Abstract
Background
Risperidone long-acting injectable was previously approved for treatment of schizophrenia as biweekly injections in the gluteal muscle only. We present data on local injection-site tolerability and safety of risperidone long-acting injectable and comparability of systemic exposure of deltoid versus gluteal injections.
Methods
Risperidone long-acting injectable was administered in an open-label, single-dose, two-way crossover study, with patients randomized to receive either 25mg gluteal/37.5mg deltoid crossover in two treatment periods or 50mg gluteal/50mg deltoid injections crossover; each treatment period was separated by an 85-day observation period (Study 1) and an open-label, multiple-dose study (4 sequential 37.5mg or 50mg deltoid injections every 2 weeks) (Study 2). The pharmacokinetic results from both the studies have already been published.
Results
In Study 1 (n=170), the majority of patients had no local injection-site findings, based on investigator and patient-rated evaluations. In Study 2 (n=53), seven of the 51 patients who received at least two deltoid injections discontinued (primary endpoint). However, none of the discontinuations were due to injection-site related reasons. The 90-percent upper confidence limit of the true proportion of injection-site issue withdrawals was 5.7 percent. No moderate or severe injection-site reactions were reported.
Conclusion
Intramuscular injections via the deltoid and gluteal sites are equivalent routes of administration of risperidone long-acting injectable with respect to local injection-site tolerability. The overall safety and tolerability profile of risperidone long-acting injectable was comparable when administered as an intramuscular injection in the deltoid (37.5mg and 50mg) and gluteal (25mg and 50mg) sites.
Keywords: Atypical antipsychotic, deltoid injections, gluteal injections, injection-site reactions, risperidone long-acting injectable, safety, tolerability
Introduction
The principal goals of treatment in schizophrenia are to achieve sustained relief from psychotic symptoms, reduce relapse rates, and to improve patient functioning and quality of life.1,2 Since the achievement of remission requires long-term maintenance treatment over a sustained time period,3 adherence is of paramount importance in view of the fact that even a brief hiatus in treatment can be detrimental, leading to a worsening in symptoms and, ultimately, relapse and rehospitalization.4–7 For this reason, the guidelines for long-acting antipsychotic treatment developed by a European Neuropsychopharmacology Consensus Conference recommend that, “Any patient for whom long-term antipsychotic treatment is indicated should be considered for long-acting agents.”8
Long-acting injectable (LAI) formulations of second-generation antipsychotics provide continuous medication delivery and reduced plasma drug-level fluctuations compared with oral agents and, importantly, enable more accurate monitoring of medication adherence.8 Patients failing to return for scheduled injections are readily identified, thereby allowing earlier intervention to prevent further worsening and escalation of psychotic symptoms.8 In a systematic review of patient preference for route of medication administration, in five out of the six studies analyzed, the majority of patients preferred the injectable formulation to the oral formulation.9 Of the different sites for administering an injection, the deltoid muscle of the arm is considered less intrusive and embarrassing for a patient due to its ease of accessibility. Thus, the option to be able to administer treatment via a deltoid versus gluteal injection may further aid patient adherence and acceptance.
Risperidone LAI, the long-acting injectable formulation of risperidone, combines the benefits of an atypical antipsychotic agent with the advantages of a long-acting formulation, and allows physicians and other healthcare providers to more rapidly detect patient nonadherence. Risperidone LAI is approved for the treatment of schizophrenia in many countries and for the treatment of bipolar I disorder in the United States (Risperdal® Consta® Prescribing Information, 2010). Previously, the only approved route of administration of risperidone LAI was intramuscular in the gluteal muscle. We have previously reported the pharmacokinetic results of the two studies10 that demonstrated the bioequivalence of equal doses of risperidone LAI following deltoid or gluteal administration and dose-proportional pharmacokinetics. The results from these served as the basis for the recent approvals of risperidone LAI deltoid administration in a number of countries worldwide including the United States, European Union, and Canada. Here we present the injection-site tolerability results of deltoid versus gluteal injection as well as the investigator-rated assessments of injection-site reactions, the patient-rated assessments of injection-site reactions, and other safety findings for both the studies.
Methods
Patients. In both studies, physically healthy patients of either sex aged 18 to 55 years, body mass index (BMI) of 18 to 35kg/m2 and fulfilling Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnostic criteria for schizophrenia were enrolled. No changes in medication or dosage were permitted during the four weeks before the screening visit, and eligible patients were required to have a Clinical Global Impressions (CGI) scale score of ≤3 (mild) in Study 1 and ≤4 in Study 2. In addition, in Study 2, patients should have been treated with at least two gluteal injections of risperidone LAI (25 or 37.5mg) and were in need of a dose increase for clinical reasons.
The exclusion criteria for both studies included a DSM-IV Axis I diagnosis other than schizophrenia, the presence of a substance dependence disorder, or any acute unstable or significant concomitant medical illness, a history of neuroleptic malignant syndrome, or an allergy to risperidone or other constituents of the formulation. In addition, patients were excluded if they were considered by the investigator to have inadequate muscle mass within the gluteal or deltoid regions to receive the intramuscular injections. Women who were not practicing an effective method for birth control and those who were pregnant or nursing were also excluded.
Both studies were conducted in accordance with the Good Clinical Practice Guidelines and the Declaration of Helsinki and its revisions. The study protocols and informed consent documents were all reviewed and approved by the Independent Ethics Committees. All patients provided informed consent before participation in either study, and after the study procedures, risks, benefits, and alternatives to participation had been fully explained.
Study design. Study 1 was a randomized, multicenter, open-label, single-dose, two-way crossover study consisting of a pretreatment screening phase (≤21 days), two treatment phases, and an end-of-study evaluation, either upon completion of all study-related procedures or following early discontinuation.
During the treatment periods, patients were randomized to one of two parallel treatment panels to receive a single intramuscular injection of risperidone LAI followed by an 85-day observation period, during which patients continued ongoing approved oral antipsychotic treatment. Patients assigned to Panel I received 25mg risperidone LAI via the gluteal muscle or 37.5mg risperidone LAI via the deltoid muscle and then were crossed over to the alternate treatment in the subsequent treatment period. Patients assigned to Panel II received 50mg risperidone LAI via the gluteal muscle or 50mg risperidone LAI via the deltoid muscle and then were crossed over in the subsequent treatment period.10
Study 2 was a multicenter, open-label, multiple-dose study, consisting of a pretreatment screening phase (≤14 days); an eight-week, open-label treatment phase, during which patients received four sequential injections of 37.5 or 50mg risperidone LAI, administered every two weeks into the deltoid muscle in alternate arms for each visit; and an end-of-study evaluation, either upon completion of all study procedures or following early discontinuation.10
Doses of risperidone LAI could be adjusted within the 37.5 to 50mg range for efficacy or general tolerability but not for reasons related to local injection-site tolerability. Patients with local injection-site issues were discontinued due to lack of tolerability. Patients continued their previous oral antipsychotic medication, if it was not excluded per protocol. To evaluate tolerability, patients needed to have a minimum of two risperidone LAI deltoid injections (to account for the learning process of receiving injections into a site other than the gluteus muscle).
Study drug administration. In both studies, risperidone LAI was administered using 2mL of diluent with a 21-gauge (1-inch) needle for injection into the deltoid muscle, and a 20-gauge (2-inch) needle for injection into the gluteus muscle. In Study 2, risperidone LAI injections were administered in alternate arms.
Tolerability and safety assessments. Local injection-site reactions were evaluated by the investigators and by the patients separately. Redness, swelling, tenderness, and induration were evaluated and rated by investigators 30 minutes before risperidone LAI injection and two hours post-injection. The evaluation was recorded using a rating scale of 0 to 3 (0=absent, 1=mild, 2=moderate, and 3=severe). In Study 1, investigators performed injection-site evaluations at 12- and 24-hours post injection and on Days 3 and 15 after each injection, and in Study 2, investigators performed their evaluations at study completion or at early discontinuation. In both studies, patient-rated local injection-site pain was assessed separately using a 100mm visual analogue scale (VAS) (0mm=no pain and 100mm=unbearable pain) and was evaluated at the same time points as the investigator-rated assessments.
The frequency, severity, and duration of all treatment-emergent adverse events (TEAEs) and their relationship to the study medication were recorded throughout both studies. Physical examinations and electrocardiograms (ECGs) were recorded at screening and at study completion or early discontinuation. Vital signs (e.g., temperature, pulse rate, respiratory rate, and blood pressure) were assessed at screening and at regular intervals throughout both studies. Clinical laboratory tests (e.g., serum chemistry, hematology, and urinalysis) were conducted at screening and at study completion or early discontinuation, and before dosing in both periods of Study 2. The clinical laboratory tests were performed at a central laboratory (Covance Central Laboratory Services [Indianapolis for sites in the United States and Geneva, Switzerland for sites in Europe]). The frequency of extrapyramidal symptoms (EPS) on the EPS Rating Scale (ESRS) was also assessed.
Statistical analysis. In Study 1, approximately 150 patients were planned for enrollment to ensure that 130 patients completed all study-related evaluations. In Study 2, for the tolerability issues related to the intramuscular nature of the injection, a sample size of 50 patients was estimated to produce a 90-percent confidence interval (CI) with a precision of 0.12, assuming a discontinuation rate of 0.5. With a corresponding point estimate of 50 percent (0.5 discontinuation rate or 50% of patients completing the study) and precision of 0.12, the lower limit of the CI would be approximately 38 percent. This was considered clinically acceptable and a positive outcome. In Study 2, approximately 60 patients were screened in order to ensure that 50 patients completed the second deltoid injection with risperidone LAI.
Patients who received at least one injection of risperidone LAI were included in the safety population. Investigator-rated local injection-site reactions were analyzed descriptively at each time point. Changes from baseline in patient-rated local injection-site pain (VAS scores) were calculated and summarized.
Clinical laboratory test data were summarized using descriptive statistics at baseline and at the end of the study, and changes from baseline results were calculated. The effects of risperidone LAI on cardiovascular variables were evaluated by means of descriptive statistics and frequency tabulations, including changes from baseline values. All important abnormalities from the ECG readings were reported.Vital signs were summarized descriptively by parameter and time point, and included change from baseline; values for individual patients were compared with normal reference ranges and predefined criteria for orthostatism. Any changes in the results of the physical examinations between screening and the end of the study or early withdrawal were recorded. If an anticholinergic medication or any other medication was prescribed for EPS, including akathisia, or such a medication was increased in dosage, an additional ESRS assessment was to be completed. The ESRS scores and subscores were tabulated and analyzed descriptively.
Results
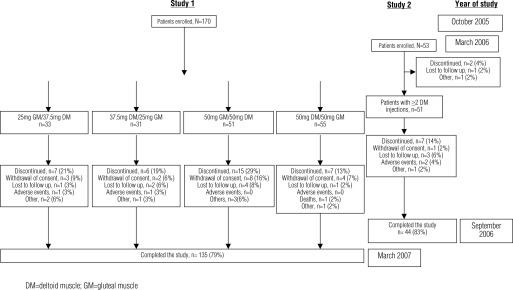
Patient disposition, demographics and baseline characteristics. Study 1 was conducted between October 2005 and March 2007 at seven sites in the United States (64% of patients) and five sites in Europe (36% of patients; 2 sites in Poland, 2 sites in Spain and 1 site in Estonia). A total of 170 patients were enrolled and 135 (79%) patients completed the study receiving both the planned single injections of risperidone LAI into the gluteal and deltoid muscle (Figure 1).
Figure 1.
Patient accounting
Study 2 was conducted between March 2006 and September 2006 at nine sites in the United States. Fifty-three patients were enrolled in the study, 51 (96%) patients received two or more injections of risperidone LAI and 44 (83%) patients completed the study (Figure 1).
Patient demographics and baseline characteristics for both studies were similar (Table 1). In Study 1, patient characteristics were well balanced across the four treatment sequence groups; overall, 66 percent of patients were men, 61 percent were white, and the mean age was 41 years. In Study 2, 72 percent of patients were men, 62 percent were white, and the mean age was 42 years.
Table 1.
Patient demographics and baseline characteristics
| DEMOGRAPHICS | STUDY 1 | STUDY 2 | ||||
|---|---|---|---|---|---|---|
| Total N=170 |
25mg GM/ 37.5mg DM n=33 |
37.5mg DM/ 25mg GM n=31 |
50mg GM/ 50mg DM n=51 |
50mg DM/ 50mg GM n=55 |
Total N=53 |
|
| Gender, n (%) | ||||||
| Men Women |
112 (66) 58 (34) |
18 (55) 15 (45) |
24 (77) 7 (23) |
32 (63) 19 (37) |
38 (69) 17 (31) |
38 (72) 15 (28) |
| Race, n (%) | ||||||
| White Black Other |
104 (61) 64 (38) 2 (1) |
18 (55) 15 (45) 0 (0) |
18 (58) 13 (42) 0 (0) |
39 (76) 12 (24) 0 (0) |
29 (53) 24 (44) 2 (4) |
33 (62) 18 (34) 2 (4)* |
| Age, years | ||||||
| Mean (SD) Median (range) |
41.4 (9.1) 42.5 (21;57) |
43.0 (8.1) 43.0 (21;55) |
41.2 (10.1) 43.0 (22;56) |
40.4 (9.9) 42.0 (21;55) |
41.5 (8.3) 43.0 (24;57) |
42.0 (10.9) 44.0 (19;59) |
| Weight, kg | ||||||
| Mean (SD) Median (range) |
85.4 (16.7) 85.8 (51;132) |
88.0 (16.3) 88.0 (54;128) |
89.3 (19.8) 88.5 (54;132) |
80.4 (13.8) 80.7 (51;108) |
86.2 (16.8) 84.8 (55;124) |
91.3 (17.6) 89.1 (61;127) |
| Height, cm | ||||||
| Mean (SD) Median (range) |
173.4 (9.9) 174.0 (148;203) |
173.7 (11.2) 175.3 (148;196) |
174.2 (9.2) 173.5 (159;196) |
173.4 (10.4) 173.0 (156;203) |
172.6 (9.2) 174.0 (148;191) |
175.0 (9.3) 175.3 (155;198) |
| BMI, kg/m2 | ||||||
| Mean (SD) Median (range) |
28.3 (4.5) 29.0 (19;38) |
29.0 (4.2) 29.4 (21;36) |
29.2 (4.7) 30.3 (19;35) |
26.8 (4.2) 26.2 (21;35) |
28.9 (4.7) 29.7 (20;38) |
29.8 (4.9) 30.6 (20;38) |
| Diagnosis, n (%) | ||||||
| Paranoid Disorganized Residual Undifferentiated |
151 (89) 1 (1) 5 (3) 13 (8) |
29 (88) 1 (3) 1 (3) 2 (6) |
27 (87) 0 0 4 (13) |
44 (86) 0 4 (8) 3 (6) |
51 (93) 0 0 4 (7) |
51 (96) 2 (4) 0 0 |
| Age at diagnosis, yrs | ||||||
| Mean (SD) Median (range) |
27.6 (10.0) 26.0 (5;54) |
27.9 (9.9) 25.0 (12;50) |
29.0 (10.6) 27.0 (15;54) |
25.7 (9.9) 22.5 (5;52) |
28.5 (9.9) 27.0 (7;49) |
25.5 (9.9) 23.0(12,48) |
| CGI scale score, n (%)** | ||||||
| Mild Moderate |
NR | NR | NR | NR | NR | 15 (28) 38 (72) |
For race in Study 2, “Other” includes Asian and others.
In Study 2, by definition of the inclusion criteria, all patients had a Clinical Global Impression scale score of 3 or less (i.e., mild or lower).
- GM
- gluteal muscle
- DM
- deltoid muscle
- SD
- standard deviation
- BMI
- body mass index
- NR
- not recorded
Tolerability and Safety
Tolerability evaluation. There were no discontinuations related to injection-site tolerability in Study 1, and the upper limit of the true proportion of withdrawals due to injection-site issues was 5.7 percent with 90-percent confidence. Fifty-one patients in Study 2 received at least two injections into the deltoid muscle and were eligible for evaluation of tolerability. Of these, 44 (86%) completed the study, considered a clinically acceptable and positive outcome (a positive outcome was defined a priori as 50 percent or more of patients continuing among those who received at least two intramuscular deltoid injections with risperidone LAI) for the study.
Investigator-rated assessments of injection-site reactions. Of the 170 patients enrolled in Study 1, injection-site reactions were rated as mild in 51 (30%) patients, and as moderate in nine (5%) patients. There were no patients with injection-site reactions that were rated as severe by the investigator (Table 2). The proportion of patients with mild or moderate injection-site reactions was lower with risperidone LAI gluteal muscle injections (25mg: 12%; 50mg: 14%) than with deltoid muscle injections (37.5mg, 21%; 50mg, 27%) (Table 2). The most common injection-site reaction was tenderness (risperidone LAI gluteal injections—25mg and 50mg: 10% each; deltoid injections—37.5mg: 18%; 50mg: 19%). Redness was observed in three percent of patients receiving 25mg and six percent of patients receiving 50mg of the risperidone LAI gluteal muscle injections; and in five percent of patients receiving 37.5mg and eight percent of patients receiving 50mg of the risperidone LAI deltoid muscle injections. There were few reports of swelling or induration at either injection site. Injection-site reactions were most frequent at two and 12 hours post injection, and resolved thereafter, with almost no injection-site reactions reported beyond Day 3 (except for one occurrence of redness reported at the end of the study following the 50mg risperidone LAI deltoid muscle injection).
Table 2.
Investigator-rated assessments of injection-site reactions in Study 1, by severity, at any time point
| SEVERITY OF REACTIONS |
Total n (%) N=170 |
25mg GM n (%) n=60 |
37.5mg DM n (%) n=57 |
50mg GM n (%) n=103 |
50mg DM n (%) n=96 |
|---|---|---|---|---|---|
| Mild Moderate |
51 (30) 9 (5) |
7 (12) 0 |
12 (21) 2 (4) |
14 (14) 3 (3) |
26 (27) 5 (5) |
None of the reactions were rated as severe by the investigator at any time point
- GM
- gluteal muscle
- DM
- deltoid muscle
In Study 2, investigators reported mild injection-site reactions at two hours post-injection in a maximum of 10 (19%) of the 53 patients (Table 3). Importantly, scores returned to ‘absent’ at the preinjection assessment of the next injection administered two weeks later. No moderate or severe injection-site reactions were reported, and no clinically relevant differences were observed between patients who received either 50mg or 37.5mg risperidone LAI.
Table 3.
Investigator-rated assessments of mild injection-site reactions in Study 2 by reaction type at 2 hours post-injection
| STUDY 2 (N=53) | ||||
|---|---|---|---|---|
| VISIT | INDURATION | REDNESS | SWELLING | TENDERNESS |
| Day 1 n Mild |
53 1 |
53 3 |
53 0 |
53 6 |
| Day 15 n Mild |
51 1 |
51 1 |
51 1 |
51 10 |
| Day 29 n Mild |
46 0 |
46 5 |
46 0 |
46 7 |
| Day 43 n Mild |
45 0 |
45 0 |
45 0 |
45 6 |
All reactions were ‘mild’ in severity and disappeared by next visit; No moderate or severe reactions were reported.
Patient-rated assessments of injection-site reactions. In Study 1, patient-rated assessments of pain at the injection-sites, based on VAS scores, indicated that for the majority of patients there were no or minimal changes at two (Table 4), 12, and 24 hours from baseline at either the gluteal or deltoid muscle injection sites. The median change from baseline was 0mm at all time points. Injection-site pain was low overall. The highest mean values for injection-site pain recorded by patients (scale of 0–100mm) were 1.30mm (25mg) and 1.56mm (50mg) for the risperidone LAI gluteus muscle injections; and 3.56mm (37.5mg) and 1.89mm (50mg) for the risperidone LAI deltoid muscle injections. The mean changes from baseline (predose on Day 1) were highest at two and 12 hours postdose and normalized thereafter. For patients who indicated that injection-site pain had increased compared with baseline, maximum changes in VAS scores were lowest for the 25mg risperidone LAI gluteus muscle injection (28.0mm), and comparable for the 37.5mg risperidone LAI deltoid muscle injections (50.0mm), the 50mg risperidone LAI gluteus muscle injections (41.0mm) and the 50mg risperidone LAI deltoid muscle injections (47.0mm). Individual patient VAS scores for patient-rated injection-site pain were similar to baseline values on Day 3 for greater than 90 percent of patients.
Table 4.
Mean patient-rated changes in injection-site pain values from baseline to 2 hours post-injection in Study 1 (100mm VAS scores)
| 25mg GM n (%) n=60 |
37.5mg DM n (%) n=57 |
50mgGM n (%) n=103 |
50mg DM n (%) n=96 |
|
|---|---|---|---|---|
| Change from baseline at 2 hrs n Mean (SD) |
60 1.1(3.3) |
57 3.4(9.6) |
102 0.5(2.2) |
96 1.1 (5.9) |
Injection-site pain was overall very low.
- DM
- deltoid muscle
- GM
- gluteal muscle
- VAS
- visual analog scale
In Study 2, patient-rated assessments of pain at the injection-site on the VAS score (scale of 0–100mm) showed mean pain values of 2.4mm at baseline, and within a range of 4.2 to 6.1mm at two hours post injection. Mean increases in pain values from pre-dose to post-dose were approximately 1 to 3mm in patients who received 37.5mg risperidone LAI, and approximately 5 to 10mm (slightly higher) in patients who received 50mg risperidone LAI. For the majority of the patients, mean pain values returned to baseline levels at the pre-dose assessment of the next injection two weeks later (no assessments in between).
Injection site-related treatment-emergent adverse events. In Study 1, the incidence of potential injection site-related TEAEs was low (≤2%) for all treatments and similar between the gluteus (25 and 50mg) and deltoid muscle (37.5 and 50mg) injection groups. The injection site-related TEAEs were all of mild severity, and there were no patient discontinuations due to these TEAEs in either of the studies. In Study 2, injection-site pain and injection-site reaction were reported as adverse events by 7.5 and 1.9 percent of patients, respectively.
Overall treatment-emergent adverse events. In Study 1, 109 (64%) patients reported one or more TEAE(s) during the study.10 The most frequently reported TEAEs were headache (15%) and nasopharyngitis (12%), which were generally considered to be mild in severity (Table 5). The overall tolerability of risperidone LAI was similar for the deltoid and gluteal sites (Table 3). Ten (6%) patients had serious TEAEs, including two patients who died. The two serious TEAEs (psychotic disorder [n=1]; moderate cough with pharyngolaryngeal pain and hydrocodone toxicity [n=1]) leading to death were considered by the investigators to be either not related (patient with psychotic disorder) or doubtfully related (patient with hydrocodone toxicity) to study drug administration. The patient with psychotic disorder completed suicide approximately 1.5 months after withdrawing from the study. Two patients were withdrawn from the study due to serious TEAEs (subdural hematoma and psychotic disorder).
Table 5.
Treatment-emergent adverse events reported by ≥5% of patients in either of the studies
| TEAEs | STUDY 1 | STUDY 2 | ||||
|---|---|---|---|---|---|---|
| Total n (%) N=170 |
25mg GM n (%) n=60 |
37.5mg DM n (%) n=57 |
50mg GM n (%) n=103 |
50mg DM n (%) n=96 |
Total n (%) N=53 |
|
| Total patients with TEAE (s) | 109 (64.1) | 30 (50.0) | 28 (49.1) | 48 (46.6) | 47 (49.0) | 21 (39.6) |
| Headache | 25 (14.7) | 10 (16.7) | 8 (14.0) | 7 (6.8) | 9 (9.4) | 0 (0) |
| Nasopharyngitis | 21 (12.4) | 5 (8.3) | 5 (8.8) | 5 (4.9) | 6 (6.3) | 0 (0) |
| Injection-site pain | 2 (1.2) | 0 (0) | 0 (0) | 1 (1.0) | 1 (1.0) | 4 (7.5) |
| Upper respiratory tract infection | 10 (5.9) | 3 (5.0) | 2 (3.5) | 3 (2.9) | 2 (2.1) | 0 (0) |
| Insomnia | 10 (5.9) | 0 (0) | 1 (1.8) | 5 (4.9) | 7 (7.3) | 1 (1.9) |
| Fatigue | 7 (4.1) | 2 (3.3) | 3 (5.3) | 1 (1.0) | 3 (3.1) | 3 (5.7) |
| Sedation | 1 (0.6) | 0 (0) | 0 (0) | 1 (1.0) | 0 (0) | 3 (5.7) |
| Anxiety | 9 (5.3) | 1 (1.7) | 0 (0) | 5 (4.9) | 3 (3.1) | 0 (0) |
| Toothache | 7 (4.1) | 3 (5.0) | 1 (1.8) | 1 (1.0) | 3 (3.1) | 0 (0) |
| Tachycardia | 7 (4.1) | 0 (0) | 3 (5.3) | 3 (2.9) | 4 (4.2) | 0 (0) |
- DM
- deltoid muscle
- GM
- gluteal muscle
- TEAE
- treatment-emergent adverse event
In Study 2, 21 (39.6%) patients reported one or more TEAEs during the study. The most frequently reported TEAEs (occurring in >5% of patients) were injection-site pain (7.5%), fatigue (5.7%), and sedation (5.7%) (Table 5). There were no deaths reported in this study. Two (4%) patients had serious TEAEs (worsening of schizophrenia). For one patient, the event resolved after concomitant administration of oral risperidone while for the other patient, it resolved after concomitant oral risperidone and oral haloperidol administration and increase of subsequent deltoid risperidone LAI injections dose to 50mg. Two (4%) patients discontinued due to a TEAE: one patient experienced mild sedation and another patient experienced mild EPS. As sedation and EPS are expected adverse events associated with risperidone use, these discontinuations are unlikely to be related to the mode of risperidone administration (injection into the deltoid muscle site).
Extrapyramidal Symptom Rating Scale scores and other clinical assessments. In Study 1, mean total ESRS scores tended to decrease (improve) during the study for all treatment groups. The mean (SE) changes from baseline in total ESRS scores on Day 36 for the risperidone LAI gluteal muscle injection groups were as follows: 25mg, −0.16 (0.34) and 50mg, −0.67 (0.21). The mean (SE) changes from baseline in total ESRS scores on Day 36 for the risperidone LAI deltoid muscle injection groups were as follows: 37.5mg, −0.02 (0.296) and 50mg, −0.16 (0.197). Overall, there were no clinically relevant differences from baseline to study endpoint in mean clinical laboratory values, vital signs, ECG parameters, or physical examinations in either study (data not shown). In Study 2, there was a slight improvement in the mean total ESRS score from baseline to study endpoint; the mean (SE) change was −0.35 (0.21). In Study 1, mean (SE) creatine kinase values increased by 38.40U/L (17.69U/L) from baseline to the end of the study. There were no TEAEs related to heart rate and rhythm in either study. Mean (SE) weight increased from baseline to the end of the study by 1.39kg (0.38kg) in Study 1 and 0.92kg (0.38kg) in Study 2. In Study 2, mean (SE) creatine kinase values increased by 59.67U/L (37.25U/L) from baseline to the end of the study.
Discussion
The results from these two studies demonstrate that injection of risperidone LAI into the deltoid muscle is generally well tolerated when administered either as single or multiple doses. The pharmacokinetic analyses from these studies have been published separately and demonstrated the bioequivalence of the deltoid to gluteal muscle injection at equal doses and the interchangeability of the injection site in terms of drug exposure.10 In Study 2, which was designed to primarily evaluate the tolerability of deltoid muscle administration after repeated doses, there were no patients who discontinued the study due to reasons related to the injection site. The upper limit of the true proportion of withdrawals due to injection-site issues was very low, with a value of 5.7 percent (with 90% confidence), which was considered a clinically acceptable outcome for this study.
The investigator reported the injection-site reactions as mild in 51 (30%) patients and as moderate in nine (5%) patients in Study 1 and as mild reactions in a maximum of 10 (19%) patients at two hours post-injection in Study 2. Injection-site reactions that did occur were primarily mild and none were classified as severe. Study drug injection sites for the majority of patients revealed no redness, swelling, tenderness, or induration at either the deltoid muscle or gluteal muscle injection sites. Patient-evaluated injection-site pain scores were generally low. Pain associated with the administration of risperidone LAI into the deltoid muscle, if any, increased at two hours after injection and for the majority of patients returned to baseline values by the next injection assessment. These findings are comparable with a previous report11 examining pain at the gluteal injection site following risperidone LAI injection, which noted either minimal or no injection-site pain.
The proportion of patients with injection-site reactions for the gluteal muscle injection was lower than for the deltoid muscle injections in Study 1. It is important to note, however, that all of these were considered to be only mild or moderate in severity, transient in duration, and that the overall frequency of any of these reactions was low.
Based on TEAE observations of injection-site reactions in Study 1 (1.2%), the tolerability of risperidone LAI was generally similar for all dosage strengths regardless of injection site. In both studies, the nature and number of TEAEs was consistent with the previous safety profile of risperidone LAI.11–15 Furthermore, no patients discontinued the treatment for reasons related to the injection site from either trial. The incidence of TEAEs with risperidone LAI deltoid muscle injection was low and there were very few serious TEAEs or withdrawals due to TEAEs, which was also comparable with the safety profile of risperidone LAI following gluteal muscle injection.11 Moreover, deltoid muscle and gluteal muscle injection of risperidone LAI at the same doses are interchangeable in terms of drug exposure.10
The low incidence of injection-site reactions and injection-site pain observed in the studies presented here is likely related to risperidone LAI's formulation, an isotonic and water-based suspension, which is easier to administer and less painful for the patient than the oil-based solvents used in conventional long-acting antipsychotic agents.16 The aqueous suspension of risperidone LAI has been associated with minimal pain, induration, and inflammation at the gluteal muscle injection site and injection-site reactions are uncommon (1–7% of patients).11,13,17,18
The principal limitation of these studies is their relatively brief duration; up to four injections in Study 1 (eight weeks) and up to two injections in Study 2 (each one followed by a 12-week observation period). These studies were designed to assess the nature and characteristics of a patient's tolerability to single and multiple injections into the deltoid muscle and to characterize the pharmacokinetic profile of these interventions. In addition, as there was no placebo control in either study, it is not possible to confirm whether aspects related to tolerability occurred as a result of the route of administration or the study medication itself.
These findings compare favorably with those reported in studies evaluating conventional long-acting antipsychotic agents.19,20 Overall, the results from both studies presented here indicate that there were no clinically relevant differences in the safety and tolerability of risperidone LAI injected into the deltoid muscle compared with gluteal muscle injections. Importantly, there were no clinically significant changes from baseline to the study endpoint for any of the clinical laboratory parameters, ECG, vital signs, or physical examination. In both studies, there was a trend for the mean total ESRS scores to decrease, indicating improvement during the study for all treatment groups.
Based on the safety and local tolerability results presented in both the single- and multiple-dose studies across all dosages, injection of risperidone LAI into the deltoid muscle can offer patients with schizophrenia an alternative route of administration to the previously approved gluteal muscle injection site. This alternative may be potentially less intrusive for patients with schizophrenia, improving ease of access to the injection site for the treating clinician and also enhance patient comfort.
Acknowledgment
The study investigators, including the principal investigators Dr. M. A. Bari (Synergy Clinical Research Center, National City, USA—Study 1) and Dr. M. Marandi (Comprehensive Neuroscience, Cerritos, USA—Study 2) are acknowledged for conducting the clinical aspects of the study.
The authors would like to thank Frances Gambling, Medicus International for her editorial assistance. Dr. Madhavi Patil and Dr. Lakshmi Venkatraman (SIRO Clinpharm Pvt. Ltd.) and Dr. Wendy P. Battisti (Johnson & Johnson Pharmaceutical Research & Development, L.L.C.) provided additional editorial support for this manuscript. Editorial assistance was funded by Johnson & Johnson Pharmaceutical Research & Development.
References
- 1.Carpenter WT, Jr., Hanlon TE, Heinrichs DW, et al. Continuous versus targeted medication in schizophrenic outpatients: outcome results. Am J Psychiatry. 1990 Sep;147(9):1138–1148. doi: 10.1176/ajp.147.9.1138. [DOI] [PubMed] [Google Scholar]
- 2.Herz MI, Glazer WM, Mostert MA, et al. Intermittent vs maintenance medication in schizophrenia. Two-year results. Arch Gen Psychiatry. 1991;48(4):333–339. doi: 10.1001/archpsyc.1991.01810280049007. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen NC, Carpenter WT, Jr., Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 4.Kane JM. Schizophrenia. N Engl J Med. 1996;334(1):34–41. doi: 10.1056/NEJM199601043340109. [DOI] [PubMed] [Google Scholar]
- 5.Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308–1315. doi: 10.4088/jcp.v64n1105. [DOI] [PubMed] [Google Scholar]
- 6.Weiden P, Glazer W. Assessment and treatment selection for “revolving door” inpatients with schizophrenia. Psychiatr Q. 1997;68(4):377–392. doi: 10.1023/a:1025499131905. [DOI] [PubMed] [Google Scholar]
- 7.Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886–891. doi: 10.1176/appi.ps.55.8.886. [DOI] [PubMed] [Google Scholar]
- 8.Kane JM, Aguglia E, Altamura AC, et al. Guidelines for depot antipsychotic treatment in schizophrenia. European Neuropsychopharmacology Consensus Conference in Siena, Italy. Eur Neuropsychopharmacol. 1998;8(1):55–66. doi: 10.1016/s0924-977x(97)00045-x. [DOI] [PubMed] [Google Scholar]
- 9.Walburn J, Gray R, Gournay K, et al. Systematic review of patient and nurse attitudes to depot antipsychotic medication. Br J Psychiatry. 2001;179:300–307. doi: 10.1192/bjp.179.4.300. [DOI] [PubMed] [Google Scholar]
- 10.Thyssen A, Rusch S, Herben V, et al. Risperidone long-acting injection: pharmacokinetics following administration in deltoid versus gluteal muscle in schizophrenic patients. J Clin Pharmacol. 2010;50(9):1011–1021. doi: 10.1177/0091270009355156. [DOI] [PubMed] [Google Scholar]
- 11.Lindenmayer JP, Eerdekens E, Berry SA, Eerdekens M. Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry. 2004;65(8):1084–1089. [PubMed] [Google Scholar]
- 12.Turner M, Eerdekens E, Jacko M, Eerdekens M. Long-acting injectable risperidone: safety and efficacy in stable patients switched from conventional depot antipsychotics. Int Clin Psychopharmacol. 2004;19(4):241–249. doi: 10.1097/01.yic.0000133500.92025.20. [DOI] [PubMed] [Google Scholar]
- 13.Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250–1257. doi: 10.4088/jcp.v64n1017. [DOI] [PubMed] [Google Scholar]
- 14.Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160(6):1125–1132. doi: 10.1176/appi.ajp.160.6.1125. [DOI] [PubMed] [Google Scholar]
- 15.Lindenmayer JP, Jarboe K, Bossie CA, et al. Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol. 2005;20(4):213–221. doi: 10.1097/00004850-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bloch Y, Mendlovic S, Strupinsky S, et al. Injections of depot antipsychotic medications in patients suffering from schizophrenia: do they hurt? J Clin Psychiatry. 2001;62(11):855–859. doi: 10.4088/jcp.v62n1104. [DOI] [PubMed] [Google Scholar]
- 17.Eerdekens M, Van Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res. 2004;70(1):91–100. doi: 10.1016/j.schres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Lasser R, Bossie CA, Gharabawi G, et al. Efficacy and safety of long-acting risperidone in stable patients with schizoaffective disorder. J Affect Disord. 2004;83(2–3):263–275. doi: 10.1016/j.jad.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Hamann GL, Egan TM, Wells BG, Grimmig JE. Injection site reactions after intramuscular administration of haloperidol decanoate 100mg/mL. J Clin Psychiatry. 1990;51(12):502–504. [PubMed] [Google Scholar]
- 20.Hay J. Complications at site of injection of depot neuroleptics. BMJ. 1995;311(7002):421. doi: 10.1136/bmj.311.7002.421. [DOI] [PMC free article] [PubMed] [Google Scholar]