Abstract
Background: Dermatology visits for the prevention and treatment of aging skin are rapidly increasing. The clinical sequelae including wrinkling, pigmentary changes, roughness, laxity, and telangiectasia can all result in the appearance of aging skin, impacting quality of life. A facial serum was developed with ingredients associated with an improvement in the appearance of fine lines and wrinkles and increase in stratum corneum barrier function. Patients were instructed to use a gentle wash before applying the formulation and a moisturizer afterwards. Objective: To assess the efficacy and tolerability of a facial serum in improving the appearance of fine lines, wrinkles, and signs of photodamage. Methods: Thirty-four female subjects (Fitzpatrick classification I–IV) with early to advanced photodamaged skin in a 12-week, single-arm, open-label clinical trial. Visits were scheduled at Baseline and Weeks 4, 8, and 12. Efficacy was assessed using visual grading of facial and periocular skin (modified 10-point scales); changes in viscoelasticity properties were assessed by cutometry. Cutaneous tolerability was evaluated both clinically and subjectively using a 4-point scale and monitoring adverse events. Digital photography documented treatment-related changes in skin appearance. Subjects completed self-assessments at Baseline and Weeks 4, 8, and 12. Results: Significant improvements in all parameters and skin condition were seen as early as Week 4 (p≤0.05). There was an 18-percent improvement in overall appearance by Week 12 (p≤0.05). Fine lines and coarse winkles improved by 27 and 15 percent, respectively (both p≤0.05). Significant improvements were also seen in uneven pigmentation, firmness/elasticity, toned/resiliency, skin radiance, tone, and tactile roughness/smoothness (10%, 11%, 18%, 21%, 16%, and 47%, respectively; allp≤0.05). By Week 12 subjects reported a 43-percent improvement in overall facial skin appearance and 24-percent reduction in mean scores for facial lines and wrinkles (bothp≤0.05). Improvements were also reported in overall skin tone, firmness, dryness, appearance of pores, appearance of brown spots/facial discoloration, skin radiance, and texture (37%, 35%, 35%, 28%, 24%, 39%, 38%, respectively; allp≤0.05). There was a 71-percent reduction in erythema and 94-percent reduction in skin dryness by Week 12 (both p≤0.05). Conclusion: The facial serum, in combination with the wash and moisturizer, may be effective and well-tolerated when treating photodamaged skin and may improve the appearance of fine lines and wrinkles. Significant improvements were seen with all grading parameters as early as four weeks of usage. A controlled study is warranted to further validate these findings.
Dermatology visits for the prevention and treatment of aging skin are rapidly increasing. Since 1997, the number of interventions for facial photodamage in the United States has increased 293 percent—with an 87-percent increase in surgical procedures and a 471-percent increase in nonsurgical procedures.1
The clinical sequelae of photodamage including wrinkling, pigmentary changes, roughness, laxity, and telangiectasia, can all result in the appearance of aging skin.2 Underlying these visible signs are various histological and cytological changes induced by acute or chronic ultraviolet (UV) exposure.3 The loss of collagen fibers, which normally provide structural stability, and the degradation of elastin fibers, which give skin its natural elasticity, result in a general breakdown of the skin's fibrous matrix. This creates an inelastic, thin, dull-appearing skin. In addition to the structural damage to the dermal extracellular matrix, chronic exposure to the sun's UV rays causes a generalized dysplasia of a variety of epidermal cell types, including keratinocytes and melanocytes. This cellular damage contributes to the mottled and/or hyperpigmented appearance of photodamaged skin.
The severity of photodamage increases with age, and wrinkling is most common in people with skin phototpyes I and II.4 Indeed the incidence of photodamage in European and North American populations with Fitzpatrick skin types I, II, and III has been reported to be as high as 80 to 90 percent.5 The effects of photodamage can have a negative impact on quality of life and can culminate in an excessive preoccupation with dieting, exercise, and thinness.6
This article describes a study using a facial serum (C8 Peptide Intensive Treatment, Kinerase ). The facial serum includes several ingredients associated with improvement in the appearance of fine lines and wrinkles and increased barrier function including a neuropeptide. The facial serum also contains kinetin, a cytokinin growth factor with gerontomodulatory effects on cells7,8; SNAP-8, a neuropeptide associated with muscle contraction9,10; beta-glucan, a cell turnover and regenerative extract that is believed to support healthy immunosurveillance; sodium hyaluronate, a humectant and nascent to extracellular matrix11; and vitamin C&E formulations and green tea, both of which are antioxidants including polyphenols. This is the first study to assess the efficacy and tolerability of the facial serum in treating fine lines, wrinkles, and signs of photodamage on facial and periocular skin.
Methods
This was a 12-week, single-arm, open-label trial performed at a single site in the United States. Subjects were required to be female, within the ages of 40 and 60 with Fitzpatrick classification I to IV with early to advanced photodamaged facial skin (Modified Glogau classification II-III). All subjects were required to provide written, informed consent.
Subjects were required to refrain from using antiaging, antiwrinkle, skin lightening, or any other topical or systemic product known to affect skin aging or dyschromia at least 30 days prior to study entry. Subjects were asked to use a Purpose Gentle Cleansing Wash (Johnson & Johnson Consumer Companies Inc.) before applying facial serum and Purpose Dual Treatment Moisture Lotion with SPF 15 (Johnson & Johnson Consumer Companies Inc.) each morning. Study treatment and wash/moisturizer was provided by Valeant Pharmaceuticals North America. Subjects and investigators were not blinded to treatment, which was provided in commercial packaging.
Efficacy parameters were assessed using visual grading of facial and periocular skin while changes in viscoelasticity properties were assessed by cutometery. Cutaneous tolerability was evaluated both clinically and subjectively using a four-point scale and by monitoring adverse event reports. As this was an exploratory analysis, no primary efficacy parameter was pre-identified.
Clinical assessment. Subjects were clinically assessed for appearance of fine lines and coarse wrinkles as well as signs of uneven pigmentation; lack of elasticity, resilience, and radiance; skin tone; tactile roughness; and overall appearance. Subjects also completed a self-assessment questionnaire to assess skin condition in terms of facial lines and wrinkles; the appearance of brown spots and facial discoloration; and overall skin tone (firmness, dryness, radiance and texture, and the appearance of pores). Using 10-point scales, clinical evaluations (where 0=none, 1–=mild, 4–6=moderate, and 7–9=severe) and subject self-assessments (where 0=most positive response and 9=most negative response) were conducted at Baseline (Visit 1) and Weeks 4, 8, and 12. Mean and average percent change from Baseline and percent of subjects showing improvement were calculated at each visit. Subject opinion of current facial skin condition was recorded at Baseline and Week 12 and improvement/ changes to skin condition since the start of the study at Weeks 4, 8, and 12.
Cutometer measurement. A Cutometer SEM 575 (Courage and Khazaka) measurement was taken on the left ocular bone, in line with the center of the eye to assess skin elasticity. Negative pressure (300mbar) was applied and the movement of the skin recorded to calculate resiliency and extensibility.
Digital photography. Three images were taken of each subject's face (right, left, and center full face) at Baseline and Weeks 4, 8, and 12 to document visible changes in the skin.
Cutaneous tolerability. Signs and symptoms of irritation were evaluated both clinically (erythema, edema, dryness, and peeling) and subjectively (burning, stinging, itching, and tingling) using a 4-point scale (where 0=none, 1=mild, 2=moderate, and 3=severe).
Statistical analysis. For the analysis, at 12 weeks, mean values for clinical grading and instrumentation measurements were compared to Baseline for statistical significance using paired t-test at the P≤0.05 significance level. A top box analysis was performed on the responses to the self-assessment questionnaires.
Results
Thirty-eight female subjects 40 to 60 years of age (Fitzpatrick classification I–IV) were entered into the study with early to advanced photodamaged facial skin (Modified Glogau classification II–III). At Week 12, 34 patients completed the study (Figure 1).
Figure 1.
Subject eligibility
Efficacy. The facial serum showed statistically significant within-group improvement in the appearance of fine lines and wrinkles, uneven skin pigmentation, skin firmness/elasticity, skin tone and resilience, skin radiance and color, smoothness, and overall appearance over 12 weeks.
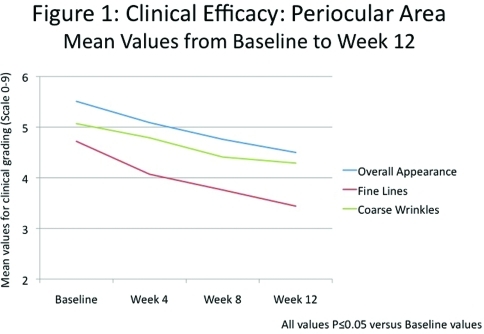
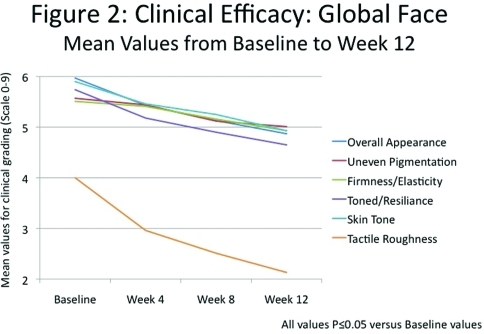
Significant improvements (P≤0.05) in all clinical grading parameters were seen as early as Week 4. By Week 12, there was an 18.4-percent improvement in overall appearance with significant improvements in the appearance of fine lines (27.1%) and coarse wrinkles (15.3%), uneven skin pigmentation (10.1%), skin firmness/elasticity (10.6%), skin tone and resilience (18.2%), skin radiance (21.0%), and color (16.4%) and smoothness (46.6%) (Figures 2 and 3).
Figure 2.
Clinical efficacy: Periocular area. Mean values from Baseline to Week 12
Figure 3.
Clinical efficacy: Global face. Mean values from Baseline to Week 12
Treatment also was associated with improvement of skin firmness (i.e., decreased extensibility) by 15.2 percent by Week 8 as measured by Cutometry (P≤0.05). There were also significant (P≤0.05) increases in measures of elasticity as early as Week 4.
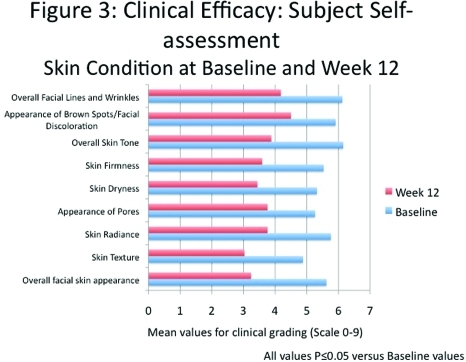
Subjects recorded a statistically significant improvement of 42.4 percent in overall facial skin appearance by Week 12 (P≤0.05). All aspects of skin condition showed significant improvements with a 31.7-percent improvement in facial lines and wrinkles, 23.8-percent improvement in facial discoloration (brown spots), 36.8-percent improvement in overall skin tone (35.1% improvement in firmness, 35.3% in dryness, 39.2% in radiance, and 37.9% in texture), and 28.4-percent improvement in appearance of pores (all P≤0.05) (Figure 4).
Figure 4.
Clinical efficacy: Subject self-assessment. Skin condition at Baseline and Week 12
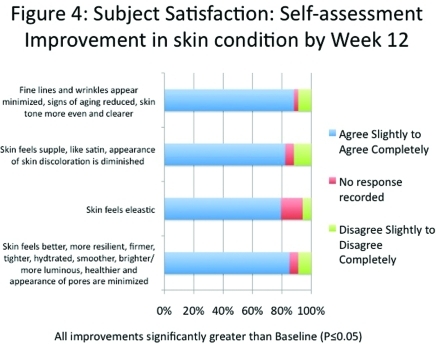
Subject satisfaction was higher than 80 percent for all aspects of skin condition after 12 weeks of treatment, including the skin feeling more elastic, more resilient, firmer, tighter, hydrated, smoother, brighter, and supple. More than 88 percent of subjects stated that their skin tone was more even and clearer, that fine lines and wrinkles appeared minimized, and signs of aging had reduced significantly at Week 12 (P≤0.05) (Figure 5).
Figure 5.
Subject satisfaction: Self-assessment. Improvement in skin condition by Week 12
Tolerability. Overall, two subjects reported adverse events over 12 weeks; neither was associated with treatment. Erythema, dryness, and, to a lesser extent, stinging were the reported signs and symptoms of irritation at Baseline. Subjects had a 70.5-percent reduction in skin redness and 93.7-percent reduction in skin dryness by Week 12 (P≤0.05). Stinging was not reported after Week 4.
Conclusion
This 12-week, single-arm, open-label study suggests that a facial serum, in combination with Purpose Gentle Wash and Purpose Dual Treatment Moisture Lotion, may be effective and well tolerated when treating photodamaged facial and periocular skin. It was observed to improve the appearance of fine lines and wrinkles, uneven skin pigmentation, elasticity, resiliency, radiance, tone smoothness, and overall appearance both through investigator and subject assessment. Statistically significant improvements were seen with all parameters as early as four weeks. Subject satisfaction and cutaneous tolerability were excellent. Overall, this study suggests that a facial serum may be an appropriate option for photodamaged facial skin. A controlled trial would be needed to confirm the findings of this study.
Acknowledgment
The authors acknowledge the support of Brian Bulley, MSc of Inergy for his editorial assistance and medical writing of this manuscript. Inergy activities pertaining to this manuscript were funded by Coria Laboratiories, a subsidiary of Valeant Pharmaceuticals North America.
References
- 1.Yaar M, Gilchrest B. Cellular and molecular mechanisms of cutaneous aging. J Dermatol Surg Oncol. 1990;16:915–22. doi: 10.1111/j.1524-4725.1990.tb01555.x. [DOI] [PubMed] [Google Scholar]
- 2.El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs photoaging: a comparative histopathological, immunohisto-chemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 3.American Society for Aesthetic Plastic Surgery (ASAPS) Cosmetic Surgery National Data Bank: 2003 Statistics. New York, NY: ASAPS; [Accessed on October 10, 2010]. http://www.surgery.org/download/2003-stats.pdf. [Google Scholar]
- 4.Nagashima H, Hanada K, Hashimoto I. Correlation of skin phototype with facial wrinkle formation. Photodermatol Photoimmunol Photomed. 1999;15(1):2–6. doi: 10.1111/j.1600-0781.1999.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 5.Maddin S, Lauharanta J, Agache P, et al. Isotretinoin improves the appearance of photodamaged skin: results of a 36-week, multicenter, double-blind, placebo- controlled trial. J Am Acad Dermatol. 2000;42:56–63. doi: 10.1016/s0190-9622(00)90009-4. [DOI] [PubMed] [Google Scholar]
- 6.Gupta MA, Gupta AK. Photodamaged skin and quality of life: reasons for therapy. J Dermatol Treat. 1996;7:261–264. [Google Scholar]
- 7.Amasino R. 1955: Kinetin arrives. The 50th anniversary of a new plan hormone. Plant Physiology. 2005;138:1174–1184. doi: 10.1104/pp.104.900160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronin H, Draelos ZD. Top 10 botanical ingredients in 2010 anti-aging creams. J Cosmet Dermatol. 2010;9(3):218–225. doi: 10.1111/j.1473-2165.2010.00516.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin AT-L. Basic pharmacology of botulinum toxin in lower respiratory tract infection. Tzu Chi Med J. 2007;19(3):109–114. [Google Scholar]
- 10.Guitierrez LM, Viniegra S, Rueda J, et al. Peptide that mimics the C-terminal sequence of SNAP-25 inhibits secretory vesicle docking in chromaffin cells. JBiol Chem. 1997;272:263. doi: 10.1074/jbc.272.5.2634. [DOI] [PubMed] [Google Scholar]
- 11.Jang S-A, Park S, Kim K-S, Joo H, et al. The role of beta-glucan receptor dectin-1 in phagocytosis and TNF-alpha-production bymacrophages. Cytokine. 2009;48(1–2):109. [Google Scholar]