Abstract
The expression pattern for tissue transglutaminase (TG2) suggests that it regulates cartilage formation. We analyzed the role of TG2 in early stages of chondrogenesis using differentiating high-density cultures of mesenchymal cells from chicken limb bud as a model. We demonstrate that TG2 promotes cell differentiation towards a pre-hypertrophic stage without inducing precocious hypertrophic maturation. This finding, combined with distinctive up-regulation of extracellular TG2 in the pre-hypertrophic cartilage of the growth plate, indicates that TG2 is an autocrine regulator of chondrocyte differentiation. We also show that TG2 regulates synthesis of the cartilaginous glycosaminoglycan (GAG)-rich extracellular matrix. Elevated levels of TG2 down-regulate xylosyltransferase activity which mediates the key steps in chondroitin sulfate synthesis. On the contrary, inhibition of endogenous transglutaminase activity in differentiating chondrogenic micromasses results in increased GAG deposition and enhancement of early chondrogenic markers. Regulation of GAG synthesis by TG2 appears independent of TGF-β activity, which is a downstream mediator of the TG2 functions in some biological systems. Instead, our data suggest a major role for cAMP/PKA signaling in transmitting TG2 signals in early chondrogenic differentiation. In summary, we demonstrate that matrix synthesis and early stages of chondrogenic differentiation are regulated through a novel mechanism involving TG2-dependent inhibition of PKA. These findings further advance understanding of cartilage formation and disease, and contribute to cartilage bioengineering.
Keywords: chondrocyte, mesenchymal cells, transglutaminase 2, glycosaminoglycan, PKA, cell differentiation
1. INTRODUCTION
Condensation of mesenchymal cells triggers their differentiation into chondrocytes and thus initiates the progression of chondrogenic differentiation through a sequential series of maturation stages, including a proliferation stage, pre-hypertrophic stage and terminal maturation characterized by cell hypertrophy, extracellular matrix (ECM) calcification and resorption. Chondrocytes synthesize large amounts of specific ECM characterized by the presence of collagen type II and aggrecan that carries extensive post-translational modifications with sulfated polysaccharide chains called glycosaminoglycans (GAGs). GAG synthesis is the highest in proliferating chondrocytes, and slowly declines with advanced maturation (Ooira et al., 1974; Kim and Conrad, 1977). Identification of the signaling pathways that regulate chondrocyte differentiation and ECM production is essential for the understanding of both skeletal development and for advancement of therapeutics for diseases such as osteoarthritis. Specific proteins expressed at a particular chondrocyte differentiation stage regulate transition to the next stage acting as auto- and paracrine factors. Recent studies have identified extracellular enzymes transglutaminases (TGases) as such regulators (Aeshlimann et al., 1997; Nurminskaya et al., 2003; Johnson et al., 2005).
Transglutaminases (TGs) catalyze Ca2+−dependent covalent ε-(γ-glytaminyl)lysyl intra- and inter-protein crosslinking. The best studied enzyme – tissue TG, also called TG2, - also possesses GTPase/ATPase and deamidating activities in addition to the crosslinking activity (reviewed by (Lorand and Graham, 2003)). Moreover, when externalized by a yet unknown mechanism, TG2 can act as an extracellular regulator of diverse signaling pathways. Thus, extracellular TG2 has been shown to regulate phospholipase Cδ (Nakaoka et al., 1994), cAMP-dependent protein kinase [PKA] (Nurminskaya et al., 2003), β-catenin (Faverman et al., 2008), integrin-dependent mitogen-activated protein kinase ((Johnson et al., 2005); (Tanaka et al., 2007)), and Src-p190/RhoGAP (Janiak et al., 2006). The diversity of the signal transduction targets for extracellular TG2 is conferred by the ability of this enzyme to interact with a number of cell surface receptors including α1B adrenergic receptors (Nakaoka et al., 1994), integrins (Akimov et al., 2000), the atypical G-protein-coupled receptor GPR56 (Xu and Hynes, 2007), VEGF receptor 2 (Dardik and Inbal, 2006), and LRP5/6 receptors (Faverman et al., 2008). Particular patterns of the TG2-interacting receptors probably determine the cell-specific effects of this multifunctional protein.
Only two transglutaminases among the nine mammalian enzymes (Lorand and Graham, 2003), are expressed in cartilaginous and osseous tissues. These are TG2 and a homodimeric form of the circulating heterodimeric coagulation factor FXIII ((Aechlimann et al, 1993); (Nurminskaya and Linsenmayer, 1996)). These enzymes have very similar substrate specificity and both act as potent enhancers of terminal maturation in differentiating osteochondral cells in vitro ((Aeschlimann 1993); (Nurminskaya et al., 2003); (Johnson et al., 2003)). In vivo, in the developing cartilaginous anlage of endochondral bones, TG2 is markedly absent at the very early stages of chondrocyte differentiaton but is expressed prior to chondrocyte hypertrophy ((Thomazy and Davies 1999); (Nurminskaya and Kaartinen 2006)), suggesting a stage-specific role for this protein. Intriguingly, no skeletal phenotype has been observed in either TG2 or FXIIIa knockout models ((Nanda 2001); (Koseki-Kuno et al., 2003)), likely due to the ability of these two enzymes to compensate for each other. (maria – are they co-expressed?) For example, in the TG2−/− knockout bone and cartilage tissues the total levels of transglutaminase activity remain unchanged due to compensatory activation of FXIIIa ((Nurminskaya and Kaartinen, 2006); (Tarantino 2009)).
To overcome the complications associated with the genetic loss-of-function models and to obtain an insight into the role of transglutaminases in chondrogenesis, we combined a gain-of-function approach with pharmacological loss-of-function approach using chondrogenesis in micromasses of chick limb bud mesenchymal cells as a model. Here we show that forced expression of TG2 promotes transition into the pre-hypertophic stage and down-regulates the key enzyme of protein glycosylation, xylosyltransferase 2, thus attenuating deposition of the cartilaginous ECM. Conversely, pharmacologic inhibition of endogenous transglutaminase activity results in increased GAG synthesis and enhanced expression of the early chondrogenic markers in the micromass cultures. These results suggest that down-regulation of GAG synthesis, characteristic of prehypertophic chondocytes may be regulated by the onset of endogenous TG2. Identification of TG2 as a novel autocrine negative regulator of matrix deposition provides the necessary background for improved cartilage regeneration and in vitro bioengineering. In addition, our results strongly implicate PKA signaling as a mediator of these effects of TG2, revealing a novel mechanism for PKA regulation in chondrocyte differentiation and further advancing the general understanding of the molecular pathways that orchestrate chondrogenesis.
2. RESULTS
2.1 TG2 is expressed in prehypertrophic chondrocytes
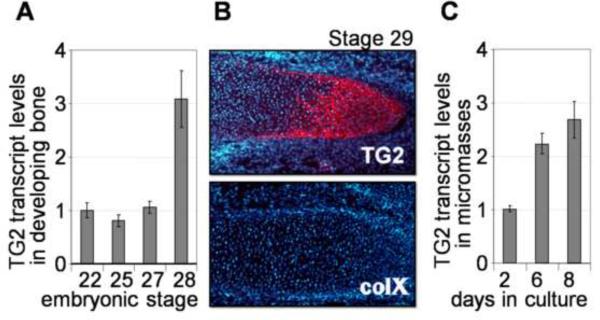
To characterize the expression pattern of TG2 during the mesenchymal-to-chondrogenic transition and in early chondrogenesis, we measured by quantitative RT-PCR the TG2 transcript levels in chicken embryonic limb buds starting with the embryonic stages 21–22 (3 ½ days) prior to mesenchymal condensation, and through the stage 28 (5 ½-6 days) when the cartilaginous anlagen are already formed. Expression of the TG2 protein at the same stages was analyzed by immunolocalisation to tissue sections. This analysis shows that TG2 mRNA is up-regulated at stage 28 concomitant with accumulation of detectable amounts of the TG2 protein in the cartilaginous anlagen. Noteworthy, at this stage there are yet no hypertrophic collagen type X–positive chondrocytes (Fig. 1A, B) (Schmid and Linsenmayer, 1985), indicating the pre-hypertrophic nature of the TG2-positive cells. Thus, TG2 expression is induced during cell transition into pre-hypertrophy around day 6 of embryonic development, and even earlier than suggested previously (Thomazy and Davies, 1999).
Figure 1.
TG2 expression in the developing chicken limbs. A –levels of TG2 mRNA in mesenchymal cells isolated from the limb buds or cartilaginous anlagen at stages 21–28 of embryonic development, analyzed by real-time RT-PCR. B – immunological detection of the TG2 protein (red, upper panel) in the prehypertrophic cartilaginous anlage of a day 6.5 (stage 29) embryo. The same anlage is negative for collagen type X protein (as indicated by the lack of red signal on the lower panel). Cell nuclei are counterstained with DAPI (blue). C –real-time RT-PCR analysis shows up-regulation of the TG2 mRNA during chondrogenic differentiation of micromass cultures (normalized to beta-actin expression).
The in vivo pattern of TG2 expression in chondrogenesis is mirrored in the high-density cell cultures, defined as micromass cultures, in which chondrogenic differentiation in mesenchymal cells occurs spontaneously. The micromass culture system recapitulates in vitro the entire process of chondrogenesis including chondrocyte differentiation (days 1–10), growth and early differentiation (days 5–12), and maturation and hypertrophy (from day 12 on) (Daumer et al., 2004). We observed a 2–3 fold induction in TG2 expression in the 6 days old avian micromass cultures which contain chondrocytes at the stages of growth and transition into prehypertrophy, and are collagen type X–negative (Fig. 1C). Based on the pattern of TG2 expression, characterized by its rapid induction prior to the onset of hypertrophy both in vivo and in vitro, we hypothesize that TG2 regulates early steps of chondrogenesis including transition of differentiating chondrocytes to prehypertrophy.
2.2 Over-expression of TG2 promotes chondrogenic differentiation in mesenchymal cells
To gain the insight into the role of TG2 at early stages of chondrogenesis, we overexpressed TG2 in the micromass cultures using the avian replication-competent retrovirus RCASBP(A). We took this approach because synthesis of the elevated amounts of TG2 by mesenchymal cells in the chondrogenic micromass cultures assures its normal externalization and cell surface/extracellular localization whereas purified TG2 has been unable to replicate some of the biological activities of the endogenous enzyme (discussed in (Akimov et al., 2000)). In addition, cellular expression of TG2 overcomes the potentially impaired diffusion of the recombinant protein through the cartilaginous matrix of differentiating micromasses in which heparin sulfate binds exogenous TG2 with high affinity (reviewed in (Leddy et al., 2008); (Scarpellini et al., 2009)). Therefore, a viral expression construct was designed to encode human TG2 tagged with the secretory pre-pro-trypsin 15aa sequence to ensure externalization of the recombinant protein, similarly to extracellular localization of endogenous TG2 in the cartilaginous matrix (Fig. 1B), ((Aeschlimann et al., 1993); (Nurminskaya and Kaartinen, 2006)),.
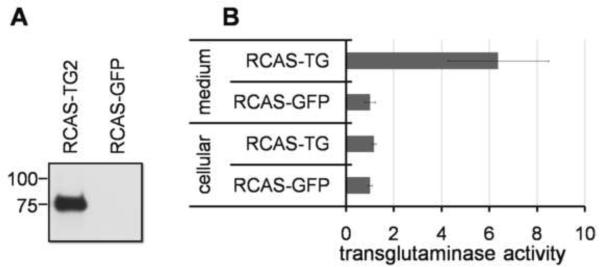
Mesenchymal cells from the day 3 chicken limb buds were mixed with concentrated retrovirus particles, and grown for up to 7 days in the high-density micromass cultures. In these experiments, RCASBP virus expressing GFP protein was used as a control for possible non-specific effects of viral infection, and to determine the time course of viral protein expression. Monitoring GFP expression with fluorescent microscopy revealed appreciable levels of the virally expressed proteins in the infected micromass cultures on the third day after plating, preceding expression of endogenous TG2 by 3 days. Efficient secretion of the virus-expressed TG2 was confirmed by Western analysis of the medium conditioned by the infected mesenchymal cells (Fig. 2A). The secreted virus- derived TG2 is catalytically active, as demonstrated by the high levels of the transglutaminase activity in the growth medium of the RCAS-TG2 infected micromass versus the RCAS-GFP infected control (Fig. 2B).
Figure 2.
Secretion of the enzymatically active virus-derived TG2. A – efficient expression and secretion of RCAS-derived TG2 confirmed by Western blot analysis of the matrix protein extracted with high salt solution. B –The secreted TG2 in the medium conditioned by the RCAS-TG2 infected micromasses is catalytically active, as compared to the RCAS-GFP-infected cells, in a TG2 enzymatic activity assay.
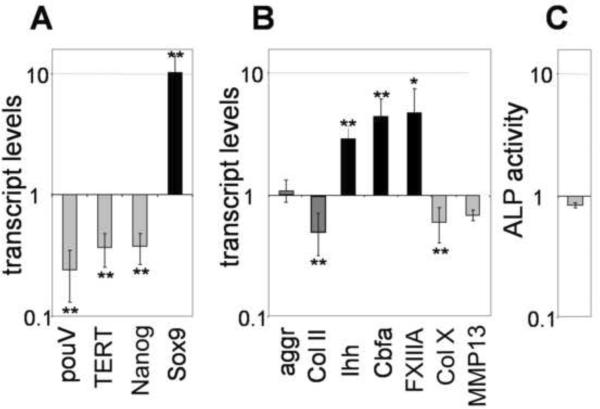
To characterize the impact of extracellular TG2 on the program of chondrogenic differentiation in micromasses, we analyzed expression of mesenchymal and chondrocyte molecular markers in 5 day-old micromass cultures infected with RCAS-TG2 using real-time PCR. Cultures infected with the control RCAS-GFP virus served as a reference. We found that extracellular TG2 stimulates mesenchymal cell differentiation, as evidenced by the reduced expression of the mesenchymal markers, pouV, TERT, Nanog, and increased expression of Sox9 which is a marker of chondrogenic commitment (Fig. 3A, gray bars versus black bar). However, expression of the molecules characteristic for chondrocyte growth, including collagen type II and aggrecan, was not markedly affected by the excess TG2. Instead, we observed up-regulation of the typical markers of pre-hypertrophic chondrogenic maturation including Ihh, Cbfa1 and plasma transglutaminase FXIIIA (Fig. 3B, black bars). Similarly, N-cadherin, which is expressed at high levels in condensing mesenchymal cells (Oberlender and Tuan, 1994), is significantly down-regulated about 50% (p<0.05) in the cells infected with RCAS-TG2 (Fig. 3C). These observations strongly suggest that excess TG2 accelerates progression of chondrocyte differentiation towards the pre-hypertrophic stage, causing accumulation of the pre-hypertrophic cells. Consistent with this model, excess TG2 does not induce markers of late hypertrophy such as the collagen type X and MMP13 even in older 8 day cultures (Fig. 3B, gray bars). Similarly, alkaline phosphatase activity is not induced by TG2 overexpression (Fig. 3D). These results demonstrate that elevated levels of TG2 do not trigger precocious chondrocyte hypertrophy prior to its normal onset at about 12th day in micromass culture (Daumer, 2004)
Figure 3.
Excess TG2 down-regulates mesenchymal markers (A) and affects expression of the chondrogenic markers (B) in 7 days old micromass cultures. Transcript levels were measured by real-time RT-PCR; C – Levels of N-cadherin transcripts are reduced in RCAS-TG2 infected micromasses as compared to the RCAS-GFP infected control cultures. (p<0.05) D – excess TG2 does not affect alkaline phosphatase (ALP) activity. Cultures infected with the RCAS-GFP virus were used as the reference to determine the changes caused by the RCAS-TG2 (*, p<0.05; **, p<0.01).
2.3 Over-expression of TG2 inhibits matrix production during micromass-induced chondrogenesis
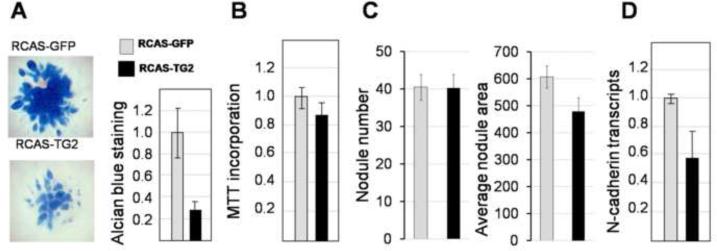
To examine the effect of TG2 in early chondrogenesis we analyzed nodule formation in the infected micromasses. First, the number of nodules and the area of the GAG-positive Alcian blue staining was analyzed in 5 day old micromasses when the nodules are small and distinct. This analysis revealed no difference in nodule number between the RCASGFP- and RCASTG2- infected micromasses (Fig. 4A), indicating that initial cellular condensation, which occurs over the first 24 hours of micromass initiation, was not affected by viral infections in agreement with accumulation of the virally expressed TG2 about 3 days after initial infection (Fig. 2). However, the average area of Alcian blue staining per nodule was 15% smaller in the TG2-overexpressing cells (Fig. 4A), indicating that nodule enlargement was reduced by the premature expression of TG2. This difference becomes even more pronounced over time, and in the 7 days micromass cultures GAG-positive deposition in the RCASTG2-infected cells is dramatically as reduced compared to control RCASGFP cells (Fig. 4B). At the same time, cell proliferation is not affected by TG2 expression (Fig. 4C), suggesting TG2 is a negative regulator of the cartilaginous ECM deposition.
Figure 4.
Inhibition of cartilaginous ECM deposition by TG2. A – nodule formation in infected micromass cultures. The average numbers of nodule per micromass are equal in the RCASTG2 and RCASGFP-infected cultures, however the average area of nodule growth shown in pixels, is decreased by overexpression of TG2 (p<0.05). B - Representative micromass showing reduced GAG deposition in 7 day old RCAS-TG2 infected micromasses, as compared to the RCAS-GFP control cultures. The staining intensity and area of the Alcian blue-positive matrix is reduced. Quantitative analysis of the Alcian blue staining after solubilization and spectrophotometric detection of the dye (normalized to the total cell number measured with Crystal violet staining) in (A) from a triplicate experiments (>3 micromasses in each) (p<0.01). C - Cell proliferation measured by MTT incorporation in RCAS-TG2 infected cultures versus the RCAS-GFP control.
2.4 TG2 down-regulates xylosyltransferases, the key enzymes responsible for sulfated proteoglycan biosynthesis
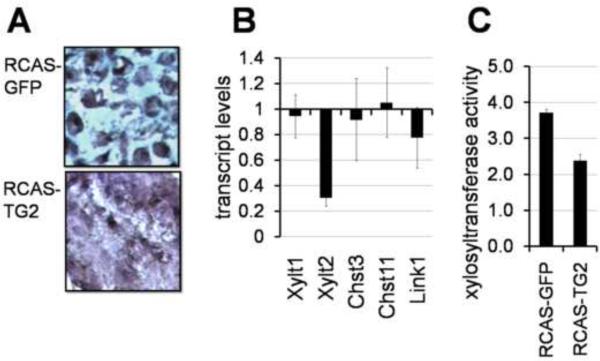
Histological analysis confirmed the reduced amount of the intercellular Alcian blue-positive extracellular matrix, and showed a dense arrangement of cells in the RCAS-TG2 infected micromass cultures. For comparison, characteristic cartilage morphology with abundant basophilic matrix separating individual cells was observed in the control RCAS-GFP infected micromasses (Fig. 5A). Taking into consideration that TG2 does not significantly affect transcript levels of aggrecan which is the core protein of the major sulfated cartilage proteoglycan (Fig. 3B), we hypothesized that TG2 negatively regulates aggrecan glycosylation and/or sulfation. We analyzed the effect of TG2 on expression of the key enzymes required for biosynthesis of glycosaminoglycan chains and for sulfation of glycosaminoglycans, including xylosyltransferases 1 and 2, chondroitin 6-sulfotransferase 3, and chondroitin 4-sulfotransferase 11, as well as the Link1 protein participating in proteoglycan assembly (Woods et al., 2007). We found that TG2 markedly down-regulates transcript levels of xylosyltransferase 2 (Xytl2) and to a lesser extent of Link1 (Fig. 5B). The xylosyltranferase enzymatic activity is also decreased in the TG2-overexpressing cultures (Fig. 5C), supporting the hypothesis that TG2 negatively regulates deposition of the cartilaginous matrix in differentiating micromass cultures by attenuating the posttranslational modification of matrix proteins such as aggrecan.
Figure 5.
TG2 attenuates synthesis of glucosaminoglycans (GAGs). A - Overexpression of TG2 disrupts cartilage-specific morphology of the micromass tissue revealed by the Alcian Blue and Hematoxilin iron staining. B – expression analysis of the GAG biosynthesis genes in RCAS-TG2 infected micromasses shows down-regulation of xylosyltransferase 2 and Link1. RCAS-GFP infected cultures served as a reference. (p<0.01) C – reduced xylosyltransferase activity in RCAS-TG2 infected micromasses. (p<0.05).
2.5 TG2 inhibits PKA signaling in mesenchymal cells undergoing chondrogenic differentiation
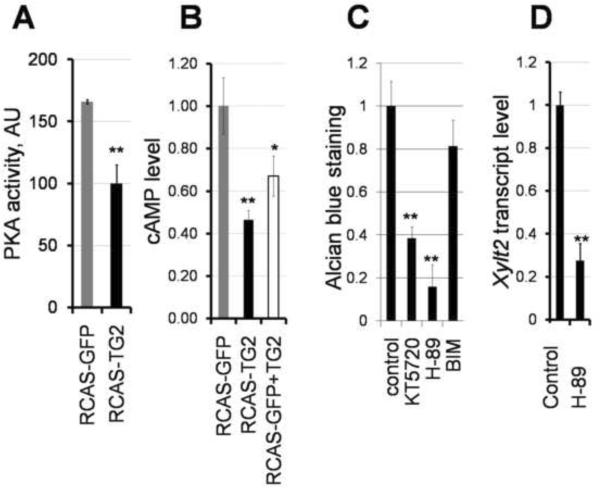
Previously, we have shown that extracellular TG2 inhibits PKA activity in osteoblasts (Nurminskaya et al., 2003). We therefore asked whether excess TG2 similarly attenuate PKA signaling in early chondrocytes and whether this pathway mediates the TG2-dependent inhibition of GAG synthesis in the micromass cultures. Using ELISA-based and radiometric assays, we detected a significant 1.7-fold decrease in the PKA activity in the RCAS-TG2 infected cultures as compared to the RCAS-GFP infected control (Fig. 6A), demonstrating a correlation between the TG2-mediated inhibition of the PKA signaling and reduced ECM deposition. Analysis of the intracellular levels of cAMP in chondrifying infected cells revealed a significant 2-fold reduction in the cAMP levels (p<0.01) in the presence of RCASTG2 (Fig. 6B, black bar versus gray RCAS-GFP bar). Moreover, a short 20 minute exposure of the RCAS-GFP-infected cells to purified TG2 also results in a similar decrease in the cAMP levels (Fig. 6B, p<0.05, white bar versus gray bar). These results implicate attenuation of the intracellular cAMP levels as a potential mechanism for the inhibition of PKA activity by extracellular TG2.
Figure 6.
TG2 modulates chondrogenesis through inhibition of the PKA pathway. A - PKA activity is reduced in total cell lysates of 6-day old micromass cultures infected with RCAS-TG2 as compared to control RCAS-GFP micromasses. B- extracellular TG2 decreases cellular levels of cAMP in differentiating chondrocytes, when either overexpressed by RCAS-TG2 virus (black bar) or added in a purified form (0.01U/ml) (open bar); C- Effect of pharmacological kinase inhibitors on deposition of the Alcian Blue-positive cartilaginous matrix in the 5 day old micromass cultures. PKA inhibitors 1 μM KT5720 and 100 nM H89,and PKC inhibitor 10 nM bisindolylmaleimide I (BIM), were continuously present in the medium from day 2 in culture. Cultures treated with DMSO vehicle served as the reference. D - Treatment of micromass cultures with PKA inhibitor H89 reduces transcript levels of xylosyltransferase 2, as measured by real-time PCR. Control cultures were treated with the vehicle (DMSO). (** p<0.01)
2.6 PKA signaling may mediate the effects of TG2 on chondrogenesis in vitro
Next, we analyzed the role of PKA signaling in the micromass-driven chondrogenesis. For this, we examined Alcian blue staining of the micromass cultures grown for 5 days in the presence of two pharmacological inhibitors of PKA: KT5720 or H-89 (Fig. 6C). Both inhibitors caused a significant 60–80% decrease in GAG deposition. While KT5720 is considered to be very specific for PKA, H-89 can also inhibit PKC, although at higher concentrations than were used in this study (Chijiwa et al., 1990). To assure that the reduction of GAG deposition by H-89 did not dependent on PKC signaling, we employed the PKC specific inhibitor bisindolylmaleimide and observed only a minor 20% decrease in chondrogenesis (Fig. 6C, BIM), implicating that strong attenuation of the cartilaginous GAG-rich matrix by H-89 is due to inhibition of PKA. We further analyzed the effect of H-89 on expression of xylosyltransferases in differentiating chondrocytes and found that, similarly to TG2, inhibition of PKA causes a drastic 75% down-regulation of Xylt 2 gene, p<0.01 (Fig. 6D).
2.7 TG2 inhibits cartilage formation independently of TGFβ
Extracellular TG2 is able to activate latent TGFβ (Nunes et al., 1997) which when overexpressed can inhibit chondrogenesis in cultured chick leg bud mesenchymal cells ((Carrington and Reddi, 1990); (Jin et al., 2008); (Mello and Tuan, 2006). Therefore, we considered a possibility that activated TGFβ signaling mediates the effects of TG2 on chondrogenesis.. To explore this hypothesis, 2 day old infected micromass cultures were transfected with the TGFβ–dependent reporter construct in which luciferase is expressed under the SMAD2/SMAD3/SMAD4-regulated promoter (SABiosciences). There was no significant difference in luciferase expression 72 hours after transfection between the RCASTG2 and RCAS-GFP infected micromasses (Fig. 7A), indicating similar levels of the matrix-bound TGFβ in micromasses with endogenous and elevated levels of TG2. In addition, we determined the levels of secreted active TGFβ in the growth medium conditioned by these micromass cultures, using Cos-7 cells stably transfected with the TGFβ-dependent luciferase reporter. Levels of luciferase activity were the same in the Cos-7 cells treated with medium from control or TG-overexpressing micromasses (data not shown). Together, these data indicate a TGFβȃindependent mechanism for the TG2-mediated regulation of the early stages of chondrogenesis.
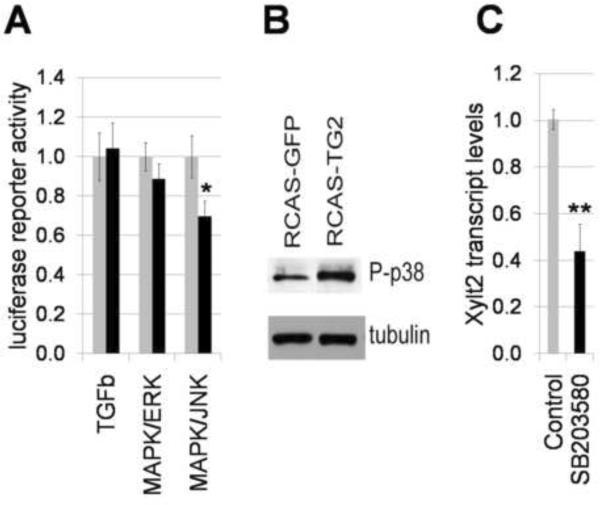
Figure 7. Regulation of MAPK signaling in differentiating micromass cultures by TG2.
A – activity of ERK and JNK pathways were analyzed with specific luciferase reporter constructs (SABiosciences). Five days-old RCAS-infected micromass cultures were transiently transfected with reporter constructs and luciferase activity was measured 72 hours later using luciferase assay kit (Promega). B – Western blot analysis of phosphorylated p38 detected with specific antibody 9212 (Cell Signaling Technologies, MA). C – treatment of micromass cultures with p38 inhibitor SB203580 (10 μM) reduces transcript levels for xyloxyltransferase 2, measured by real-time RT-PCR (**, p<0.01).
2.8 Role of MAPK signaling in TG2-dependent regulation of early chondrogenic differentiation
Because extracellular TG2 can affect MAPK signaling (Janiak et al., 2006; Johnson et al., 2005), we sought to investigate the potential effect of the elevated TG2 on the ERK1/2, p38, and JNK pathways which are the major conduits of the MAPK signaling pathway. Activity of MAPK/ERK and MAPK/JNL was analyzed using specific luciferase reporters (SABiosciences). Excess TG2 has minor, if any, effect on MAPK/ERK activity in the 5 days old micromasses, while causing an approximately 30% inhibition of JNK activity (Fig. 7A). The activity status of the p38 pathway was determined by Western blot analysis for phosphorylated p38 protein. In the RCAS-TG2-infected cultures the p38 pathway is activated (Fig. 7B). Next, we analyzed whether p38 kinase regulates xylosyltransferase expression similarly to TG2. Pharmacological inhibition of p38 results in a significant 58% down-regulation of Xylt2 mRNA, p<0.01 (Fig. 7C)., indicating that p38 is a positive regulator of Xylt2 in contrast to TG2. These data suggest that the TG2-dependent regulation of GAG synthesis is not mediated by either ERK or p38 signaling pathways. Further experiment will access the role of JNK inhibition in the TG2-reliant chondrocyte differentiation.
2.9 Loss-of-function analysis
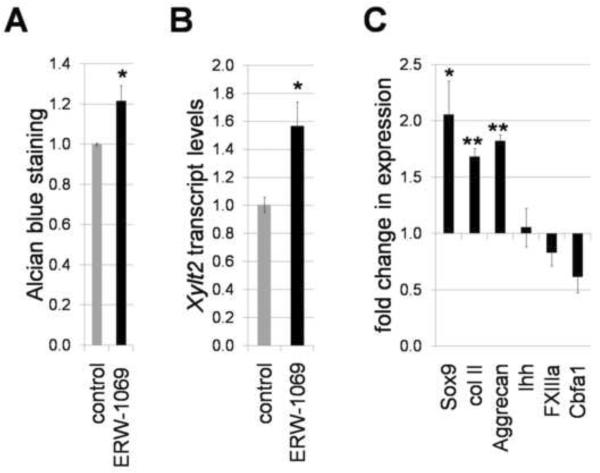
To examine whether the effects of the overexpressed human TG2 on chondrocyte differentiation reflect the role of endogenous enzyme, we complemented the gain-of-function studies with the loss-of-function approach by inhibiting the endogenous transglutaminase activity with small-molecule irreversible TGase inhibitor ERW-1069 (Watts et al., 2006). The 30μM ERW1069 inhibits total transglutaminase activity in the micromasses down to approximately 60% (from 28+/− 4.3 to 17.4+/−1.2 U/mg, p=0.007). Continuous presence of the same concentration of ERW1069 in growing micromass cultures starting from day 2 causes a 20% increase in GAG deposition (p<0.05) (Fig. 8A), and a significant increase in Xylt2 expression (p<0.05) (Fig. 8B). These results are in accordance with the TG2-dependent inhibition of both Xylt2 expression and GAG deposition in the RCAS-TG2 infected micromasses and indicate that endogenous TG2 signaling regulates these processes (Fig. 4B, 5B).
Figure 8.
Loss-of-function analysis on the role of endogenous TG2 in cartilage formation. Enzymatic activity of TG2 was inhibited with specific compound ERW-1069 (30 μM). A – deposition of the GAG-rich matrix is enhanced by inhibiting endogenous TG2. Alcian blue staining normalized to total cell number detected with Crystal violet vital dye. B – Quantitative RT-PCR analysis of the expression of xylosyltransferase 2, normalized to housekeeping gene Gusb in micromasses cultures grown in the presence of ERW-1069. C – inhibition of endogenous TG2 augments expression of early chondrogenic molecular markers suggesting chondrocyte blocking in early immature stage of differentiation. Real-time PCR analysis, normalization to housekeeping gene Gusb (*, p<0.05, **, p<0.01).
Next, we analyzed the effects of blocking the TGase activity on the progression of chondrocyte differentiation. Real-time PCR analysis revealed up-regulation of the early chondrogenic molecular markers, including Sox9, collagen type II and aggrecan, in the inhibitor-treated as compared to control untreated micromasses, while markers of prehypertrophy, such as Ihh, Cbfa1 and FXIIIa, were either slightly down-regulated or remained unaffected (Fig. 8C). These results together with conclusions from the gain-of-function experiments (section 2.2) demonstrate that TG2 regulates chondrocytes differentiation by promoting transition into pre-hypertrophy.
2.10 In vivo effects of TG2 on skeletal development
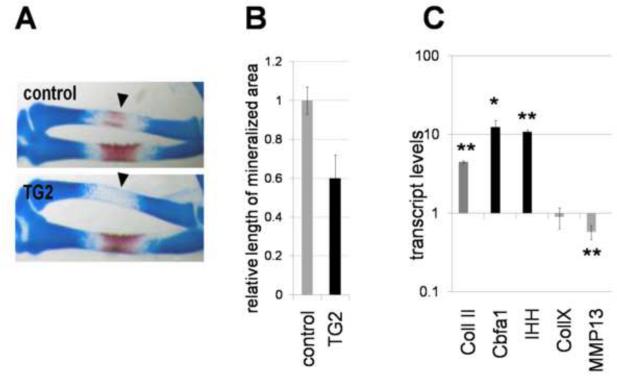
To extend the above findings to in vivo analysis, we determined the consequences of TG2 misexpression on long bone formation in the limb. To infect limbs, cell pellets of fibroblasts infected with retrovirus were micrografted into the wing bud between stages 18 and 19 (prior to the condensation of the cartilaginous anlagen). In these studies, contra lateral uninfected wing buds served as control. Embryos were collected at day 12 for anatomical analysis of skeletal development. Control grafting with RCAS-GFP virus revealed that expression of the target protein was found in the long bones, and surrounding mesenchyme, without any effect on their development (data not shown). On the contrary, grafting with RCAS-TG2 caused deregulation of long bone formation. One prominent phenotype consistently observed was the delay/disruption of ossification, as indicated by the absence or reduction of Alizarin Red staining (Fig. 9A). On average, TG2 induced a 60% reduction in the length of the ossifying region (n=11) (Fig. 9B), indicating retardation of endochondral ossification. The TG2-induced blocking of cells at more immature stage of pre-hypertrophy without acceleration of further development into hypertrophic cells would account for such phenotype. Consistent with this model, in the RCAS-TG2 infected right wing (compared to uninfected left wing) the pre-hypertrophic markers Ihh and Cbfa1 are significantly induced (Fig. 9C) while hypertrophic markers MMP13 and collagen type X are slightly inhibited if changed at all. Thus, TG2 promotes chondrocyte transition into pre-hypertrophic stage both in vivo in the growth plate and in vitro in micromass cultures.
Figure 9.
Morphological changes in chicken limbs induced by retroviral expression of secreted TG2. Calcified areas are revealed with Alizarin Red; cartilaginous matrix is counterstained with Alcian Blue. A – impaired endochondral ossification in the RCAS-TG2 infected anlagen (arrowheads). B – reduction in length of the mineralized area in radius by excess TG2. The RCAS-TG2 infected wings were compared to the contralateral uninfected control wings (black versus gray bars), n=11. (p<0.05). C - Excess TG2 affects expression of the chondrogenic markers in the central part of the RCAS-TG2 infected radii versus control non-infected side. Transcript levels were measured by real-time RT-PCR normalized to beta-actin levels (*, p=0.014, **, p<0.01).
2. DISCUSSION
In this study, we show that extracellular enzyme transglutaminase 2 (TG2) is a novel autocrine regulator of cartilage formation. Previous work of the spatial and temporal expression pattern of TG2 in the embryonic growth plate implied that this protein promotes the transition of the differentiating chondrocytes into pre-hypertrophic stage. We addressed this hypothesis using the mesenchymal micromass-based model of the in vitro chondrogenesis which precisely recapitulates both the progression of chondrocyte differentiation and the TG2 expression profile. As a gain-of-function approach we employed the virus-driven expression of secreted TG2 several days earlier than induction of endogenous enzymes in differentiating micromasses. These studies were complemented with the loss-of-function approach by inhibiting the endogenous transglutaminases with a specific small molecule compound ERW-1069 (Watts et al., 2006). Premature expression of TG2 up-regulates pre-hypertrophic markers including Ihh, Cbfa1 and FXIIIa, while inhibition of endogenous transglutaminase promotes the expression of early chondrogenic markers, including Sox9, coll II and aggrecan. Cell proliferation is not affected by TG2 indicating that this enzyme regulates cell differentiation by promoting transition into the pre-hypertrophic stage. However, further maturation is not accelerated as evident by the inability of viral TG2 to induce hypertrophic markers collagen type X and MMP-13 in vitro and by the delay in endochondral ossification in vivo in the embryonic limbs infected with RCAS-TG2. The latter phenotype indicates blocking the chondrocytes at more immature stage of pre-hypertrophy without progression into hypertrophy. Similar up-regulation of the pre-hypertrophic molecular markers is also observed in vivo in the embryonic limbs infected with RCAS-TG2. Interestingly, collagen type II expression in vivo is to some extent induced by TG2, suggesting accumulation of both resting and pre-hypertrophic chondrocytes in the growth plate with disturbed chondrocyte maturation. Together, these data identify extracellular TG2 as a positive regulator of chondrocyte differentiation which promotes transition into the pre-hypetrophic stage. This activity of TG2 determines the importance of the TG2 paucity during the early stages of chondrogenic differentiation in the embryonic growth plate and in articular chondrocytes which remain in a resting state not undergoing full differentiation and in which TG2 normally is not expressed.
In addition to regulating gene expression in differentiating chondrocyte, TG2 regulates formation of the cartilaginous matrix. Premature expression of TG2 results in reduced deposition of the GAG-rich ECM, however, expression of the main ECM proteins collagen type II and aggrecan is not affected. This result suggests that TG2 attenuates ECM production by a specific mechanism, rather than by merely reducing the numbers of the cartilage-producing cells. Here we demonstrate that this mechanism involves TG2-induced down-regulation of the key enzyme of protein glycosylation - xylosyltransferase 2 (Xylt2), which regulates the initial step of GAG attachment to Ser residue of the core protein. The TG2-dependent coordinated down-regulation of xylosyltransferase activity and promotion of chondrocyte transition into pre-hypertrophic stage can explain, at least partially, the previously described reduced capacity for polysaccharide chain synthesis in maturing versus proliferating chondrocytes (Ooira et al., 1974; Kim and Conrad, 1977).
In this study, we have identified that PKA signaling is a major molecular mechanism that mediates the TG2-dependent regulation of chondrogenic differentiation. First, we detect the TG2-induced reduction in cAMP levels and inhibition of PKA activity in differentiating micromasses. Not clear. A plausible model for the TG2-dependent inhibition of PKA, which needs further experimental evidence, suggests an involvement of yet unidentified cell-surface receptors that regulate intracellular adenylate cyclase activity controlling the intracellular levels of cAMP and are capable to bind extracellular TG2. The adrenergic receptors are credible candidates because they regulate cAMP levels (Cassel and Selinger, 1978) and bind TG2 (Chuang, 1984; Nakaoka et al., 1994). Binding to TG2 induces internalization of adrenergic receptors (Chuang, 1984), thus lowering the receptor levels on cell surface and resulting in a decrease in cAMP synthesis and in PKA inhibition. Next, we demonstrate that inhibition of PKA with specific pharmacological compounds is sufficient to mimic the effects of TG2 on Xylt2 expression and GAG deposition, in agreement with PKA signaling being the main intracellular intermediary of extracellular TG2.
In addition, inhibition of PKA may lead to activation of p38 kinase detected in the RCASTG2 infected micromass cultures, since PKA is known to destabilize the integrin-cytoskeletal complex required for efficient MAPK signaling (reviewed in (Juliano, 2002)). Although the p38 pathway is a positive regulator of chondrocyte differentiation (reviewed in (Bobick and Kulyk, 2008)) and its activation is expected to correlate with the TG2-induced cell progression into pre-hypertrophic stage, the p38 pathway appears to positively regulate Xylt2 expression in contrast to TG2. This suggests that the TG2-induced reduction in GAG synthesis in mesenchymal cells undergoing chondrogenic differentiation is independent from p38 kinase, and that the p38 pathway may be a secondary target of extracellular TG2 downstream of PKA inhibition. This is in contrast to articular chondrocytes in which the TG2 effects appear to be mediated by the integrin-p38 pathway (Johnson et al., 2005), and may be a reflection of the fact that signaling processes are often highly cell-context dependent. In the micromass model of chondrogenic differentiation, both endogenous TG2 and virus-derived TG2 are expressed several days after the initial condensation in early chondrogenesis, a process regulated by integrins. This pattern of expression suggests that TGase-mediated regulation of chondrocyte differentiation may be integrin-independent. Indeed, overexpression of TG2 does not activate either ERK1/2 or JNK kinases, in contrast to typical activation of these pathways by the integrin signaling (reviewed in (Juliano, 2002)). Moreover, we detect a TG2-dependent significant 30% inhibition in JNK activity in the chondrifying micromasses. Further experiments will address the role of JNK inhibition and possible interactions between PKA and JNK pathways in the TG2-reliant chondrocyte differentiation.
The novel ability of TG2 to regulate protein glycosylation, described here, may underlie the variety of biological activities of this enzyme, associated with normal development, wound healing, neurological and cardiovascular disease, as well as with cancer. The TG2-induced reduction in glycosylation may indirectly affect various signaling pathways since many transmembrane proteins engaged in signal transduction are glycosylated (reviewed in (Juliano, 2002)). In addition, matrix GAG chains play a significant role in retention of various growth factors and morphogens. For example, GAGs have been implicated in the biological activity of hedgehog (Hh) proteins by localizing them to the extracellular space and conveying the Hh signal (Koziel et al., 2004; Dierker et al., 2009). It is reasonable to suggest, that in the RCAS-TG2-infected micromasses reduced GAG content may lower the activity of Ihh even though this hypertrophy-promoting factor is up-regulated by TG2, and thus mediate blocking of the chondrocytes in the pre-hypertrophic stage without acceleration of cell hypertrophy.
In conclusion, our data provide an important insight into the mechanisms regulating proteoglycan synthesis in chondrogenesis. We have identified extracellular TG2 as a novel negative regulator of GAG synthesis. The proper deposition of the GAG-rich extracellular matrix determines cartilage stiffness to compression and its resilience. In addition, GAGs influence the function of numerous growth factors which affect chondrocytes. Thus, our findings highlight the importance of the TG2 paucity during the earliest stages of chondrogenesis and suggest that biological activity of TG2 in this developmental process must be tightly regulated, in agreement with in vivo expression of TG2 being restricted to pre-hypertrophic cartilage ((Thomazy and Davies, 1997); (Nurminskaya and Kaartinen, 2006)). The described inhibitory effect of TG2 on the deposition of cartilaginous matrix should be considered when TG2 is used in bioengineering as “biological glue” (Ehrbar et al., 2007); (Jones and Messersmith, 2007); (McHale et al., 2005)) or in other capacity. PKA signaling is an important regulator of chondrogenesis in differentiating mesenchymal cells ((Yoon et al., 2000); (Za'ka'ny, 2002); (Ciarmatori, 2007)), and identification of TG2 as a novel negative regulator of this pathway contributes to general understanding of the complex regulatory network orchestrating cartilage development and disease. Manipulations with TG2 and/or with the identified TG2-dependent signaling pathways will likely lead to advances in bioengineering of cartilage implants, and may prove instrumental for correction of developmental cartilage malformations.
4. EXPERIMENTAL PROCEDURES
4.1 Chick embryos
fertilized wild-type chicken eggs (White Leghorn; Poyndon Farm, UK; Charles River, USA) were incubated at 38°C and staged according to Hamburger and Hamilton (Hamburg and Hamilton, 1951).
4.2 Viral construct for expression of secreted TG2
was created by inserting the coding sequence for the preprotrypsin secretory peptide SALLILALVGAAVAA at the 5'-end of TG2 ORF, and insertion of the myc epitope-coding sequence GLEQKLISEEDL at the 3'-end of ORF. For this, the human TG2 ORF was modified using PCR-directed mutagenesis, by replacing the initiation Met codon with the Not I cleavage site and by replacing the termination codon with the Eag I cleavage site. The Not I/Eag I fragment comprising the TG2 ORF was cloned into the Cla I site of the RCASBP(A) vector with the help of synthetic double-stranded oligonucleotide adaptors TACGTGGGACGTGCAGCCGACCACCATGTCTGCACTTCTGATCCTAGCTCTTGTT GGAGCTGCAGTTGC (ligated at the 5'-end of ORF) and TGGAACAAAAACTCATCTCAGAAGAGGATCTGTAG (at the 3'-end). The adaptors had appropriate overhangs matching the sticky ends produced by endonuclease cleavage of the TG2 ORF fragment and vector. This procedure resulted in in-frame fusion of the TG2 ORF with sequences coding for the secretory peptide preceded by the new initiation Met codon, and for the myc epitope ending with the new termination codon. Placing the chimeric secreted myc-tagged TG2 ORF into the Cla I site of RCASBP(A) poised it for expression as a spliced mRNA driven by the ASLV promoter. The integrity of sequence cloned in RCASBP(A) was verified by sequencing. As a control for non-specific effects of viral infection, we used an RCASBP virus that encodes GFP (Clontech).
4.3 Micromass cultures
were carried out as described (Logan and Francis-West 2008) using stage 20/21 wing buds. Cells were plated at high density in the suspension of TG2- or GFP-encoding retrovirus (3–7×108 pfu/ml). The effect of TG2 overexpression on chondrogenesis was evaluated by staining the cartilaginous nodules in six-day-old cultures with Alcian Blue. Briefly, cultures were fixed in 4% paraformaldehyde, washed and stained with 1% Alcian blue (8GX) in 0.1N HCl for 1 hour. For quantitative assessment, the bound Alcian blue was extracted with 1 ml 4M guanidine hydrochloride for 1 hour with shaking and optical density of the extract measured at 590 nm. Experiments were repeated in triplicate, with at least 3 micromasses in each replicate.
4.4 Cell proliferation
was measured using the formazan-based color assay kit (MTT) (Promega) according to manufacturer's instructions.
4.5 Synthetic inhibitors
The protein kinase inhibitors (EMD Biosciences) were used as culture medium supplements throughout the micromass culture. Inhibitors were used either at their reported IC50 for cultured cells or, if IC50 values were not reported, at their Ki. Specifically, the following concentrations were used: the p38 kinase inhibitor SB203580 (10 μM), PKA inhibitors KT5720 (1 μM) and H-89 (100 nM), and PKC inhibitor bisindolylmaleimide I (10 nM) (all from Calbiochem). Transglutaminase inhibitor ERW-1069 (quinolin-3-ylmethyl (S)-1-(((S)-3-Bromo-4,5-dihydroisoxazol-5-yl)methylamino)-3-(5-fluoro-1H-indol-3-yl)-1-oxopropan-2-ylcarbamate), kindly provided by Dr. C. Khosla (Stanford University) (Watts et al., 2006), was used at 30μM. Less than 10% cytotoxicity of the employed inhibitor concentrations was confirmed using lactate dehydrogenase release assay reagents (Roche).
4.6 PKA activity
in the micromass cultures was assayed with SigmaTECT® Protein Kinase Assay System (Promega) and with ELISA-based kinase activity assay (EMD Biosciences) according to manufacturer's instructions.
4.7 Transglutaminase activity
in the cultured cells and in the medium was measured by incorporation of EZ-biotinpentilamine (Promega) into casein in microplates (Trigwell et al, 2004).
4.8 Alkaline phosphatase activity
in the cultured cells was measured by hydrolysis of p-Nitrophenyl Phosphate (Sigma), according to manufacturer's instructions.
4.9 Quantitative assay for biologically active TGFβ
was performed with a stable Cos-7 cell line that we established using the lentiviral TGFβ dependent luciferase Cignal reporter (Invitrogen). These reporter cells cells were treated for 48 hours with medium conditioned by the RCAS-TG2 or RCAS-GFP infected micromass cultured of chick limb bud mesenchymal cells. Luciferase activity in the total cell lysates was measured with luciferase assay (Promega).
4.10 Analysis of cell signaling
including TGFβ, MAPK/ERK, and MAPK/JNK pathways was performed using the plasmid-based Cignal luciferase reporters (Invitrogen). Mesenchymal cells were infected with RCAS-TG2 or RCAS-GFP, allowed to undergo chondrogenic differentiation for 7 days, and then transfected with the Cignal reporter plasmids according to the manufacturer's protocol. The β-galactosidase internal control pSV40-β-gal construct (Promega) was used as the transfection efficiency reference.
4.11 Real-time RT- PCR
Primers for detection of transcripts for TG2, N-cadherin, actin, L-19, xylosyltransferases 1 and 2, chondroitin 6-sulfotransferase 3, chondroitin 4-sulfotransferase, PouV, TERT and Nanog, coll II, coll X, F13, sox9, Cbfa1, MMp13, osteopontin, and beta-actin were designed with Oligo4.0 software. Four to five micromasses from independent experiments were analyzed in triplicates, using SYBR Green chemistry (Applied Biosystems) and iCycler IQ apparatus (Bio-Rad). Gene expression was normalized to beta-actin levels. Paired Student's t-test was used for statistical analysis.
4.12 cAMP assay
Mesenchymal cells were infected with either GFP-RCAS or TG2-RCAS and grown for 7 days. Some samples of infected cells were additionally exposed to 3 μg/ml purified guinea pig liver TG2 (Sigma) for 30 minutes. Cellular levels of cAMP were detected with cAMP-Glo assay kit (Promega)
4.13 Xylosyltransferase assay
Micromass cultures were lyzed in a buffer containing 1% Nonidet P-40, 150 mM NaCl, and 50mM tris-HCl, pH 7.8. Enzymatic activity was measured with the previously described mass spectrometric assay using a modified bikunin peptide as a substrate (Kuhn et al., 2006).
4.14 Immunohistological analysis
Micromass or embryonic limbs were fixed for 15 min in 4% paraformaldehyde and 9–10μm sections were cut with cryostat. For immunohistological analysis, sections were treated for 30min at room temperature with 0.06% hyaluronidase solution to reveal collagen epitopes and blocked with 1% bovine serum albumin for 20 min. Anti-human TG2 antibody (Upstate, Inc.), was employed Rhodamine-conjugated secondary antibodies (Pierce) were used for visualization with fluorescent Nikon microscope. For histological analysis sections were stained with hematoxylin and Alcian blue.
4.15 Culture and grafting of the retrovirus
Chick embryonic (O-line) fibroblasts (CEFs) were transfected with retroviral DNA (encoding TG2 or a control construct) as described previously (Logan and Francis-West 2008). After seven days when the cells were fully infected, the CEFs were trypsinised from the culture plate, pelleted and allowed to consolidate for one hour. The cell pellet was grafted into the right wing bud of stage 18/19 embryos and the embryos allowed to develop until day 10–14 during which time the replication-competent retrovirus spreads throughout the limb. Following manipulation, embryos were fixed at various time points, for skeletal analysis by Alcian blue/Alizarin Red S staining (McLeod, 1980). The lengths of the mineralized areas were measured in the treated and contralateral control limbs and the differences in the lengths were analyzed using a paired Student's t-test. For real-time PCR analysis radius bones were dissected from the right RCAS-TG2 infected and left control wings. The central parts of the radii, in which changes in mineralization were observed, were used for mRNA isolation and gene expression analysis, after confirmation of the virus-derived human TG2 expression by PCR.
ACKNOWLEDGEMENTS
We would like to thank Christoph Lichtenberg and Anika Wolf for technical assistance, and Dr. Chaitan Khosla for providing transglutaminase inhibitor ERW1069. This study was supported by the American Health Foundation, European Science Foundation, Maryland Stem Cell Foundation and by grants from the National Institutes of Health (to M.N.), and by ARC (to P.F-W).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aeschlimann D, Wetterwald A, Fleisch H, Paulsson M. Expression of tissue transglutaminase in skeletal tissues correlates with events of terminal differentiation of chondrocytes. J. Cell Biol. 1993;120:1461–1470. doi: 10.1083/jcb.120.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J. Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenghat FJ, Tytell JD, Thodeti CK, Derrien A, Ingber DE. Mechanical control of cAMP signaling through integrins is mediated by the heterotrimeric Galphas protein. J Cell Biochem. 2009;106:529–538. doi: 10.1002/jcb.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobick BE, Kulyk WM. Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling. Birth Defects Res C Embryo Today. 2008;84:131–54. doi: 10.1002/bdrc.20126. [DOI] [PubMed] [Google Scholar]
- Carrington JL, Reddi AH. Temporal changes in the response of chick limb bud mesodermal cells to transforming growth factor beta-type 1. Exp Cell Res. 1990;186:368–373. doi: 10.1016/0014-4827(90)90318-5. [DOI] [PubMed] [Google Scholar]
- Cassel D, Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978;75:4155–9. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p -bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Chuang DM. Beta-adrenergic receptor internalization and processing: role of transglutaminase and lysosomes. Mol Cell Biochem. 1984;58:79–89. doi: 10.1007/BF00240607. [DOI] [PubMed] [Google Scholar]
- Ciarmatori S, Kiepe D, Haarmann A, Huegel U, Tonshoff B. Signaling mechanisms leading to regulation of proliferation and differentiation of the mesenchymal chondrogenic cell line RCJ3.1C5.18 in response to IGF-I. J Mol Endocrinol. 2007;38:493–508. doi: 10.1677/jme.1.02179. [DOI] [PubMed] [Google Scholar]
- Dardik R, Inbal A. Complex formation between tissue transglutaminase II (tTG) and vascular endothelial growth factor receptor 2 (VEGFR-2): proposed mechanism for modulation of endothelial cell response to VEGF. Exp Cell Res. 2006;312:2973–2982. doi: 10.1016/j.yexcr.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Daumer KM, Tufan AC, Tuan RS. Long-term in vitro analysis of limb cartilage development: involvement of Wnt signaling. J Cell Biochem. 2004;93:526–541. doi: 10.1002/jcb.20190. [DOI] [PubMed] [Google Scholar]
- Dierker T, Dreier R, Migone M, Hamer S, Grobe K. Heparan sulfate and transglutaminase activity are required for the formation of covalently cross-linked hedgehog oligomers. J Biol Chem. 2009;284:32562–71. doi: 10.1074/jbc.M109.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrbar M, Rizzi SC, Schoenmakers RG, Miguel BS, Hubbell JA, Weber FE, Lutolf MP. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules. 2007;8:3856–3866. doi: 10.1021/bm070228f. [DOI] [PubMed] [Google Scholar]
- Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 2008;582:1552–1557. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1951;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Lee SY, Jung JC, Bang OS, Kang SS. TGF-beta3 inhibits chondrogenesis of cultured chick leg bud mesenchymal cells via downregulation of connexin 43 and integrin beta4. J Cell Physiol. 2008;214:345–53. doi: 10.1002/jcp.21202. [DOI] [PubMed] [Google Scholar]
- Janiak A, Zemskov EA, Belkin AM. Cell surface transglutaminase promotes RhoA activation via integrin clustering and suppression of the Src-p190RhoGAP signaling pathway. Mol Biol Cell. 2006;17:1606–1619. doi: 10.1091/mbc.E05-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, van Etten D, Nanda N, Graham RM, Terkeltaub RA. Distinct transglutaminase 2-independent and transglutaminase 2-dependent pathways mediate articular chondrocyte hypertrophy. J. Biol. Chem. 2003;278:18824–18832. doi: 10.1074/jbc.M301055200. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Terkeltaub RA. External GTP-bound transglutaminase 2 is a molecular switch for chondrocyte hypertrophic differentiation and calcification. J. Biol. Chem. 2005;280:15004–15012. doi: 10.1074/jbc.M500962200. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Rose DM, Terkeltaub RA. Factor XIIIA mobilizes transglutaminase 2 to induce chondrocyte hypertrophic differentiation. J Cell Sci. 2008;121(Pt 13):2256–64. doi: 10.1242/jcs.011262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Messersmith PB. Facile coupling of synthetic peptides and peptide-polymer conjugates to cartilage via transglutaminase enzyme. Biomaterials. 2007;28:5215–5224. doi: 10.1016/j.biomaterials.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL. Signal transduction by cell adhesion. Receptors and the cytoskeleton: funtions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Conrad HE. Properties of cultured chondrocytes obtained from histologically distinct zones of the chick embryo tibiotarsus. J Biol Chem. 1977;252:8292–9. [PubMed] [Google Scholar]
- Kluppel M, Wight TN, Chan C, Hinek A, Wrana JL. Maintenance of chondroitin sulfation balance by chondroitin-4-sulfotransferase 1 is required for chondrocyte development and growth factor signaling during cartilage morphogenesis. Development. 2005;132:3989–4003. doi: 10.1242/dev.01948. [DOI] [PubMed] [Google Scholar]
- Koseki-Kuno S, Yamakawa M, Dickneite G, Ichinose A. Factor XIII A subunit-deficient mice developed severe uterine bleeding events and subsequent spontaneous miscarriages. Blood. 2003;102:4410–2. doi: 10.1182/blood-2003-05-1467. [DOI] [PubMed] [Google Scholar]
- Koziel L, Kunath M, Kelly OG, Vortkamp A. Ext1-Dependent Heparan Sulfate Regulates the Range of Ihh Signaling during Endochondral Ossification. Developmental Cell. 2004;6:801–813. doi: 10.1016/j.devcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Kuhn C. Prante, Schön S, Götting C, Kleesiek K. Measurement of fibrosis marker xylosyltransferase I activity by HPLC electrospray ionization tandem mass spectrometry. Clin. Chem. 2006;52:2243–2249. doi: 10.1373/clinchem.2006.071167. [DOI] [PubMed] [Google Scholar]
- Logan C, Francis-West P. Gene transfer in avian embryos using replication-competent retroviruses. Methods Mol Biol. 2008;461:363–76. doi: 10.1007/978-1-60327-483-8_26. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Mello MA, Tuan RS. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095–2105. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276:20673–8. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–1596. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- Nunes I, Gleizes PE, Metz CN, Rifkin DB. Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta. J Cell Biol. 1997;136:1151–63. doi: 10.1083/jcb.136.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminskaya M, Linsenmayer TF. Identification and characterization of up-regulated genes during chondrocyte hypertrophy. Dev. Dyn. 1996;206:60–271. doi: 10.1002/(SICI)1097-0177(199607)206:3<260::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Nurminskaya MV, Linsenmayer TF. Immunohistological analysis of transglutaminase factor XIIIA expression in mouse embryonic growth plate. J Orthop Res. 2002;20:575–8. doi: 10.1016/S0736-0266(01)00146-2. [DOI] [PubMed] [Google Scholar]
- Nurminskaya M, Kaartinen MT. Transglutaminases in mineralized tissues. Frontiers in Biosciences. 2006;11:1591–1606. doi: 10.2741/1907. [DOI] [PubMed] [Google Scholar]
- Nurminskaya M, Magee C, Nurminsky D, Linsenmayer TF. Plasma transglutaminase in hypertrophic chondrocytes: expression and cell-specific intracellular activation produce cell death and externalization. J. Cell Biol. 1998;142:1135–1144. doi: 10.1083/jcb.142.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminskaya M, Magee C, Faverman L, Linsenmayer TF. Chondrocyte-derived transglutaminase promotes maturation of preosteoblasts in periosteal bone. Dev. Biol. 2003;263:139–152. doi: 10.1016/s0012-1606(03)00445-7. [DOI] [PubMed] [Google Scholar]
- Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120:177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- Ooira A, Kimata K, Suzuki S, Takata K, Suzuki I. A correlation between synthetic activities for matrix macromolecules and specific stages of cyto-differentiation in developing cartilage. J. Biol Chem. 1974;249(5):1637–45. [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpellini A, Germack R, Lortat-Jacob H, Muramatsu T, Billett E, Johnson T, Verderio EA. Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J Biol Chem. 2009;284(27):18411–23. doi: 10.1074/jbc.M109.012948. 2009 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid TM, Linsenmayer TF. Developmental acquisition of type X collagen in the embryonic chick tibiotarsus. Dev. Biol. 1985;107:373–381. doi: 10.1016/0012-1606(85)90319-7. [DOI] [PubMed] [Google Scholar]
- Tarantino U, Oliva F, Taurisano G, Orlandi A, Pietroni V, Candi E, Melino G, Maffulli N. FXIIIA and TGF-beta over-expression produces normal musculo-skeletal phenotype in TG2−/− mice. Amino Acids. 2009;36:679–84. doi: 10.1007/s00726-008-0133-7. [DOI] [PubMed] [Google Scholar]
- Thomazy VA, Davies PJ. Expression of tissue transglutaminase in the developing chicken limb is associated both with apoptosis and endochondral ossification. Cell Death. Differ. 1999;6:146–154. doi: 10.1038/sj.cdd.4400464. [DOI] [PubMed] [Google Scholar]
- Trigwell SM, Lynch PT, Griffin M, Hargreaves AJ, Bonner PL. An improved colorimetric assay for the measurement of transglutaminase (type II)-(gamma-glutamyl) lysine cross-linking activity. Anal Biochem. 2004;330:164–6. doi: 10.1016/j.ab.2004.03.068. [DOI] [PubMed] [Google Scholar]
- Watts RE, Siegel M, Khosla C. Structure-activity relationship analysis of the selective Inhibition of transglutaminase 2 by dihydroisoxazoles. J. Med. Chem. 2006;49:7493–7501. doi: 10.1021/jm060839a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Khan S, Beier F. C-type natriuretic peptide regulates cellular condensation and glycosaminoglycan synthesis during chondrogenesis. Endocrinology. 2007;148:5030–41. doi: 10.1210/en.2007-0695. [DOI] [PubMed] [Google Scholar]
- Xu L, Hynes RO. GPR56 and TG2: possible roles in suppression of tumor growth by the microenvironment. Cell Cycle. 2007;6:160–5. doi: 10.4161/cc.6.2.3760. [DOI] [PubMed] [Google Scholar]
- Yoon YM, Oh CD, Kang SS, Chun JS. Protein kinase A regulates chondrogenesis of mesenchymal cells at the post-precartilage condensation stage via protein kinase C-alpha signaling. J Bone Miner Res. 2000;15:2197–2205. doi: 10.1359/jbmr.2000.15.11.2197. [DOI] [PubMed] [Google Scholar]
- Za'ka'ny R, Szucs K, Bako' E, Felszeghy S, Czifra G, Bi'ro' T, Mo'dis L, Gergely P. Protein phosphatase 2A is involved in the regulation of protein kinase A signaling pathway during in vitro chondrogenesis. Exp Cell Res. 2002;275:1–8. doi: 10.1006/excr.2002.5487. [DOI] [PubMed] [Google Scholar]
- Zhen X, Wei L, Wu Q, Zhang Y, Chen Q. Mitogen-activated protein kinase p38 mediates regulation of chondrocyte differentiation by parathyroid hormone. J Biol Chem. 2001;276:4879–85. doi: 10.1074/jbc.M004990200. [DOI] [PubMed] [Google Scholar]