Abstract
Rationale
Cannabinoid CB1 receptor agonists vary in efficacy in vitro; however, relationships between efficacy and behavioral effects are unclear.
Objective
This study examined the relationship between apparent CB1 agonist efficacy and in vivo effects.
Methods
Male C57BL/6J mice responded for food under a fixed ratio 30 schedule; rectal temperature was measured. Sensitivity of the mice to cannabinoid agonists (rank order efficacy in vitro reported to be CP 55940>anandamide>Δ9-tetrahydrocannabinol; Δ9-THC) and a non-cannabinoid (the benzodiazepine midazolam) was determined before, during, and after discontinuation of daily Δ9-THC treatment (32 mg/kg/day, i.p.). Rimonabant was combined with cannabinoids to examine whether CB1 receptors mediated effects on response rate.
Results
Δ9-THC, CP 55940, anandamide, and midazolam decreased responding at doses smaller than those producing hypothermia. Rimonabant antagonized the rate-decreasing effects of Δ9-THC and CP 55940, but not those of anandamide. Δ9-THC treatment produced tolerance for both rate-decreasing and hypothermic effects. Δ9-THC treatment did not change sensitivity to the rate-decreasing effects of CP 55940, but produced cross-tolerance to CP 55940 for hypothermic effects. Δ9-THC treatment did not modify sensitivity to anandamide and midazolam.
Conclusions
CB1 receptors mediate the operant rate-decreasing effects of Δ9-THC and CP 55940, but not anandamide, in mice. CB1 agonist efficacy is an important determinant of in vivo effects, especially with regard to the magnitude of tolerance and cross-tolerance resulting from daily Δ9-THC treatment. This applies not only to different cannabinoids when measuring the same effect but also to the same cannabinoid when measuring different effects.
Keywords: Agonist efficacy, Cannabinoid, CB1, Cross-tolerance, Delta-9-tetrahydrocannabinol, Hypothermia, Mouse, Schedule-controlled behavior, Tolerance
Introduction
Smoking or ingestion of Cannabis sativa preparations such as marijuana (leaves and flowering tops) can result in sedation, cognitive dysfunction, short-term memory disruption, altered time assessment, perceptual changes, motor in-coordination, and impaired executive function (Dewey 1986; Hollister 1986; Pertwee 1988; Abood and Martin 1992). The effects of Cannabis result from actions of Δ9-tetrahydrocannabinol (Δ9-THC) (Mechoulam and Gaoni 1965). In addition to Δ9-THC, a number of structurally diverse cannabinoid agonists, some (e.g., anandamide) derived from brain and others (e.g., CP 55940) not found in nature, bind to two cannabinoid receptor subtypes: CB1 and CB2. The receptor subtypes differ in amino acid sequence, signaling mechanisms, and tissue distribution (Howlett 2002). CB1 receptors are seven transmembrane-spanning receptors coupled to the Gi/o class of G-proteins, are widely distributed in brain (e.g., Herkenham et al. 1991; Gifford et al. 1999), and are generally thought to mediate the high produced by marijuana as well as most other centrally mediated effects of the cannabinoids.
CB1 receptor agonists vary in the maximum to which they stimulate G-protein-coupled signaling (i.e., agonist efficacy), as evidenced by [35S] guanosine 5′–3′thiotriphosphate (GTPγS) binding in rodent brain tissue, with the following rank order: CP 55940>anandamide>Δ9-THC (Breivogel and Childers 2000; Childers 2006). However, in vivo, even low efficacy agonists such as Δ9-THC often produce the same maximum effect as that obtained with high efficacy agonists (e.g., Fan et al. 1994), perhaps reflecting the large number of cannabinoid receptors (i.e., spare receptors) in the central nervous system (Gifford et al. 1999). According to receptor theory (Kenakin 1997, 2002), low efficacy agonists occupy more CB1 receptors than high efficacy agonists at equal levels of effect. In addition to agonists differing in efficacy, different in vivo effects might require increasing levels of CB1 receptor activation or efficacy which, in turn, could result in a decrease in potency. In other words, when the CB1 agonist efficacy required for an effect is relatively low, smaller doses are sufficient, whereas larger doses might be required to produce an effect requiring relatively high efficacy. If cannabinoid efficacy is important in vivo, then loss of receptor function should be accompanied by a greater decrease in sensitivity (i.e., tolerance and cross-tolerance) to a low efficacy agonist as compared with a higher efficacy agonist. Moreover, loss of sensitivity to an effect requiring high efficacy will be greater as compared to another effect requiring lower efficacy. Numerous mechanisms have been implicated in loss of CB1 receptor function including receptor dimerization, changes in G-proteins and other proteins involved in second messenger signaling, receptor internalization, and receptor loss (Smith et al. 2010). Because chronic CB1 receptor agonist treatment has been repeatedly shown to decrease CB1 receptor function (Dill and Howlett 1988; Oviedo et al. 1993; Rodríguez de Fonseca et al. 1994; Coutts et al. 2001), such treatment was chosen in the current study to examine relationships between efficacy and effect.
The current study compared not only in vivo effects of cannabinoids (Δ9-THC, CP 55940, and anandamide) that vary in agonist efficacy in vitro (Breivogel and Childers 2000; Childers 2006) but also two in vivo effects (decreases in schedule-controlled responding and hypothermia) that vary in their sensitivity to cannabinoids and, perhaps, efficacy required for agonist activity. Male C57BL/6J mice were trained to respond under an FR30 schedule of food presentation; rectal temperature was obtained during operant sessions. Sensitivity of the mice to the cannabinoids and a non-cannabinoid (the benzodiazepine midazolam) was determined before, during, and after discontinuation of Δ9-THC treatment (32 mg/kg/day i.p.). In a previous study (Giuffrida and McMahon 2010), the CB1 antagonist rimonabant dose-dependently antagonized the hypothermic effects of Δ9-THC and CP 55940, demonstrating involvement of CB1 receptors. However, even though anandamide is a CB1 agonist (Devane et al. 1992), its hypothermic effects were not antagonized by rimonabant (Adams et al. 1998; Giuffrida and McMahon 2010). In the current study, rimonabant was combined with the agonists to evaluate the role of CB1 receptors in cannabinoid-induced decreases in fixed ratio responding in mice. Collectively, these studies tested the hypothesis that daily Δ9-THC treatment would produce a greater loss of sensitivity to low as compared with high efficacy CB1 agonists and, further, would produce a greater loss of sensitivity to effects requiring high CB1 agonist efficacy as compared with effects requiring lower agonist efficacy.
Materials and methods
Subjects
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were purchased at 5 weeks of age (approximately 15 g) and were housed individually on a 14/10-h light/dark cycle. Mice were maintained at 85% of free-feeding weight and received approximately 1 cm3 of a 1-to-1 mixture of condensed milk and tap water during experimental sessions and 2.5 g of food (Dustless Precision Pellets 500 mg, Rodent Grain-Based Diet; Bio-Serv, Frenchtown, NJ, USA) per day after sessions; water was available ad libitum in the home cage. Mice were habituated to the experimental room for 7 days before the first experimental session, and testing was conducted during the light period. Mice were maintained, and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the “Principles of Laboratory Animal Care” and the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
Apparatus
Commercially available mouse operant conditioning chambers (MedAssociates, St. Albans, VT, USA) were placed in ventilated, sound-attenuating enclosures. The ceiling of each operant conditioning chamber contained a light (i.e., house light) and the side of one wall contained a recessed hole (2.2-cm diameter). The center of the hole was positioned 1.6 cm from the floor. The hole contained a photo beam, a light, and a dipper to which 0.01 cm3 of condensed milk could be delivered from a tray positioned outside the operant conditioning chamber. An interface (MedAssociates) connected the operant conditioning chambers to a computer, and experimental events were controlled and recorded with Med-PC software. Temperature was measured by inserting a probe 2 cm into the rectum; a digital recording was obtained from a commercially available thermometer (model BAT-7001H; Physitemp Instruments, Inc., Clifton, NJ, USA).
Operant conditioning procedure
Sessions were conducted once daily 7 days per week. Mice initially responded under a schedule of continuous reinforcement. The hole containing the photobeam was illuminated and mice could insert their snouts to disrupt the photobeam; disruption of the photobeam resulted in 10-s access to 0.01 cm3 of milk. During 10-s access to milk, the light in the recessed hole was turned off and the house light was illuminated. Disruptions of the photobeam during the 10-s period of milk availability had no programmed consequence. Training under the schedule of continuous reinforcement was conducted for three sessions; sessions ended when 100 reinforcers were earned or after 30 min, whichever occurred first. Thereafter, the fixed ratio requirement was increased every three sessions to an FR3, FR10, and FR30. After three sessions at FR30, sessions were divided into 20-min, consecutive cycles. Each cycle began with a 15-min pretreatment, during which time the chamber was dark and responding had no programmed consequence. The 15-min pretreatment was followed by a 5-min period of milk availability under the FR30 schedule. Mice received intraperitoneal injections of vehicle in the first minute of the first cycle, followed by vehicle or sham injections (dull pressure applied to abdominal area) non-systematically in the first minute of subsequent cycles. Training was conducted until mean response rate during a session was ±20% of the mean response rate for all cycles in the five preceding sessions. After satisfying this criterion, drugs were administered during sessions; successive drug tests were conducted only when responding during the immediately preceding non-drug session was ±20% of the mean rate for all cycles during the five previous non-drug sessions.
Tests with rimonabant in combination with cannabinoid agonists
The effects of rimonabant in combination with Δ9-THC, CP 55940, and anandamide were examined using a within-subjects design. The effects of Δ9-THC in combination with rimonabant were examined in seven mice. Three of these mice and four other pharmacologically naïve mice (i.e., not tested with Δ9-THC) were used to examine the effects of rimonabant in combination with CP 55940 and anandamide. A control dose–response curve (i.e., agonist alone) was determined first, followed by studies with that agonist in the presence of three doses (1, 3.2, and 10 mg/kg, i.p.) of rimonabant; the order of testing with various doses of rimonabant was non-systematic. A second control dose–response curve was determined after studies with rimonabant. This sequence of testing (i.e., agonist alone, followed by agonist in combination with rimonabant, followed by agonist alone) was completed for one agonist before initiating the sequence with a second agonist. Agonist dose–response functions were determined by administering vehicle or a dose of rimonabant at the beginning of the first cycle, followed by cumulative doses of agonist increasing by 0.25 or 0.5 log unit per cycle. The number of cycles for a test was determined by the number of cycles required to complete the dose–effect curve (i.e., from an ineffective dose to a dose that resulted in fewer than 30 responses during a cycle).
Tests with cannabinoids and a non-cannabinoid before, during, and after discontinuation of daily Δ9-THC treatment
The four mice that had been tested with rimonabant in combination with acute Δ9-THC (see above), and three other pharmacologically naïve mice, were used to examine the effects of daily Δ9-THC treatment. Mice were trained to respond under an FR30 schedule of milk presentation during six, consecutive 20-min cycles as described above. Before drug tests, mice received saline or sham at the beginning of cycles and rectal temperature was measured at the end of each cycle until responding stabilized, defined as five consecutive days with response rate within ±20% of the mean rate for those days. After satisfying this criterion, control dose–response curves for Δ9-THC, CP 55940, anandamide, and midazolam were determined by administering vehicle at the beginning of the first cycle followed by cumulative doses in subsequent cycles. Successive drug tests were conducted only when responding during the immediately preceding non-drug session was ±20% of the mean rate for all cycles during the five previous non-drug sessions. The number of cycles for a test depended on the number of cycles required to complete a dose–effect curve for hypothermic effects, i.e., from an ineffective dose to a dose that produced maximum hypothermia as determined previously (Giuffrida and McMahon 2010). The order of testing with the various drugs was non-systematic.
For studies with daily Δ9-THC, a dose of 32 mg/kg/day was chosen. When administered acutely, this dose (32 mg/kg) of Δ9-THC suppressed responding for up to 4 h; therefore, a 6-h pretreatment was chosen so that mice would respond during experimental sessions. Fifty-six days elapsed between the first and last day of Δ9-THC treatment; however, on days when drugs were administered in cumulative doses during the session, Δ9-THC treatment was omitted (i.e., mice received an injection of vehicle 6 h before the session). On days when Δ9-THC (32 mg/kg) was administered 6 h before sessions, mice received vehicle or sham at the beginning of two to six cycles with the number of cycles varying non-systematically across days. Dose–response curves were determined in a single session by administering vehicle in the first cycle followed by cumulative doses in subsequent cycles. Dose–response curves for Δ9-THC were determined on days 8, 15, and 54 of daily treatment. For other drugs, dose–response curves were determined between days 15 and 54 of daily treatment and when response rate during the immediately preceding session was ±20% of the mean rate for all cycles during the five previous sessions, excluding days on which Δ9-THC (32 mg/kg) treatment was omitted. Drugs were studied up to doses that markedly decreased response rate; doses larger than 56 mg/kg Δ9-THC and 3.2 mg/kg CP 55940 were not studied to minimize disruption in operant responding on the following day. During Δ9-THC treatment, the order of testing with CP 55940, anandamide, and midazolam was non-systematic.
Daily Δ9-THC treatment was discontinued by administering vehicle instead of sham before daily sessions; vehicle or sham was administered at the beginning of cycles on days when drugs were not administered. A dose–response curve was re-determined for Δ9-THC on day 9 of the discontinuation period. Thereafter, dose–response curves for CP 55940, anandamide, and midazolam were determined in non-systematic order when response rate during the immediately preceding session was ±20% of the mean rate for all cycles during the five previous non-drug sessions.
Drugs
Rimonabant and Δ9-THC (100 mg/ml in absolute ethanol) were obtained from The Research Technology Branch, National Institute on Drug Abuse (Rockville, MD, USA); CP 55940 was obtained from Tocris (Ellisville, MO, USA). Anandamide was synthesized according to a protocol developed by Giuffrida and Piomelli (1998a); the final product was analyzed with gas chromatography and mass spectrometry as described (Giuffrida and Piomelli 1998b; Hardison et al. 2006). The ethanol was evaporated and rimonabant, Δ9-THC, CP 55940, and anandamide were dissolved in a mixture of one part propylene glycol (Sigma), one part Tween 80 (Sigma), and 18 parts physiologic saline. Midazolam hydrochloride (5 mg/ml in physiologic saline; Bedford Laboratories, Bedford, OH, USA) was diluted in physiologic saline as needed. All drugs were administered i.p. in a volume of 10 ml/kg and dose was expressed as the weight of the forms listed above in milligrams per kilogram of body weight.
Data analysis
Control rate of responding (responses per second) was calculated as the average rate for all cycles in the five non-drug (i.e., vehicle) sessions immediately preceding a drug session. Control response rate, with and without measurement of rectal temperature, was compared with a paired t test (p<0.05). Response rate data were plotted and analyzed as a percentage of the control response rate. The effects of drugs to decrease body temperature were plotted as the change from the vehicle control in degrees Celsius. Differences in maximum hypothermic effect among drugs were analyzed with repeated measures oneway ANOVA and Tukey all pair-wise comparison procedure (p<0.05). To compare the potency of drugs to produce hypothermia, dose–response data for each drug were converted to a percentage of the maximum effect determined for each respective drug before daily Δ9-THC treatment. Potency was calculated by simultaneously fitting straight lines to individual dose–effect data by means of GraphPad Prism version 5.0 for Windows (San Diego, CA, USA) with linear regression. Straight lines were fitted to the linear portion of dose–effect curves. The slopes of dose–effect curves were compared with an F-ratio test using GraphPad; if the slopes were not significantly different, then a common, best-fitting slope was used for further analyses (Kenakin 1997). Doses corresponding to the 50% level of the effect (ED50 values), potency ratios, and their 95% confidence limits were calculated with parallel line analyses of data from individual subjects (Tallarida 2000). The potencies were considered significantly different when the 95% confidence limits of the potency ratio did not include 1.
Relatively large doses of rimonabant tended to decrease response rate when administered before cumulative doses of agonist; therefore, the effects of rimonabant in combination with Δ9-THC, CP 55940, and anandamide were analyzed by expressing data as a percentage of the response rate following each respective dose of rimonabant alone.
Results
Control performance and rate-decreasing effects of cannabinoid agonists alone and in combination with rimonabant
When response rate for five non-drug sessions were averaged for individual mice, the median value of absolute response rate among mice (n=14) was 2.9 responses per second; the range was 1.1–4.8 responses per second. Response rate did not significantly vary as a function of whether rectal temperature was measured during the experimental session (n=7; mean=2.9 responses per second; SEM=0.41) or not (n=7; mean=3.1 responses per second; SEM=0.51) (p>0.05).
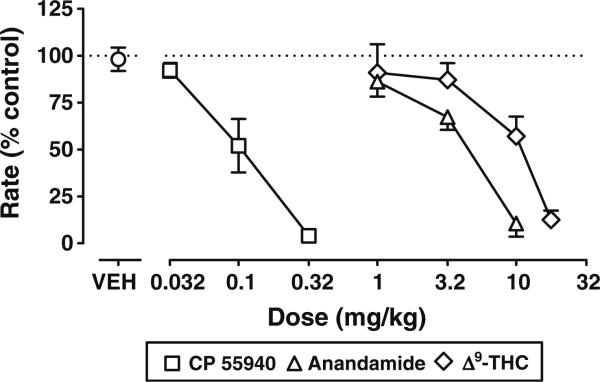
Vehicle i.p. did not significantly modify response rate, whereas Δ9-THC, CP 55940, and anandamide dose-dependently decreased response rate (Fig. 1). For example, response rate was decreased to less than 20% of control by Δ9-THC at a dose of 17.8 mg/kg, CP 55940 at a dose of 0.32 mg/kg, and anandamide at a dose of 10 mg/kg. The two control agonist dose–response functions (i.e., ED50 values), one determined before and the other after the agonist was combined with various doses of rimonabant, were not significantly different from each other (p>0.05); therefore, the data were averaged for graphic presentation (Fig. 1) and further analysis. The slopes of the dose– response curves were not significantly different (p>0.05); CP 55940 (ED50 value=0.10 mg/kg) was 39-fold more potent than anandamide (ED50 value=3.9 mg/kg), which was 2-fold more potent than Δ9-THC (ED50 value=7.5 mg/kg) (Table 1).
Fig. 1.
Effects of CP 55940, anandamide, and Δ9-THC to decrease responding for food in mice. Abscissae vehicle (VEH) or i.p. dose in milligrams per kilogram of body weight. Ordinates mean (±SEM) rate of responding expressed as a percentage of control
Table 1.
ED50 values and 95% confidence limits for the effects of Δ9-THC, CP 55940, and anandamide, alone and in combination with rimonabant, to decrease rate of operant responding
| ED50 in mg/kg (95% confidence limits) | Potency ratioa (95% confidence limits) | |
|---|---|---|
| Agonists alone | ||
| CP 55940 | 0.10 (0.072–0.14) | |
| Anandamide | 3.86 (2.85–5.23) | vs. CP 39 (24–59) |
| Δ9-THC | 7.54 (5.41–10.5) | vs. CP 74 (46–120) |
| Δ9-THC | vs. Anand 2.0 (1.2–3.1) | |
| Rimonabant antagonismb | ||
| Δ9-THC alone | 7.54 (5.41–10.5) | |
| +Rimonabant (1 mg/kg) | 12.7 (7.66–21.0) | 1.7 (1.0–3.0) |
| +Rimonabant (3.2 mg/kg) | 17.8 (11.0–28.8) | 2.4c (1.4–4.1) |
| + Rimonabant (10 mg/kg) | 6.19 (2.79–13.7) | 0.8 (0.4–1.6) |
| CP 55940 alone | 0.10 (0.072–0.14) | |
| + Rimonabant (1 mg/kg) | 0.46 (0.11–1.85) | 4.6c (1.2–17) |
| +Rimonabant (3.2 mg/kg) | 0.15 (0.09–0.23) | 1.5 (0.8–2.6) |
| + Rimonabant (10 mg/kg) | 0.20 (0.067–0.63) | 2.0 (0.6–6.7) |
| Anandamide alone | 3.86 (2.85–5.23) | |
| + Rimonabant (1 mg/kg) | 2.80 (1.74–4.51) | 0.7 (0.4–1.2 0) |
| +Rimonabant (3.2 mg/kg) | 1.50 (0.72–3.10) | 0.4d (0.2–0.7) |
| + Rimonabant (10 mg/kg) | 2.83 (1.40–5.76) | 0.7 (0.4–1.5) |
Potency ratios and 95% confidence limits are the ED50 values of agonist divided by the ED50 value of CP 55940 or anandamide, and the ED50 values of the agonists in combination with an antagonist divided by the ED50 value of the agonist alone
Agonist dose–response data were expressed and analyzed as a percentage of the response rate following each respective dose of rimonabant alone
Significant antagonism
Significant increase in potency
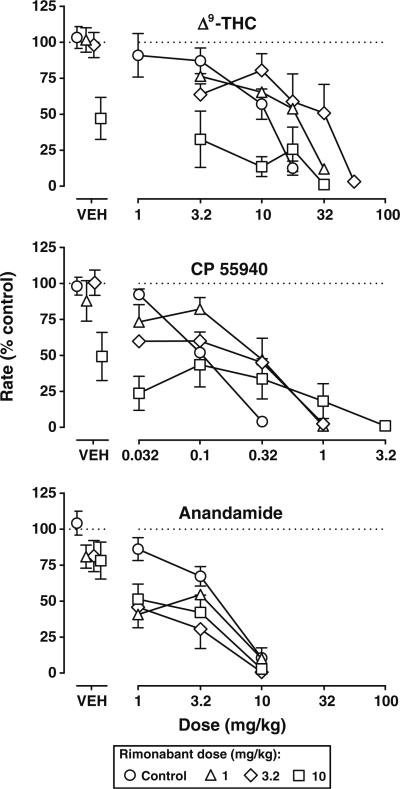
Rimonabant, up to the largest dose (10 mg/kg) studied, decreased response rate to a maximum of 47% (Fig. 2, top, square above VEH). The slopes of the Δ9-THC dose–response curves alone and in combination with various doses of rimonabant were not significantly different (p> 0.05). Rimonabant dose-dependently antagonized the effects of Δ9-THC to decrease responding, albeit the magnitude of antagonism was relatively small (Fig. 2, top). Rimonabant (3.2 mg/kg) significantly decreased the potency of Δ9-THC 2.4-fold (Table 1); the smaller and larger doses (1 and 10 mg/kg, respectively) of rimonabant did not produce significant antagonism. The slopes of the CP 55940 dose–response curves alone and in combination with various doses of rimonabant were not significantly different. Rimonabant produced similar antagonism of the rate-decreasing effects of CP 55940. There was a tendency for each dose of rimonabant to produce some antagonism of the rate-decreasing effects of CP 55940 (Fig. 2, middle); however, only a dose of 1 mg/kg of rimonabant significantly increased the ED50 value of CP 55940 (Table 1). The slopes of the anandamide dose–response curves alone and in combination with rimonabant did not significantly differ; however, in contrast to Δ9-THC and CP 55940, rimonabant did not antagonize the rate-decreasing effects of anandamide (Fig. 2, bottom). Rather, a dose of 3.2 mg/kg of rimonabant significantly increased the potency of anandamide (Table 1).
Fig. 2.
Effects of rimonabant alone and in combination with Δ9-THC (top), CP 55940 (middle), and anandamide (bottom) on responding for food in mice. Abscissae vehicle (VEH) or i.p. dose in milligrams per kilogram of body weight. Ordinates mean (±SEM) rate of responding expressed as a percentage of control. Points above VEH represent the average response rate after vehicle or a dose (1–10 mg/kg i.p.) of rimonabant alone. Control data are re-plotted from Fig. 1. Rimonabant was administered prior to cumulative doses of the agonists
Rate-decreasing and hypothermic effects of drugs before, during, and after discontinuation of daily Δ9-THC (32 mg/kg/day)
When examined for each drug separately, the slopes of the dose–response curves determined before, during, and after discontinuation of daily Δ9-THC (32 mg/kg/day) treatment were not significantly different. Similarly, for hypothermia, the slopes of the anandamide dose–effect curves did not significantly differ; however, the slopes were significantly different for the dose–response curves for Δ9-THC and CP 55940 to produce hypothermia (p<0.05). Before daily treatment, Δ9-THC, CP 55940, and anandamide dose-dependently decreased response rate (Fig. 3, top). The ED50 values were 5.64 mg/kg for Δ9-THC, 0.12 mg/kg for CP 55940, and 5.45 mg/kg for anandamide (Table 2). These ED50 values were comparable to those obtained when not measuring rectal temperature (see Table 1). Δ9-THC, CP 55940, and anandamide dose-dependently decreased body temperature (Fig. 3, bottom); the largest doses tested (i.e., 32 mg/kg, 3.2 mg/kg, and 56 mg/kg, respectively) also produced the maximum decrease in rectal temperature, which was 2.6°C for Δ9-THC, 8.9°C for CP 55940, and 5.4°C for anandamide. The maximum hypothermic effect of Δ9-THC was significantly less than the maximum obtained with anandamide which, in turn, was significantly less than the maximum produced by CP 55940 (F2,12=52.84; p<0.001). For each drug, the ED50 value for decreasing rectal temperature was greater (i.e., between 1.8- and 4.0-fold) than its respective ED50 value for decreasing response rate (Table 2).
Fig. 3.
Effects of Δ9-THC (left), CP 55940 (middle), and anandamide (right) to decrease responding for food (top panels) and to produce hypothermia (bottom panels) before (circles), during (triangles), and after discontinuation (squares) of daily Δ9-THC (32 mg/kg) treatment in mice. Abscissae vehicle (VEH) or i.p. dose in milligrams per kilogram of body weight. Ordinates mean (±SEM) rate of responding expressed as a percentage of control (top) and mean (±SEM) change in body temperature as degrees Celsius (bottom)
Table 2.
ED50 values and 95% confidence limits for drugs to decrease rate of operant responding (Response Rate) and rectal temperature (Temperature) before, during, and after discontinuation of daily Δ9-THC (32 mg/kg) treatment
| ED50 in mg/kg (95% confidence limits) | Potency ratioa (95% confidence limits) | |
|---|---|---|
| Δ9-THC response rate | ||
| Before | 5.64 (3.52–9.03) | |
| During | 46b (3.7–570) | 8.2 (1.4–48) |
| After | 17.9b (7.23–44.5) | 3.2 (1.4–7.5) |
| Δ9-THC temperature | ||
| Before | 10.2 (7.06–14.8) | |
| During | c | |
| After | 23.0b (15.8–33.5) | 2.3 (1.4–3.7) |
| CP 55940 response rate | ||
| Before | 0.12 (0.069–0.21) | |
| During | 0.20 (0.11–0.35) | 1.7 (0.8–3.5) |
| After | 0.17 (0.049–0.60) | 1.4 (0.5–4.1) |
| CP 55940 temperature | ||
| Before | 0.48 (0.37–0.67) | |
| During | c | |
| After | 2.01b (1.26–3.18) | 4.2 (2.5–7.2) |
| Anandamide response rate | ||
| Before | 5.45 (3.42–8.69) | |
| During | 7.46 (3.23–17.2) | 1.4 (0.6–2.9) |
| After | 5.25 (3.32–8.31) | 1.0 (0.5–1.8) |
| Anandamide temperature | ||
| Before | 19.2 (15.3–24.2) | |
| During | 20.4 (14.7–28.4) | 1.1 (0.7–1.6) |
| After | 16.1 (12.4–20.7) | 0.8 (0.6–1.2) |
| Midazolam response rate | ||
| Before | 4.03 (2.54–6.41) | |
| During | 23.3 (13.1–41.3) | d |
| After | 4.37 (2.32–8.23) | 1.1 (0.5–2.2) |
| Midazolam temperature | ||
| Before | 11.0 (6.65–18.3) | |
| During | 22.4 (8.25–60.7) | 2.0 (0.8–5.4) |
| After | 9.77 (5.65–16.9) | 0.9 (0.4–1.8) |
Potency ratios and 95% confidence limits are ED50 values determined during or after discontinuation of Δ9-THC treatment divided by the ED50 value determined before Δ9-THC treatment
Significantly different from Before
ED50 value not determined; the largest dose did not produce greater than 50% effect
Potency ratio not calculated because slopes were significantly different
Administration of 32 mg/kg of Δ9-THC 6 h before the session decreased response rate to 19% of control (Fig. 4; top, compare day 1 of During to Before) and also decreased temperature by 2.1°C relative to temperature measured the day before (i.e., when vehicle was administered; Fig. 4, bottom). Response rate continued to be decreased between days 2 and 7 of Δ9-THC (32 mg/kg/day) treatment. From day 11 until the last day of treatment, there was an incomplete loss of sensitivity to the rate-decreasing effects of Δ9-THC, which appeared to be steady throughout that period; the median response rate was 63% of control and the range was 43–78% of control. In contrast, loss of sensitivity to the hypothermic effects of Δ9-THC occurred more rapidly (i.e., by day 2 of treatment); from day 2 until the end of treatment, the median decrease in temperature was 0.6°C and the range was 0.1–1.1°C.
Fig. 4.
Response rate (top) and rectal temperature (bottom) before, during, and after discontinuation of daily Δ9-THC (32 mg/kg) treatment in mice. Abscissae consecutive days before, during, and after discontinuation of daily Δ9-THC treatment. Ordinates mean (±SEM) rate of responding expressed as a percentage of control (top) and mean (±SEM) body temperature as degrees Celsius (bottom)
During Δ9-THC treatment (32 mg/kg/day), there was a significant decrease in the potency of Δ9-THC to reduce response rate. For example, on day 15 of treatment, the potency of Δ9-THC was decreased 8.2-fold (Fig. 3, top left, compare triangles to circles). On days 8 and 54 of treatment, potency also was significantly decreased 7.6-and 1.9-fold, respectively. In contrast, the potency of CP 55940 and anandamide to decrease response rate were not significantly modified by Δ9-THC treatment (Fig. 3, top middle and right, respectively; Table 2). The slopes of the midazolam dose–response curves determined before and during Δ9-THC treatment were significantly different (p<0.05) (Fig. 5, top); due to the relatively steep slope of the curve determined during treatment as compared with that determined before treatment, the ED50 values could not be readily compared.
Fig. 5.
Effects of midazolam to decrease responding for food presentation (top panels) and to produce hypothermia (bottom panels) before (circles), during (triangles), and after discontinuation (squares) of daily Δ9-THC (32 mg/kg) treatment in mice. Abscissae vehicle (VEH) or i.p. dose of midazolam in milligrams per kilogram of body weight. Ordinates mean (±SEM) rate of responding expressed as a percentage of control (top) and mean (±SEM) change in body temperature as degrees Celsius (bottom)
The potency of Δ9-THC to produce hypothermia also was decreased during Δ9-THC treatment; up to the largest dose (56 mg/kg), the maximum decrease in temperature was 0.7°C (Fig. 3, bottom left, compare triangles to circles). In contrast to the absence of a change in sensitivity to the rate-decreasing effects of CP 55940, sensitivity to the hypothermic effects of CP 55940 was markedly decreased during Δ9-THC treatment (Fig. 3, bottom middle). Up to the largest dose (3.2 mg/kg), the maximum decrease in temperature was −1.6°C. The ED50 values of Δ9-THC and CP 55940 during treatment were not calculated because the maximum effect was not greater than 50% of that established before treatment. The ED50 values for anandamide and midazolam to produce hypothermia were not significantly modified during Δ9-THC treatment (Fig. 3, bottom right and Fig. 5, bottom, respectively; Table 2).
When Δ9-THC treatment was discontinued, response rate returned to control levels (Fig. 4, After). On day 9 of the discontinuation period, the potency of Δ9-THC to decrease response rate and body temperature was greater than it was during Δ9-THC treatment, although the potency remained significantly less than the control (i.e., that determined before treatment) (Fig. 3, top and bottom left, compare squares and circles; Table 2). The ED50 value of CP 55940 to decrease response rate after discontinuation of Δ9-THC treatment was not significantly different from control. Although the potency of CP 55940 to produce hypothermia after discontinuation was significantly less than control (Table 2), the potency was greater than it was during Δ9-THC treatment (Fig. 3, bottom middle, compare squares and circles). The ED50 values for anandamide and midazolam, determined for both rate-decreasing and hypothermic effects after discontinuation of treatment, did not significantly differ from control (Fig. 3, right and Fig. 5, respectively; Table 2).
Discussion
In C57BL/6J mice, the effects of Δ9-THC and CP 55940 to decrease fixed ratio responding for food were antagonized by the CB1 antagonist rimonabant; however, antagonism was limited by the rate-decreasing effects of rimonabant alone, as has been reported previously in rats (Järbe et al. 2003; De Vry and Jentzsch 2004). In contrast, the rate-decreasing effects of anandamide were not blocked by rimonabant, suggesting that CB1 receptors were not involved. Daily Δ9-THC treatment produced tolerance but not cross-tolerance to CP 55490 when measuring rate-decreasing effects, consistent with CP 55940 having higher agonist efficacy than Δ9-THC in vitro (Breivogel and Childers 2000). For hypothermic effects, both tolerance to Δ9-THC and cross-tolerance to CP 55940 developed. The greater loss of sensitivity to the hypothermic as compared to the rate-decreasing effects of CP 55940 suggests that the former requires greater CB1 agonist efficacy as compared with the latter. Δ9-THC treatment did not modify sensitivity to anandamide or a benzodiazepine (midazolam). Collectively, these results suggest that the magnitude of tolerance/cross-tolerance to cannabinoids is a function of the CB1 efficacy of the agonists to which tolerance/cross-tolerance develops, and also as a function of the efficacy required to produce different effects in vivo.
Receptor theory predicts that loss of receptor function differentially impacts sensitivity to agonists on the basis of efficacy (Kenakin 1997). For an effect to be maintained when receptor function is decreased, percent receptor occupancy (i.e., fractional occupancy) and dose increases (i.e., tolerance and cross-tolerance develop). However, because a low efficacy agonist occupies more receptors than a higher efficacy agonist when their effects are equivalent, loss of receptor function produces a disproportionately greater increase in fractional occupancy (i.e., tolerance/cross-tolerance) for a low as compared to a high efficacy agonist. Previous studies have provided evidence for a relationship between CB1 agonist efficacy and in vitro effects (i.e., inhibition of adenylyl cyclase and contraction of smooth muscle; Selley et al. 2004; Pertwee et al. 1993). Here, the greater tolerance to Δ9-THC as compared with cross-tolerance to CP 55940 provides evidence that differences in the capacity of these agonists to stimulate inhibitory G proteins (i.e., CB1 agonist efficacy; Breivogel and Childers 2000) are important for behavioral effects. Although the current study did not measure CB1 receptor function directly, an assumption is made, based on several previous studies, that daily Δ9-THC treatment decreased G-protein signaling at CB1 receptors as a result of receptor loss and/or internalization and also desensitization resulting from receptor dimerization and changes to both G- and non-G-proteins (Sim et al. 1996; Breivogel et al. 1999, 2003; Selley et al. 2004; Falenski et al. 2010; Smith et al. 2010).
CP 55940 was more potent than Δ9-THC (current results; Martin et al. 1991) and the potency of both for decreasing operant responding was greater than that for producing hypothermic effects. This difference in potency for producing two effects might reflect differential binding to multiple receptor subtypes that differentially mediate the two effects. However, rimonabant antagonized the rate-decreasing and hypothermic effects of Δ9-THC and CP 55940 (current results; Giuffrida and McMahon 2010), albeit antagonism of rate-decreasing effects was less orderly and of a lesser magnitude than antagonism of hypothermia. Less orderly antagonism of rate-decreasing effects could reflect actions of rimonabant at a subset of brain cannabinoid receptors that are more prominently involved in rate-decreasing effects than hypothermic effects; however, mechanisms underlying the effects of rimonabant are not known. Alternatively, if both rate-decreasing and hypothermic effects are mediated by the same receptor type (e.g., CB1), then the difference in potency might be due to a difference in the magnitude of CB1 receptor stimulation (i.e., the efficacy requirement) required for the effects (i.e., hypothermic>rate-decreasing effects). The marked cross-tolerance from Δ9-THC to CP 55940 that developed for hypothermic effects in the absence of a similar cross-tolerance from Δ9-THC to CP 55940 for rate-decreasing effects is consistent with a difference in the efficacy requirement. Likewise, the greater tolerance to Δ9-THC for hypothermic effects as compared with rate-decreasing effects is consistent with a difference in the CB1 agonist efficacy required for the two effects (se also De Vry et al. 2004 for differential tolerance to CP 55940). These results provide empirical support for receptor theory, which predicts that loss of sensitivity is greater for an effect associated with high receptor occupancy and few spare receptors as compared to an effect associated with lower receptor occupancy and more spare receptors.
Anandamide reportedly has CB1 agonist efficacy intermediate to that of Δ9-THC and CP 55940 (Childers 2006) and was included for study to better assess the relationship between efficacy and tolerance/cross-tolerance. However, the effects of anandamide were not mediated by Δ9-THC-and CP 55940-sensitive receptors as evidenced by failure of rimonabant to antagonize the rate-decreasing and hypothermic effects of anandamide (current results; Giuffrida and McMahon 2010) and, further, by failure of daily Δ9-THC treatment to produce cross-tolerance to anandamide. Even though anandamide did not act at CB1 receptors, its inclusion with midazolam further strengthened the notion that Δ9-THC treatment selectively decreased the effects of CB1 agonists. The results of drug discrimination studies strongly suggest that the in vivo effects of anandamide diverge from prototypic cannabinoid agonists (Wiley et al. 1995, 1997; Järbe et al. 2001; Solinas et al. 2007; Giuffrida and McMahon 2010). However, exceptions have been noted previously; anandamide shared discriminative stimulus effects with Δ9-THC in monkeys (McMahon 2009) and tolerance to Δ9-THC was accompanied by lesser cross-tolerance to anandamide and chemical analogs of anandamide (Wiley et al. 2006). One parsimonious explanation is that the rate of anandamide metabolism and subsequent contribution of non-CB1 receptor-acting metabolites (Willoughby et al. 1997; Wiley et al. 2006) both vary as a function of species and/or route of administration. Unless strategies are used to inhibit metabolism, anandamide appears to have limited utility as a probe for CB1 receptors, at least when administered systemically in rodents. Accordingly, by decreasing anandamide metabolism through transgenic deletion or pharmacologic inhibition of fatty acid amide hydrolase, the effects of anandamide are more clearly CB1 receptor-mediated (Cravatt et al. 2001; Solinas et al. 2007) to include lesser cross-tolerance from Δ9-THC to anandamide than tolerance to Δ9-THC (Falenski et al. 2010), a finding consistent with the current results. In addition to the magnitude of tolerance/ cross-tolerance being related to CB1 efficacy, other interpretations have been provided (Wiley et al. 2006) to include differential changes in brain anandamide levels (Di Marzo et al. 2000) which could, in turn, have different effects on sensitivity to anandamide versus Δ9-THC and other cannabinoid agonists not derived from brain.
In summary, the results of this study show a relationship between the magnitude of tolerance/cross-tolerance and the apparent CB1 agonist efficacy of cannabinoids to which tolerance/cross-tolerance develops. Marijuana smoking or oral Δ9-THC administration presumably decreases CB1 receptor function, and the current results indicate that loss of sensitivity to cannabinoids will be greater for low efficacy cannabinoid agonists, such as Δ9-THC, than to higher efficacy cannabinoid agonists. In addition to differential changes in sensitivity among agonists that vary with efficacy, this study further suggests that receptor occupancy and efficacy can vary for different in vivo effects of the cannabinoids. In particular, as the dose needed for effect increases, the CB1 agonist efficacy requirement also increases. Loss of CB1 receptor function will produce a greater decrease in sensitivity to effects that require high levels of CB1 receptor stimulation as compared to effects requiring less CB1 receptor stimulation. Therapeutic effects (e.g., antinociceptive) of CB1 agonists that appear to require relatively high efficacy might be more susceptible to the development of tolerance than other effects associated with lower efficacy, which might include effects (discriminative stimulus and subjective effects) associated with the abuse liability of CB1 agonists.
Acknowledgments
Supported by U.S. Public Health Service Grants DA19222 and DA26781.
References
- Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends Pharmacol Sci. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- Adams IB, Compton DR, Martin BR. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther. 1998;284:1209–1217. [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000;295:328–336. [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic Δ9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR. The effects of Δ9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. Eur J Pharmacol. 2003;459:139–150. doi: 10.1016/s0014-2999(02)02854-6. [DOI] [PubMed] [Google Scholar]
- Childers SR. Activation of G-proteins in brain by endogenous and exogenous cannabinoids. AAPS J. 2006;8:E112–E117. doi: 10.1208/aapsj080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, Pertwee RG, Irving AJ. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci. 2001;21:2425–2433. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR. Partial agonist-like profile of the cannabinoid receptor antagonist SR141716A in a food-reinforced operant paradigm. Behav Pharmacol. 2004;15:13–20. doi: 10.1097/00008877-200402000-00002. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR, Kuhl E, Eckel G. Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behav Pharmacol. 2004;15:1–12. doi: 10.1097/00008877-200402000-00001. [DOI] [PubMed] [Google Scholar]
- Dewey WL. Cannabinoid pharmacology. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- Dill JA, Howlett AC. Regulation of adenylate cyclase by chronic exposure to cannabimimetic drugs. J Pharmacol Exp Ther. 1988;244:1157–1163. [PubMed] [Google Scholar]
- Di Marzo V, Berrendero F, Bisogno T, González S, Cavaliere P, Romero J, Cebeira M, Ramos JA, Fernández-Ruiz JJ. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of delta9-tetrahydrocannabinol-tolerant rats. J Neurochem. 2000;74:1627–1635. doi: 10.1046/j.1471-4159.2000.0741627.x. [DOI] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, Sim-Selley LJ. FAAH−/− mice display differential tolerance, dependence, and cannabinoid receptor adaptation after Δ9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology. 2010;35:1775–1787. doi: 10.1038/npp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between Δ9-tetrahydrocannabinol, CP 55, 940 and WIN 55, 212. J Pharmacol Exp Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Lan R, Makriyannis A, Volkow ND. Large receptor reserve for cannabinoid actions in the central nervous system. J Pharmacol Exp Ther. 1999;288:478–483. [PubMed] [Google Scholar]
- Giuffrida A, McMahon LR. In vivo pharmacology of endocannabinoids and their metabolic inhibitors: therapeutic implications in Parkinson's disease and abuse liability. Prostaglandins Other Lipid Mediat. 2010;91:90–103. doi: 10.1016/j.prostaglandins.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Piomelli D. Purification and high-resolution analysis of anandamide and other fatty acylethanolamides. In: Laychock SG, Rubin RP, editors. Lipid second messengers. CRC; Boca Raton: 1998a. pp. 113–133. [Google Scholar]
- Giuffrida A, Piomelli D. Isotope dilution GC/MS determination of anandamide and other fatty acylethanolamides in rat blood plasma. FEBS Lett. 1998b;422:373–376. doi: 10.1016/s0014-5793(98)00046-5. [DOI] [PubMed] [Google Scholar]
- Hardison S, Weintraub ST, Giuffrida A. Quantification of endocannabinoids in rat biological samples by GC/MS: technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 2006;81:106–112. doi: 10.1016/j.prostaglandins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology. 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Liu Q, Makriyannis A. (R)-Methanandamide and Δ9-tetrahydrocannabinol-induced operant rate decreases in rats are not readily antagonized by SR-141716A. Eur J Pharmacol. 2003;466:121–127. doi: 10.1016/s0014-2999(03)01491-2. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic analysis of drug–receptor interaction. Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- Kenakin T. Efficacy at G-protein-coupled receptors. Nat Rev Drug Discov. 2002;1:103–110. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. Psychopharmacology. 2009;203:219–228. doi: 10.1007/s00213-008-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. A total synthesis of DL-delta-1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies; Washington: 2003. [PubMed] [Google Scholar]
- Oviedo A, Glowa J, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The central neuropharmacology of psychotropic cannabinoids. Pharmacol Ther. 1988;36:189–261. doi: 10.1016/0163-7258(88)90106-4. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Stevenson LA, Griffin G. Cross-tolerance between Δ9-tetrahydrocannabinol and the cannabimimetic agents, CP 55, 940, WIN 55, 212-2 and anandamide. Br J Pharmacol. 1993;110:1483–1490. doi: 10.1111/j.1476-5381.1993.tb13989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Gorriti MA, Fernández-Ruiz JJ, Palomo T, Ramos JA. Downregulation of rat brain cannabinoid binding sites after chronic Δ9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav. 1994;47:33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Selley DE, Cassidy MP, Martin BR, Sim-Selley LJ. Long-term administration of Δ9-tetrahydrocannabinol desensitizes CB1-, adenosine A1-, and GABAB-mediated inhibition of adenylyl cyclase in mouse cerebellum. Mol Pharmacol. 2004;66:1275–1284. doi: 10.1124/mol.104.000604. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with Δ9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TH, Sim-Selley LJ, Selley DE. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery? Br J Pharmacol. 2010;160:454–466. doi: 10.1111/j.1476-5381.2010.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces Δ9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose–effect data analysis. Chapman and Hall; Boca Raton: 2000. [Google Scholar]
- Wiley J, Balster R, Martin B. Discriminative stimulus effects of anandamide in rats. Eur J Pharmacol. 1995;276:49–54. doi: 10.1016/0014-2999(95)00010-i. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Golden KM, Ryan WJ, Balster RL, Razdan RK, Martin BR. Evaluation of cannabimimetic discriminative stimulus effects of anandamide and methylated fluoroanandamide in rhesus monkeys. Pharmacol Biochem Behav. 1997;58:1139–1143. doi: 10.1016/s0091-3057(97)00327-4. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR. Evaluation of the role of the arachidonic acid cascade in anandamide's in vivo effects in mice. Life Sci. 2006;80:24–35. doi: 10.1016/j.lfs.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Moore SF, Martin BR, Ellis EF. The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther. 1997;282:243–247. [PubMed] [Google Scholar]