5 (1 to 4) |
| BDI-II | 5.0 (1 to 8) | 1.5 (1 to 5) | 2.0 (0 to 5) | 1.0 (0 to 7) |
THI score before active treatment was equal to baseline THI for patients that were randomized to receive active treatment first, and was equal to post wash-out THI for patients that received active treatment in the second arm of the study. The same reasoning was used to assess THI before sham. Pre-sham THI was equal to baseline THI score if sham was the first arm of treatment, and was equal to post wash-out THI when sham was the second arm of treatment.
The distribution of change in THI scores after active and sham treatment is shown in Figure 3. The active treatment reduced the THI score with a median reduction (median ΔTHIactive) of 5 points and 95% CI (0 to 14). The change in THI score post sham (ΔTHIsham) had a median reduction in THI of 6 points and 95% CI (−2 to 12). The Primary Efficacy Parameter Δ(ΔTHI) = ΔTHIactive − ΔTHIsham, ranged from as low as 34 points reduction in THI score after active treatment when compared to THI score after sham, to an increase of 22 points, with a median Δ(ΔTHI) reflecting an increase of only 1 point and 95% CI around the median (−6 to 4). There was no relationship between hearing loss and changes in THI scores.
Figure 3.
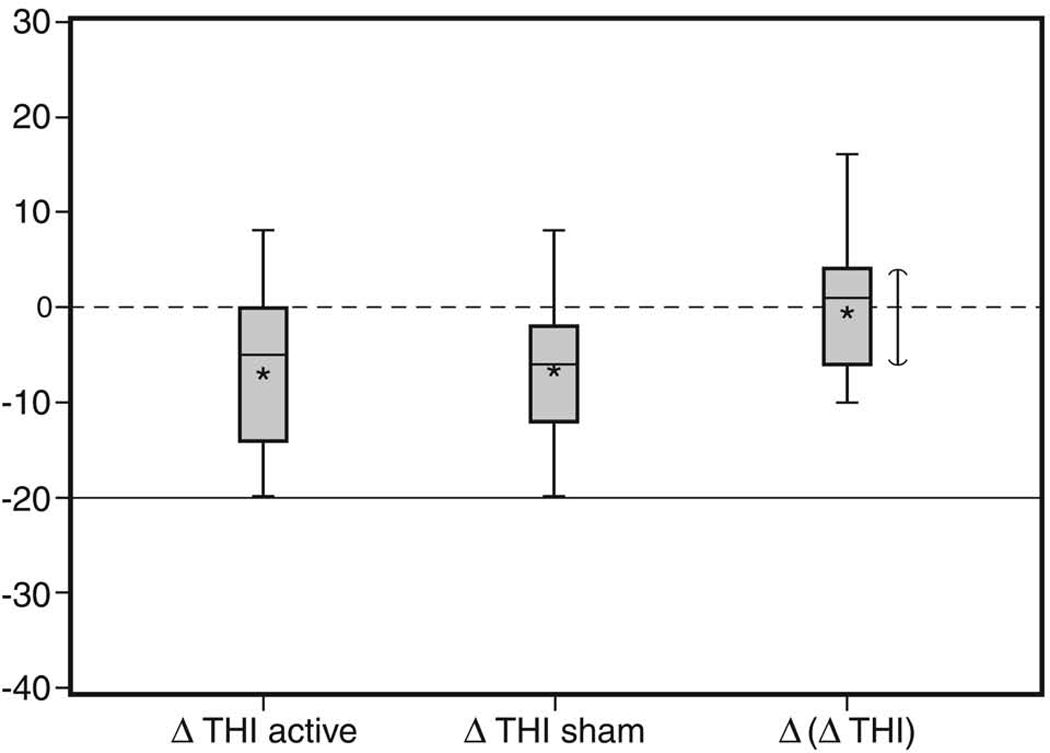
Box And Whisker Plot Of Change In THI Score
Footnote: Asterisks report mean, horizontal black line represents median, and upper and lower boundaries of box represent 75th and 25th percentile. Horizontal dash and solid lines represent statistical and clinical significant levels, respectively. Vertical line represents 95% confidence interval for the median.
Secondary efficacy parameters included the overall response to treatment assessed at the end of each treatment arm. “Poor” response to active treatment was reported by 12 (86%) patients and 11 (79%) at the end of sham (Figure 4). The number of patients that reported “Fair” was the same 2(14%) after active and sham treatment. “Good” response was reported by only 1 (7%) subject post sham.
Figure 4.
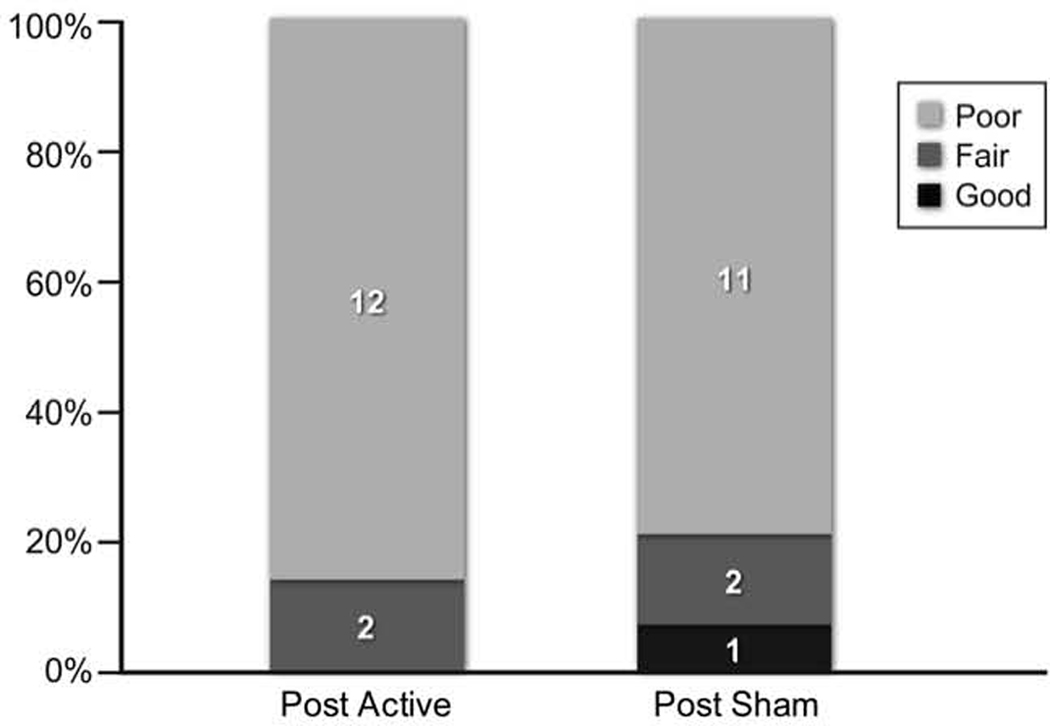
Comparison of Patient’s Global Impression of Change Post-Active and Post-Sham Treatment.
The patients’ median Body Symptom Index-18 score was 3.0, 95% CI (2 to 5) at baseline, 1.5 (0 to 4) after active treatment, and 1.5 (0 to 5) after sham treatment. The median Beck Depression Index at baseline was 5 with 95% CI (1 to 8) and dropped to 1.5 (1 to 5) after active treatment and 2.0 (0 to 5) after sham. None of these differences were statistically significant.
All patients were asked the following question “All persons have their own unique problems and attach different importance to these problems. Please indicate the overall amount of disturbance or "bother" that you experience in your life as a result of your tinnitus” at baseline, after each intervention, and at 4-week follow-up. As is shown in Figure 5, the number of patients reporting to be “Extremely Bothered” varied from 2 (14%) at baseline to a maximum of 3 (21%) post active treatment arm, to only 1 (7%) post sham and 4-week follow-up assessment. “Bothered a lot” was reported from 7 (50%) patients at baseline, 6 (43%) post-active treatment and 7 (50%) post sham. When asked if they would recommend this treatment to a friend, 4 (29%) responded “Definitely Yes”, 6 (43%) ”Maybe Yes”, 3 (21%) “Unsure”, and only 1 (7%) answered “Maybe No”.
Figure 5.
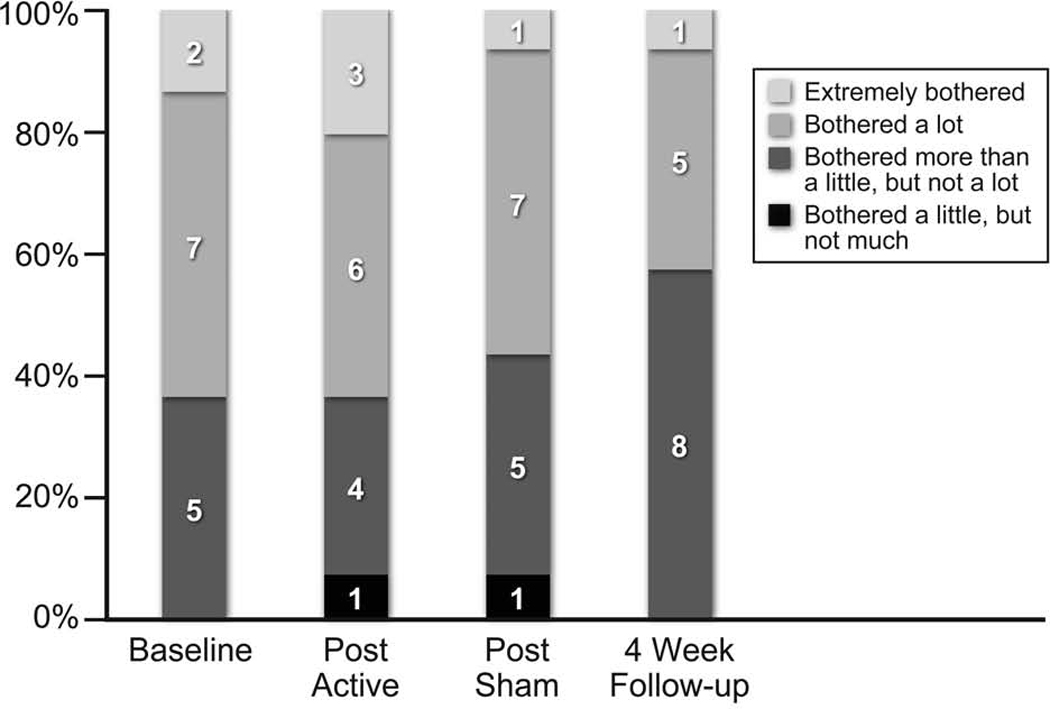
Comparison of Patients’ Response to the question “Please Indicate The Overall Amount Of Disturbance Or "Bother" That You Experience In Your Life As A Result Of Your Tinnitus” at Different Time-Points in the Study.
To assess the quality of the sham, patients were asked at the end of Arms 1 and 2 what was their “best guess” of which treatment, active or sham, they believe they received. Interestingly, all but one of the patients (93%) guessed that they received sham during Arm 1. When the same question was asked at the end of study (end of Arm 2), 3 of them changed their guess from sham in the first arm, to active treatment in the first arm and sham in the second. When the patients’ guess, at the end of study, was compared to the actual treatment received, only 50% of the patients guessed the correct arm to which they were randomized. This result was not different from what would be expected by chance alone, thus confirming the successful nature of the sham magnet.
There were no serious adverse events reported in this study (Table 3). The most common adverse events included jaw twitch (5 mild and 1 moderate) and neck or shoulder tightness or twitch (4 mild and 1 severe). The number of adverse events reported were greater during the active treatment (11) then during sham (8), but this difference was not statistically significant (p = 0.42).
Table 3.
Description of Adverse Events
| Severity | Number of Patients |
|---|---|
| Mild | |
| Jaw twitch | 5 |
| Neck or shoulder tightness or twitch | 4 |
| Facial twitch | 3 |
| Headache | 2 |
| Bilateral eye twitching | 1 |
| Facial tingling | 1 |
| Jaw joint pain | 1 |
| Lightheadedness | 1 |
| Moderate | |
| Tinnitus louder/worse after TMS | 2 |
| Facial twitch | 2 |
| Jaw twitch | 1 |
| Arm twitch | 1 |
| Temple pain | 1 |
| Severe | |
| Shoulder muscle twitch | 1 |
DISCUSSION
The primary goal of this study was to examine the effectiveness and safety of applying low-frequency rTMS in a cohort of patients with bothersome tinnitus. The target site was the temporoparietal junction. In this study we found that two weeks of low-frequency rTMS to the left temporoparietal cortex was no more effective than placebo for the treatment of chronic bothersome tinnitus. Because response to rTMS treatment was so poor, the trial biostatistician recommended early termination of the trial and no additional patients be recruited. All patients tolerated active and sham rTMS without significant complaints and there were no drop-outs or withdrawals.
These results are at odds with prior reports in the published literature35–44,57,58 and may reflect differences in treatment site and duration of stimulation, efficacy of sham, and patient selection.57 All prior studies reporting positive results in the treatment of tinnitus with rTMS and targeted auditory cortex lacked adequate controls or did not guard against placebo effects. Unlike these prior studies, we used a cross-over double-blind protocol with active and sham treatments, which enabled within patient controls. Especially important, we used a sham magnet that looked, felt, and sounded like the active magnet. Post-treatment reports indicated no probable placebo effects as patients were unable to reliably discern any differences between active and sham treatments. Additionally, the procedures used to align the magnet position for different patients and across sessions were not clearly described in prior studies, making reproduction of their results nearly impossible. The template used in the present study had great accuracy for magnet placement across patients and sessions. The template was created by an experienced radiology technician under the direction of one of the authors (MM). Given the size of the stimulation field, we feel confident that the intended auditory cortex stimulation was achieved.
It is possible that the duration of treatment used in this protocol was too short and longer duration of treatment is needed. Previous clinical studies in depression59–62 suggest that longer stimulation is more effective than shorter duration. To address this issue, our current protocol extends treatment to four weeks of active and sham rTMS. Another possibility is that our patients were too severely bothered to obtain relief from rTMS alone. A THI score of 38 or greater represents the 75th percentile of bother (JFP personal communication with S. Jacobson). Furthermore, our results are not generalizable to most tinnitus patients as we had to screen 285 people to obtain 22 who were so severely bothered (THI score ≥ 38) yet were not depressed (Beck Depression Index < 14). In addition, rTMS localized to a single site may not be effective for tinnitus because the neurocognitive symptoms reflect more widespread alterations in cortical networks. Consequently, treatments suited for localized brain areas might not effect a sufficient alteration in synaptic circuits involved in a broad-based network. And finally, since the THI has only three response categories, (Yes, No, Sometimes), real changes in tinnitus may not have been detected. While missing a true effect is possible, we do not believe it was a likely explanation for our results as we also failed to detect a significant impact of treatment based on our secondary outcomes.
We believe rTMS is a very promising tool for the treatment of tinnitus, but more basic, clinical, and translational research is needed to identify the correct treatment parameters. Future studies may significantly benefit from bringing emerging imaging techniques and stimulation protocols together to determine the most optimal site for targeting rTMS stimulation. Finally, administration of neuropsychological assessments may be a profitable avenue of investigation into the attentional factors that may be interfering with severely bothered patients’ ability to habituate to their tinnitus in the manner that other tinnitus patients achieve.
Acknowledgment
The principal investigator (JFP) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This research was supported by a grant from the National Institutes of Deafness and Other Communication Disorders (R01 DC009095)
Footnotes
This work was presented at the 4th International Tinnitus Research Initiative Tinnitus Conference, Dallas, Texas, June 10, 2010
Trial Registration: NCT00567892 http://clinicaltrials.gov/
REFERENCES
- 1.Lenarz T, Schreiner C, Snyder RL, Ernst A. Neural mechanisms of tinnitus. European Archives of Oto-Rhino-Laryngology. 1993;249:441–446. doi: 10.1007/BF00168851. [DOI] [PubMed] [Google Scholar]
- 2.Kaltenbach JA. Neurophysiologic mechanisms of tinnitus. Journal of the American Academy of Audiology. 2000;11:125–137. [PubMed] [Google Scholar]
- 3.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear.Res. 1998;117:149–160. doi: 10.1016/s0378-5955(98)00008-2. [DOI] [PubMed] [Google Scholar]
- 5.Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear.Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 6.Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear.Res. 2003;183:137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- 7.Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL. 1996;58:195–199. doi: 10.1159/000276835. [DOI] [PubMed] [Google Scholar]
- 8.Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 9.Muhlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giraud AL, Chery-Croze S, Fischer G, et al. A selective imaging of tinnitus. NeuroReport. 1999;10:1–5. doi: 10.1097/00001756-199901180-00001. [DOI] [PubMed] [Google Scholar]
- 11.Mirz F, Pedersen B, Ishizu K, et al. Positron emission tomography of cortical centers of tinnitus. Hear. Res. 1999;134:133–144. doi: 10.1016/s0378-5955(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 12.Andersson G, Lyttkens L, Hirvela C, Furmark T, Tillfors M, Fredrikson M. Regional cerebral blood flow during tinnitus: a PET case study with lidocaine and auditory stimulation. Acta Oto-Laryngologica. 2000;120:967–972. doi: 10.1080/00016480050218717. [DOI] [PubMed] [Google Scholar]
- 13.Gardner A, Pagani M, Jacobsson H, et al. Differences in resting state regional cerebral blood flow assessed with 99mTc-HMPAO SPECT and brain atlas matching between depressed patients with and without tinnitus. Nuclear Medicine Communications. 2002;23:429–439. doi: 10.1097/00006231-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Mirz F, Ovesen T, Ishizu K, et al. Stimulus-dependent central processing of auditory stimuli: a PET study. Scandinavian Audiology. 1999;28:161–169. doi: 10.1080/010503999424734. [DOI] [PubMed] [Google Scholar]
- 15.Mirz F, Gjedde A, Ishizu K, Pedersen CB. Cortical networks subserving the perception of tinnitus--a PET study. Acta Otolaryngol Suppl. 2000;543:241–243. doi: 10.1080/000164800454503. [DOI] [PubMed] [Google Scholar]
- 16.Mirz F, Gjedde A, Sodkilde-Jrgensen H, Pedersen CB. Functional brain imaging of tinnitus-like perception induced by aversive auditory stimuli. Neuroreport. 2000;11:633–637. doi: 10.1097/00001756-200002280-00039. [DOI] [PubMed] [Google Scholar]
- 17.Adjamian P, Sereda M, Hall DA. The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hear.Res. 2009;253:15–31. doi: 10.1016/j.heares.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Melcher JR, Sigalovsky IS, Guinan JJ, Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–1072. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- 19.Melcher JR, Levine RA, Bergevin C, Norris B. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear. Res. 2009;257:63–74. doi: 10.1016/j.heares.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanting CP, De K, Bartels H, Van D. Functional imaging of unilateral tinnitus using fMRI. Acta Oto-Laryngologica. 2008;128:415–421. doi: 10.1080/00016480701793743. [DOI] [PubMed] [Google Scholar]
- 21.Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llinas R, Ribary U, Jeanmonod D, et al. Thalamocortical dysrhythmia I. Functional and imaging aspects. Thalmus & Related Systems. 2001;1:237–244. [Google Scholar]
- 23.Hallam RS, Rachman S, Hinchcliffe R. Psychological aspects of tinnitus. In: Rachman S, editor. Contributions to Medical Psychology, vol. 3. Oxford: Pergamon; 1984. pp. 31–54. [Google Scholar]
- 24.Hallam RS, McKenna L, Shurlock L. Tinnitus impairs cognitive efficiency. International Journal of Audiology. 2004;43:218–226. doi: 10.1080/14992020400050030. [DOI] [PubMed] [Google Scholar]
- 25.Stevens C, Walker G, Boyer M, Gallagher M. Severe tinnitus and its effect on selective and divided attention. International Journal of Audiology. 2007;46:208–216. doi: 10.1080/14992020601102329. [DOI] [PubMed] [Google Scholar]
- 26.Delb W, Strauss DJ, Low YF, et al. Alterations in Event Related Potentials (ERP) associated with tinnitus distress and attention. Applied Psychophysiology & Biofeedback. 2008;33:211–221. doi: 10.1007/s10484-008-9065-y. [DOI] [PubMed] [Google Scholar]
- 27.Budd RJ, Pugh R. The relationship between locus of control, tinnitus severity, and emotional distress in a group of tinnitus sufferers. Journal of Psychosomatic Research. 1995;39:1015–1018. doi: 10.1016/0022-3999(95)00512-9. [DOI] [PubMed] [Google Scholar]
- 28.Newman CW, Wharton JA, Jacobson GP. Self-focused and somatic attention in patients with tinnitus. Journal of the American Academy of Audiology. 1997;8:143–149. [PubMed] [Google Scholar]
- 29.Folmer RL, Griest SE, Meikle MB, Martin WH. Tinnitus severity, loudness, and depression. Otolaryngol.Head Neck Surg. 1999;121:48–51. doi: 10.1016/S0194-5998(99)70123-3. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds P, Gardner D, Lee R. Tinnitus and psychological morbidity: a cross-sectional study to investigate psychological morbidity in tinnitus patients and its relationship with severity of symptoms and illness perceptions. Clinical Otolaryngology & Allied Sciences. 2004;29:628–634. doi: 10.1111/j.1365-2273.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- 31.Vanneste S, Plazier M, der LE, de Heyning PV, Congedo M, De RD. The neural correlates of tinnitus-related distress. Neuroimage. 2010;52:470–480. doi: 10.1016/j.neuroimage.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 33.Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 34.Chen R, Tam A, Butefisch C, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. Journal of Neurophysiology. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- 35.Plewnia C, Bartels M, Gerloff C. Transient suppression of tinnitus by transcranial magnetic stimulation. Annals of Neurology. 2003;53:263–266. doi: 10.1002/ana.10468. [DOI] [PubMed] [Google Scholar]
- 36.Langguth B, Eichhammer P, Wiegand R, et al. Neuronavigated rTMS in a patient with chronic tinnitus. Effects of 4 weeks treatment. NeuroReport. 2003;14:977–980. doi: 10.1097/00001756-200305230-00014. [DOI] [PubMed] [Google Scholar]
- 37.Eichhammer P, Langguth B, Marienhagen J, Kleinjung T, Hajak G. Neuronavigated repetitive transcranial magnetic stimulation in patients with tinnitus: a short case series. Biological Psychiatry. 2003;54:862–865. doi: 10.1016/s0006-3223(02)01896-6. [DOI] [PubMed] [Google Scholar]
- 38.Kleinjung T, Eichhammer P, Langguth B, et al. Long-term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngology - Head & Neck Surgery. 2005;132:566–569. doi: 10.1016/j.otohns.2004.09.134. [DOI] [PubMed] [Google Scholar]
- 39.De Ridder D, Verstraeten E, Van der KK, et al. Transcranial magnetic stimulation for tinnitus: influence of tinnitus duration on stimulation parameter choice and maximal tinnitus suppression. Otology & Neurotology. 2005;26:616–619. doi: 10.1097/01.mao.0000178146.91139.3c. [DOI] [PubMed] [Google Scholar]
- 40.Fregni F, Marcondes R, Boggio PS, et al. Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. European Journal of Neurology. 2006;13:996–1001. doi: 10.1111/j.1468-1331.2006.01414.x. [DOI] [PubMed] [Google Scholar]
- 41.Folmer RL, Carroll JR, Rahim A, Shi Y, Hal MW. Effects of repetitive transcranial magnetic stimulation (rTMS) on chronic tinnitus. Acta Oto-Laryngologica Supplement. 2006;126:96–101. doi: 10.1080/03655230600895465. [DOI] [PubMed] [Google Scholar]
- 42.Plewnia C, Reimold M, Najib A, Reischl G, Plontke SK, Gerloff C. Moderate therapeutic efficacy of positron emission tomography-navigated repetitive transcranial magnetic stimulation for chronic tinnitus: a randomised, controlled pilot study. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78:152–156. doi: 10.1136/jnnp.2006.095612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi S, De Capua A, Ulivelli M, et al. Effects of repetitive transcranial magnetic stimulation on chronic tinnitus. A randomised, cross over, double blind, placebo-controlled study. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:857–863. doi: 10.1136/jnnp.2006.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langguth B, Kleinjung T, Marienhagen J, et al. Transcranial magnetic stimulation for the treatment of tinnitus: effects on cortical excitability. BMC Neurosci. 2007;8:45. doi: 10.1186/1471-2202-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Londero A, Langguth B, De RD, Bonfils P, Lefaucheur JP. Repetitive transcranial magnetic stimulation (rTMS): a new therapeutic approach in subjective tinnitus? Neurophysiol.Clin. 2006;36:145–155. doi: 10.1016/j.neucli.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Archives of Otolaryngology -- Head & Neck Surgery. 1996;122:143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 48.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- 49.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 50.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (CVLT) Adult version (Research ed.) San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 51.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1989. [Google Scholar]
- 52.Visch-Brink EG, Denes G. A European Base-line Test for Word-Picture Processing. In: Stachowiak FJ, editor. Developments in the assessment and rehabilitation of brain-damaged patients. Tubingen: Gunter Narr Verlag; 1993. [Google Scholar]
- 53.Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Percept.Motor Skills. 1958;8:271–276. [Google Scholar]
- 54.Klove H. Clinical neuropsychology. In: Forster FM, editor. The medical clinics of North America. New York: Saunders; 1963. pp. 1647–1658. [PubMed] [Google Scholar]
- 55.Wechsler D. Wechsler Adult Intelligence Scale - III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 56.Piccirillo JF, Finnell J, Vlahiotis A, Chole RA, Spitznagel E., Jr Relief of idiopathic subjective tinnitus: is gabapentin effective? Archives of Otolaryngology -- Head & Neck Surgery. 2007;133:390–397. doi: 10.1001/archotol.133.4.390. [DOI] [PubMed] [Google Scholar]
- 57.Marcondes RA, Sanchez TG, Kii MA, et al. Repetitive transcranial magnetic stimulation improve tinnitus in normal hearing patients: a double-blind controlled, clinical and neuroimaging outcome study. Eur.J.Neurol. 2010;17:38–44. doi: 10.1111/j.1468-1331.2009.02730.x. [DOI] [PubMed] [Google Scholar]
- 58.Khedr EM, bo-Elfetoh N, Rothwell JC, El-Atar A, Sayed E, Khalifa H. Contralateral versus ipsilateral rTMS of temporoparietal cortex for the treatment of chronic unilateral tinnitus: comparative study. Eur.J.Neurol. 2010;17:976–983. doi: 10.1111/j.1468-1331.2010.02965.x. [DOI] [PubMed] [Google Scholar]
- 59.Couturier JL. Efficacy of rapid-rate repetitive transcranial magnetic stimulation in the treatment of depression: a systematic review and meta-analysis. J Psychiatry Neurosci. 2005;30:83–90. [PMC free article] [PubMed] [Google Scholar]
- 60.Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. Psychiatr.Pract. 2002;8:270–275. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Lam RW, Chan P, Wilkins-Ho M, Yatham LN. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can.J Psychiatry. 2008;53:621–631. doi: 10.1177/070674370805300909. [DOI] [PubMed] [Google Scholar]
- 62.Loo CK, Mitchell PB. A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. J.Affect.Disord. 2005;88:255–267. doi: 10.1016/j.jad.2005.08.001. [DOI] [PubMed] [Google Scholar]