Abstract
Efficient ligninolytic systems of wood-degrading fungi include not only oxidizing enzymes, but also low-molecular-weight effectors. The ability of Bjerkandera fumosa to secrete oxalic acid and versatile peroxidase (VP) in nitrogen-rich and nitrogen-limited media was studied. Higher activity of VP was determined in the nitrogen-limited media but greater concentration of oxalic acid was observed in the cultures of B. fumosa without nitrogen limitation. Ferric ions chelating ability of Bjerkandera fumosa studied in ferric ions limited media was correlated with the increased level of oxalic acid. The presence of hydroxamate-type siderophores in B. fumosa media were also detected. Oxalate decarboxylase was found to be responsible for regulation of oxalic acid concentration in the tested B. fumosa cultures.
Keywords: Oxalic acid, White rot fungi, Bjerkandera fumosa, CAS, Versatile peroxidase, Oxalate decarboxylase
Introduction
Bjerkandera fumosa belongs to white rot fungi which are considered as the main decomposers of dead and fallen trees. This ecological group of fungi is able to degrade all wood components, which makes them very important elements of the recycling cycles of carbon in ecosystems (Gadd 1999). White rot Basidiomycetes secrete an array of different oxidoreductases to degrade the lignin, the most recalcitrant component of wood (Shah and Nerud 2002). The best characterized of these lignolytic enzymes are laccase (Lac; EC 1.10.3.2), lignin peroxidase (LiP; EC 1.11.1.14), manganese peroxidase (MnP; EC 1.11.1.13) and versatile peroxidase (VP; EC 1.11.1.16) (Martinez et al. 2005). Besides role of lignolytic enzymes in wood degradation, they are employed in numerous applications like: e.g. wine and juice clarification, dyes decolourisation, organic synthesis, cotton fiber whitening or in biosensor designing (Mayer and Staples 2002).
The fungal enzymatic machinery is regulated by nutrients and among them nitrogen posses a strong regulating effects (Kachlishvili et al. 2006; Kapich et al. 2004; Mester and Field 1997). Activity of white rot fungi oxidative enzymes is also facilitated by low-molecular-weight compounds, which are necessary to initiate the ligninolysis (Eriksson et al. 1990). The important elements of these compounds are organic acids secreting by fungal cells. The predominant organic acid detected in the wood-rotting fungal strains is oxalic acid (Dutton and Evans 1996; Shimada et al. 1997). Oxalic acid plays multiple roles in the fungal metabolism and among other things serves as the donor or the acceptor of electrons, as the metal chelator involved in manganese peroxidase catalytic cycle, as the part of the reactive oxygen species generation pathways via Fenton reaction or quinine/semiquinone cycles, or as the osmotic and pH regulator (Goodel et al. 1997; Shimada et al. 1997; Hofrichter 2002).
In our previous paper, we have also demonstrated elevated secretion of oxalic acid by basidiomycetous fungi as the response to the stress conditions induced by the presence of heavy metals (Jarosz-Wilkołazka and Grąz 2006; Grąz et al. 2009). Due to important function of oxalic acid, very significant is learning and recognition of factors affecting oxalate secretion and regulations of its concentration in fungal vicinity as well as its possible functions, which helps to design efficient ways to utilize fungal abilities.
Materials and methods
Fungal strain and cultivation conditions
The fungal strain used was Bjerkandera fumosa obtained from Fungal Collection (FCL) of the Department of Biochemistry Maria Curie-Skłodowska University, Lublin, Poland (strain FCL 137). Stock cultures were maintained on 2% (w/v) malt agar slants at 4°C. The inoculation material was precultivated on 2% (w/v) malt extract agar at 25°C. The experiments were performed using basic liquid medium (BLM) in two versions—limited in nitrogen sources (N-limited BLM) and sufficient in nitrogen sources (N-rich BLM). The composition of BLM was following (per 1 l): 5 g glucose, 2.5 g l-asparagine (in N-rich BLM) or 0.25 g l-asparagine (in N-limited BLM), 3 g NaNO3 (in N-rich BLM) or 0.3 g NaNO3 (in N-limited BLM), 0.50 g KCl, 0.45 g KH2PO4, 0.17 g Na2HPO4, 0.50 g MgSO4·7H2O, 0.0005 g thiamine, 0.0005 g CuSO4, 0.01 g MnCl2, 0.002 g ZnSO4, and 0.005 g FeSO4 (Lindeberg and Holm 1952). Stationary cultures were incubated at 25°C in 100-ml Erlenmayer flasks containing 50 ml of N-rich or N-limited BLM. To investigate the influence of ferric ions on the oxalate and siderophores secretion, N-rich BLM with 100-times reduced amount of FeSO4 (50 μg per liter) was performed (N-rich BLMFe). Induction of oxalate decarboxylase was made after the 6th day of B. fumosa cultivation on N-rich BLM by oxalic acid addition in a sterile mode to the final concentration 5 mM. The extracellular samples for all measurements were collected by separating culture fluids from mycelia through Miracloth (Calbiochem).
Determination of enzymes activities
Versatile peroxidase activity
Manganese-dependent activity of versatile peroxidase (VP) was measured at 270 nm by monitoring the formation of Mn3+-malonate complex in 50 mM sodium malonate buffer at pH 4.5 in the presence of 19 mM H2O2 (Wariishi et al. 1992). The VP activity was expressed in U/ml. One unit of versatile peroxidase was defined as the amount of enzyme required to form 1 μmol Mn3+-malonate complex per minute (ε = 1.159 × 104 M−1 cm−1).
Oxalate decarboxylase (EC 4.1.1.2) activity
Oxalate decarboxylase (ODC) activity was assayed using the method of Magro et al. (1988). In the first step of this assay the mixture containing the enzyme and oxalate was incubated at pH 3 for 15 min before being neutralized with phosphate-citrate buffer pH 8 to stop ODC reaction. In the second step of assay, formic acid as the product of the first reaction, was determined spectrophotometrically at 340 nm using formate dehydrogenase (EC 1.2.1.2) (Sigma) in the presence of NAD. One unit of enzyme activity was defined as the amount of enzyme transformed 1 μmol of substrate to product per 1 min.
Determination of organic acids using capillary electrophoresis
Analyses were performed on Thermo Capillary Electrophoresis, Crystal 100 (Thermo Separation Products, San Jose, USA). The separation and detection conditions were prepared according to Chen et al. (1997). The buffer solution contained phthalic acid, cetyltrimethylammonium bromide (CTAB, Sigma) and methanol and the detection was performed at 210 nm. All samples, buffer solution, and conditioning solutions were filtered through 0.22 μm syringe filters before use. Peaks identification was done by spiking with commercially available standards of organic acids (formic, acetic, glyoxylic, oxalic, malic and tartaric acids; Sigma).
Detection of siderophores
Chrome Azurol S (CAS) assay for the total amount of siderophores
0.5 ml of Chrome Azurol S (CAS, Sigma) assay solution prepared according to Schwyn and Neilands (1997) was added to 0.5 ml of culture supernatant as sample. Then 10 μl of 0.2 M 5-sulfosalicylic acid (SSA) as the shuttle solution was added and after 5 min the absorbance at 630 nm was measured. The N-rich BLM or the N-rich BLMFe was used as the blanks. The N-rich BLM or N-rich BLMFe respectively with addition of CAS assay solution and SSA was used as reference sample. The results were expressed as percentage of siderophore units (% U sid):
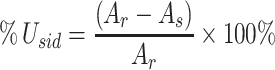 |
where A r is the absorbance of the reference sample and A s is the absorbance of the sample.
Ferric perchlorate assay for hydroxamate-type siderophores
0.5 ml of culture supernatant was mixed with 2.5 ml ferric perchlorate reagent (5 mM (Fe(ClO4)3 in 0.1 M HClO4). The absorbance of nascent orange colour complex was measured at 450 nm according to Payne (1994). The calibration curve was made using desferroxamine (Sigma) as the standard
Arnow assay for catecholate-type siderophores
To 1 ml of culture supernatant 1 ml of 1 mol HCl and 1 ml of nitrite-molybdate reagent (10 g sodium nitrate and 10 g sodium molybdate dissolved in 100 ml of water) was added and mixed. Catecholate siderophores produce a yellow colour derivatives and their absorbance was measured at 510 nm (Payne 1994). The calibration curve was made using 3,4-dihydroxybenzoic acid (Fluka) as the standard.
Results
Versatile peroxidase activity and oxalic acid secretion
The concentration of nitrogen source can influence the enzymatic activities detected in the cultures of white rot basidiomycetes fungi. B. fumosa was cultivated in N-rich and N-limited BLM and the manganese-dependent activity of versatile peroxidase was detected in both of them (Fig. 1). Higher VP activities were observed in N-limited media where enzyme reached its maximum activity 33 U/ml after 21 days of cultivation. In N-rich media the activity of VP achieved only 9 U/ml during entire period of B. fumosa cultivation.
Fig. 1.
Changes in the activity of extracellular versatile peroxidase (VP) and in oxalate concentration during B. fumosa growth on N-rich BLM and N-limited BLM. Data points are the means and the standard deviations of three repetitions
The activity of fungal peroxidase can be affected by the presence of carboxylic acids and therefore their presence and their concentrations in N-rich and N-limited media were determined during the growth of B. fumosa. Oxalic acid was the only organic acid detected in tested cultures of B. fumosa and higher concentration was observed in N-rich BLM than in N-limited BLM. The maximum concentration of oxalate concentration was 6.5 mM in N-rich BLM after 21 days of cultivation and 1.2 mM in N-limited BLM after 28 days of cultivation (Fig. 1).
Fe3+-chelating ability of B. fumosa media
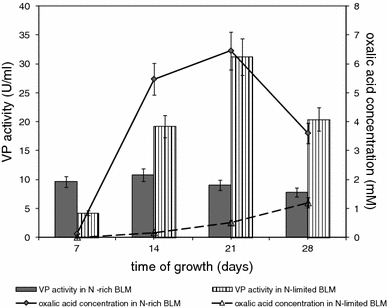
The nonspecific CAS assay was applied to investigate the ability of B. fumosa to the Fe3+ ions chelation and therefore the fungus was cultivated with ferric ions limitation (N-rich BLMFe) and without limitation (N-rich BLM). Total chelating ability of fungal cultures can be influencing by oxalate accumulation in growth media. To investigate this correlation, the level of oxalic acid was monitored in cultures of B. fumosa and distinct differences between tested (N-rich BLMFe) and control (N-rich BLM) cultures in chelation ability were observed (Fig. 2). In tested cultures the chelation ability defined as percentage of siderophore units (% U sid) was at the high, constant level during 21 days of growth (in the range between 71.6 and 84.6% U sid) and became reduced to 38% U sid on the 28th day of growing. In control cultures the tested parameter oscillated during the time of fungal growth between 9.2 and 54% U sid with the exception on 7th day of cultivation when it reached value of 77.5% U sid. The highest differences in ferric ions chelating ability between tested and control cultures of B. fumosa were detected on 14th day of fungal growth and were nine times greater in tested cultures with Fe3+-limitation (81.4% U sid) than in controls without Fe3+-limitation (9.2% U sid). This higher rate of CAS reaction was correlated with enhanced oxalate secretion in Fe3+-limited cultures (N-rich BLMFe) (Fig. 2). Oxalic acid in Fe3+-limitation conditions remained at higher level in comparison with control cultures during entire period of cultivation.
Fig. 2.
Total ability to ferric ions chelation (% U sid) and concentration of oxalic acid during B. fumosa growth on N-rich BLM and N-rich BLMFe. Data points are the means and the standard deviations of three repetitions
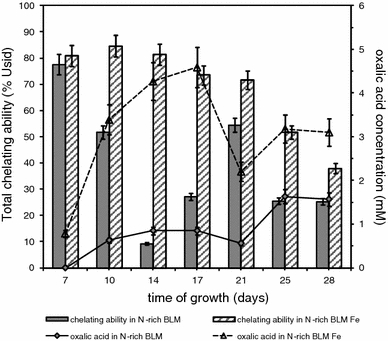
The efforts made to determine the chemical character of secreted siderophores revealed presence of hydroxamates type siderophores in higher concentration in culture with Fe3+-limitation than in control one (Fig. 3). There was very low level of catecholate type siderophores detected in both tested and control cultures (data not shown).
Fig. 3.
The level of hydroxamate siderophores detected during B. fumosa growth on N-rich BLM and N-rich BLMFe. Data points are the means and the standard deviations of three repetitions
Degradation pathways of oxalic acid by B. fumosa
Concentration of oxalic acid around fungal vicinity is precisely controlled by fungi. Because of inducible character of oxalate degrading enzymes cultures of B. fumosa growing on N-rich BLM were stimulated by addition of oxalic acid to establish the pathway of regulation of oxalate concentration. No extracellular activity of oxalate decarboxylase was detected in post-cultivated media of B. fumosa. Figure 4 presents oxalic acid degradation rate in fungal growth media after fungal culture induction with oxalic acid. During the first 24 h after induction the rapid drop of oxalic acid concentration (from 5.0 to 3.3 mM) was observed what was correlated with maximum concentration of formate—the product of oxalate decarboxylation.
Fig. 4.
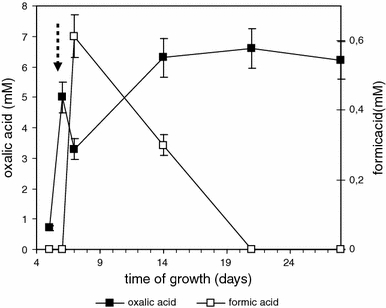
Changes in oxalic and formic acids level during B. fumosa growth after induction with 5 mM oxalic acid on the 6th day of cultivation (arrow). Data points are the means and the standard deviations of three repetitions
Discussion
Influence of nitrogen concentration on VP and oxalate secretion in B. fumosa cultures
It is well established that species of Bjerkandera secrete MnP (Mester and Field 1997; Hofrichter 2002) and VP, which is a novel class of lignolytic peroxidase described in recent years (Martinez et al. 2005; Moreira et al. 2005). In our earlier study we have ascertained that B. fumosa, strain used in this study, secretes VP which in fact share LiP and MnP catalytic properties (Rodakiewicz-Nowak et al. 2006). Process of lignin degradation can be stimulated by nitrogen limited conditions (Kirk and Farrell 1987) and in this study we have observed higher activity of extracellular VP under nitrogen limitation. In studies made by Mester and Field (1997) Bjerkandera sp. strain BOS55, a MnP producer, was defined as nitrogen-unregulated fungus. In contrast to this strain, production of MnP by Phanerochaete chrysosporium, the model organism of white rot fungi study is suppressed by high nitrogen concentration in artificial medium (Reddy and D’Souza 1994). Activity of peroxidase can be affected by oxalic acid, but this effect remains not fully explained (Mester and Field 1998; Hofrichter 2002). Coexistence of both MnP activity and oxalic acid secretion is well documented in fungal cultures (Hofrichter et al. 1999; Nüske et al. 2002; Hakala et al. 2005). Correlation between oxalic acid and MnP activity include chelation and stabilization of Mn3+ ions produced in catalytic cycle of this enzyme (Kuan and Tien 1993). Versatile peroxidase from Bjerkandera sp. strain BOS55 characterized by Mester and Field (1998) was highly stimulated by glycolate, glyoxalate and oxalate under manganese-deficient conditions, but it was also established that excess of oxalate could inhibit MnP activity (Shimada et al. 1997). In our study higher activities of VP were detected in media with N-limitation, where less oxalate was accumulated, than in N-rich growing conditions, where oxalate was secreted at greater extent. We have also observed decreasing of manganese-dependent VP activity after exogenous oxalic acid addition to the B. fumosa growth media (data not shown).
Reports about other organic acids detected during Basidiomycetes cultivation, for example malic, succinic, fumaric and acetic acid were described by Takao (1965), Galkin et al. (1998), Hofrichter et al. (1999), but oxalate is still considered as the main organic acid secreted to fungal vicinity. The medium composition can affect oxalate secretion, and the content of nitrogen source is an important factor (Kirk and Farrell 1987). Akamatsu et al. (1994) have observed accumulation of higher amounts of oxalate by white rot fungi in media with greater nitrogen content than in fungal cultures with lower nitrogen concentration. The sort of nitrogen source can also influence on oxalate secretion as describe by Gharieb and Gadd (1999). They detected higher oxalic acid accumulation in cultures contained nitrate than ammonium as a sole nitrogen source. Additional factor which induce secretion of oxalic acid is the presence of heavy metals (Gadd 2007). In our earlier study we found that oxalate crystals were produced by B. fumosa during cultivation on ZnO, Co3(PO4)2, CaCO3-amended media without N-limitation (Jarosz-Wilkołazka and Gadd 2003).
B. fumosa ferric ions chelating ability
Iron is an essential ion for almost all organisms but it exists in nature predominantly in the insoluble Fe3+ form, which is not readily available for assimilation (Winkelmann 2007). Many of fungal strains with the exception of budding and fission yeast produce siderophores in order to solubilize and sequester iron ions (Neilands 1995). Siderophores and oxalic acid belong to small agents capable to penetrate solid wood in early stages of decay (Milagres et al. 2002) and oxalic acid secreted by fungi to ambient environment can also serve as a weak iron chelator (Dutton and Evans 1996; Gadd 1999). Higher CAS reaction in N-rich BLMFe media observed in this study, showing the presence of compounds with Fe3+ ions chelating ability and it was correlated with elevated extracellular oxalate content. Hydroxamate-type siderophores determined in this study in B. fumosa post-cultivated media are typical for many fungal strains (Renshaw et al. 2002). The correlation between chelating ability and oxalic acid concentration was observed in N-rich BLMFe up to the 25th day of cultivation. However, the noticeable sharp increase in hydroxamate siderophores concentration on the 28th day of cultivation was not linked with distinct increase of the fungal total chelating ability (CAS assay) and of the level of oxalic acid (Figs. 2, 3). This lack of correlation may be explained by the presence of B. fumosa metabolites in the later days of cultivation. The natural metabolites such as proteins, free amino acids, phenolic compounds interfere with the CAS reaction and consequently gives inaccurate results (Raaska and Mattila-Sandholm 1995). Ferric perchlorate assay seems to be more reliable to determine the level of siderophores in the later days of B. fumosa cultures when a lot of secondary metabolites are presented in the growing media. Machuca et al. (2001) also detected hydroxamate derivatives and oxalic acid in extracts of low molecular mass compounds of brown rot fungus Wolfiporia cocos and white rot fungus Trametes versicolor but only extracts of Wolfiporia cocos caused positive CAS reaction. In the study of Milagres et al. (2002) catecholate and hydroxamate derivatives together with oxalic acid secretion were produced by Wolfiporia cocos, Gleophyllum trabeum, Trametes versicolor, Poria medulla-panis and all these tested fungi caused color change of CAS-blue agar.
Regulation of oxalic acid secretion
Data obtained in this study have shown inducible character of ODC which is in accordance with studies of Mäkelä et al. (2002) and Micales (1997). In the present study no extracellular ODC activity was detected in B. fumosa cultures what possible point at intracellular or membrane character of the enzyme in this strain. Mäkelä et al. (2002) detected intracellular oxalate decarboxylase activity in Dichomitus squalens, Phanerochaete sanguinea, Trametes ochracea and Trametes versicolor. Micales (1997) localized oxalate decarboxylase from Postia placenta as inducible enzyme which was associated with hyphal surface and hyphal sheath. Existence in fungal vicinity both oxalic and formic acid as the product of its decarboxylation, can form a better buffer system than presence of this acids separately (Micales 1995). It was observed that oxalic acid added to B. fumosa cultures was preserved to the end of cultivation period and act as chelator and buffer system. Probably, the main pool of this acid is found as different salts. High level of oxalate accumulation observed in this study also in non-induced by oxalic acid addition cultures, could be linked to high pH value of B. fumosa tested cultures which oscillated during cultivation time between pH 7 and pH 8 (data not shown). High value of pH could inhibit expression of oxalate degrading enzymes, because the optimal for oxalate degrading enzymes is acidic conditions (Micales 1995). Observed high pH values could be caused by other compounds secreted by this strain, which effectively buffered the medium (Dutton et al. 1993; Milagres et al. 2002). Lowering pH of growth medium correlated with oxalic acid accumulation was demonstrated during cultivation of brown rot fungi Postia placenta and Wolfiporia cocos and white rot strain Physisporinus rivulosus (Espejo and Agosin 1991; Hakala et al. 2005). Generally white rot fungi, in contrast to brown rot, are considered as ODC produced organisms (Dutton and Evans 1996). Recently there are reports about possible fungal oxidative pathway of oxalate degradation via oxalate oxidase action (Escutia et al. 2005; Grąz et al. 2009). It is worth mention also possible role of MnP in oxalate degradation by basidiomycetes fungi as a pathway of hydrogen peroxide production (Urzua et al. 1998). In our study concentration of oxalic acid was lower in the N-limited medium where VP activity was higher, but the role of VP in oxalate transformation in B. fumosa requires further studies.
In conclusion it is worth emphasizing diverse role of oxalate in fungal metabolism especially in white rot strains. In this work we have demonstrated that oxalate concentration and VP activity were affected by the level of nitrogen in fungal growth media. Oxalate can facilitate chelation ability of ferric ions by B. fumosa. We also demonstrated that in regulation of oxalate level in B. fumosa the oxalate decarboxylase is involved.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Akamatsu Y, Takahashi M, Shimada M. Production of oxalic acid by wood-rotting basidiomycetes grown on low and high nitrogen culture media. Mater Org. 1994;28:251–264. [Google Scholar]
- Chen J, Preston BP, Zimmerman MJ. Analysis of organic acids in industrial samples. Comparison of capillary electrophoresis and ion chromatography. J Chromatogr A. 1997;781:205–213. doi: 10.1016/S0021-9673(97)00374-9. [DOI] [Google Scholar]
- Dutton MV, Evans CS. Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment. Can J Microbiol. 1996;42:881–895. doi: 10.1139/m96-114. [DOI] [Google Scholar]
- Dutton MV, Evans CS, Atkey PT, Wood DA. Oxalate production by Basidiomycetes, including the white-rot species Coriolus versicolor and Phanerochaete chrysosporium. Appl Microbiol Biotechnol. 1993;39:5–10. [Google Scholar]
- Eriksson KEL, Blanchette RA, Ander P. Microbial and enzymatic degradation of wood and wood components. Berlin: Springer-Verlag; 1990. [Google Scholar]
- Escutia MR, Bowater L, Edwards A, Bottrill AR, Burrell MR, Polanco R, Vicuna R, Bornemann S. Cloning and sequencing of two Ceriporiopsis subvermispora bicupin oxalate oxidase allelic isoforms: implications for the reaction specificity of oxalate oxidases and decarboxylase. Appl Environ Microbiol. 2005;71:3608–3616. doi: 10.1128/AEM.71.7.3608-3616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo E, Agosin E. Production and degradation of oxalic acid by brown rot fungi. Appl Environ Microbiol. 1991;57:1980–1986. doi: 10.1128/aem.57.7.1980-1986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd GM. Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. Adv Microb Physiol. 1999;41:47–92. doi: 10.1016/S0065-2911(08)60165-4. [DOI] [PubMed] [Google Scholar]
- Gadd GM. Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res. 2007;111:3–49. doi: 10.1016/j.mycres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Galkin S, Vares T, Kalsi M, Hatakka A. Production of organic acids by different white-rot fungi as detected using capillary zone electrophoresis. Biotechnol Techn. 1998;12:267–271. doi: 10.1023/A:1008842012539. [DOI] [Google Scholar]
- Gharieb MM, Gadd GM. Influence of nitrogen source on the solubilization of natural gypsum (CaSO4·2H2O) and the formation of calcium oxalate by different oxalic and citric acid-producing fungi. Mycol Res. 1999;103:473–481. doi: 10.1017/S0953756298007382. [DOI] [Google Scholar]
- Goodel B, Jellison J, Liu J, Daniel G, Paszczynski A, Fekete F, Krishnamurthy S, Jun L, Xu G. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J Biotechnol. 1997;53:133–162. doi: 10.1016/S0168-1656(97)01681-7. [DOI] [Google Scholar]
- Grąz M, Jarosz-Wilkołazka A, Pawlikowska-Pawlęga B. Abortiporus biennis tolerance to insoluble metal oxides: oxalate secretion, oxalate oxidase activity, and mycelial morphology. Biometals. 2009;22:401–410. doi: 10.1007/s10534-008-9176-1. [DOI] [PubMed] [Google Scholar]
- Hakala TK, Lundell T, Galkin S, Maijala P, Kalkkinen N, Hatakka A. Manganese peroxidase, laccase and oxalic acid from the selective white-rot fungus Physisporinus rivulosus grown on spurce wood chips. Enz Microb Technol. 2005;36:461–468. doi: 10.1016/j.enzmictec.2004.10.004. [DOI] [Google Scholar]
- Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP) Enz Microb Technol. 2002;30:454–466. doi: 10.1016/S0141-0229(01)00528-2. [DOI] [Google Scholar]
- Hofrichter M, Vares T, Kalsi M, Galkin S, Scheibner K, Fritsche W, Hatakka A. Production of manganese peroxidase and organic acids and mineralization of (14C-DHP) during solid-state fermentation of wheat straw with the white rot fungus Nematoloma frowardii. Appl Environ Microbiol. 1999;65:1864–1870. doi: 10.1128/aem.65.5.1864-1870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz-Wilkołazka A, Gadd GM. Oxalate production by wood-rotting fungi growing in toxic metal-amended medium. Chemosphere. 2003;52:541–547. doi: 10.1016/S0045-6535(03)00235-2. [DOI] [PubMed] [Google Scholar]
- Jarosz-Wilkołazka A, Grąz M. Organic acids production by white rot Basidiomycetes in the presence of metallic oxides. Can J Microbiol. 2006;52:779–785. doi: 10.1139/w06-032. [DOI] [PubMed] [Google Scholar]
- Kachlishvili E, Penninckx MJ, Tsiklauri N, Elisashvili V. Effect of nitrogen source on lignocellulolytic enzyme production by white-rot basidiomycetes under solid-state cultivation. World J Microbiol Biotechnol. 2006;22:391–397. doi: 10.1007/s11274-005-9046-8. [DOI] [Google Scholar]
- Kapich AN, Prior BA, Botha A, Galkin S, Lundell T, Hatakka A. Effect of lignocellulose-containing substrates on production of ligninolytic peroxidases in submerged cultures of Phanerochaete chrysosporium ME-446. Enz Microb Technol. 2004;34:187–195. doi: 10.1016/j.enzmictec.2003.10.004. [DOI] [Google Scholar]
- Kirk TK, Farrell RL. Enzymatic “combustion”: the microbial degradation of lignin. Ann Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kuan I-C, Tien M. Stimulation of Mn peroxidase activity: possible role for oxalate in lignin biodegradation. Proc Natl Acad Sci USA. 1993;90:1242–1246. doi: 10.1073/pnas.90.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg G, Holm G. Occurence of tyrosinase and laccase in fruit bodies of mycelia of some hymenomycetes. Physiol Plant. 1952;5:100–114. doi: 10.1111/j.1399-3054.1952.tb08234.x. [DOI] [Google Scholar]
- Machuca A, Napoleao D, Milagres AMF. Detection of metal-chelating compounds from wood-rotting fungi Trametes versicolor and Wolfiporia cocos. World J Microbiol Biotechnol. 2001;17:687–690. doi: 10.1023/A:1012929112523. [DOI] [Google Scholar]
- Magro P, Marciano P, De Lenna P. Enzymatic oxalate decarboxylation in isolates of Sclerotonia sclerotiorum. FEMS Microb Lett. 1988;49:49–52. doi: 10.1111/j.1574-6968.1988.tb02680.x. [DOI] [Google Scholar]
- Mäkelä M, Galkin S, Hatakka A, Lundell T. Production of organic acids and oxalate decarboxylase in lignin-degrading white rot fungi. Enz Microb Technol. 2002;30:542–549. doi: 10.1016/S0141-0229(02)00012-1. [DOI] [Google Scholar]
- Martinez AT, Speranza M, Ruiz-Dueñas FJ, Ferreira P, Camerero S, Guillén F, Martinez MJ, Gutiérrez A, del Rio JC. Biodegradation of ligninocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol. 2005;8:195–204. [PubMed] [Google Scholar]
- Mayer AM, Staples RC. Laccase: new function for an old enzyme. Phytochemistry. 2002;60:551–565. doi: 10.1016/S0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- Mester T, Field JA. Optimization of manganese peroxidase production by the white rot fungus Bjerkandera sp. strain BOS55. FEMS Microb Lett. 1997;155:161–168. doi: 10.1111/j.1574-6968.1997.tb13873.x. [DOI] [Google Scholar]
- Mester T, Field JA. Characterization of a novel manganese peroxidase-lignin peroxidase hybrid isozyme produced by Bjerkandera species strain BOS55 in the absence of manganese. J Biol Chem. 1998;273:15412–15417. doi: 10.1074/jbc.273.25.15412. [DOI] [PubMed] [Google Scholar]
- Micales JA. Oxalate decarboxylase in the brown-rot wood decay fungus Postia placenta. Mater Org. 1995;29:177–186. [Google Scholar]
- Micales JA. Localization and induction of oxalate decarboxylase in the brown-rot wood decay fungus Postia placenta. Int Biodeterior Biodegrad. 1997;39:125–132. doi: 10.1016/S0964-8305(97)00009-7. [DOI] [Google Scholar]
- Milagres AMF, Arantes V, Medeiros CL, Machuca A. Production of metal chelating compounds by white and brown-rot fungi and their comparative abilities for pulp bleaching. Enz Microb Technol. 2002;30:562–565. doi: 10.1016/S0141-0229(02)00015-7. [DOI] [Google Scholar]
- Moreira PR, Duez C, Dehareng D, Antunes A, Almeida-Vara E, Frere JM, Malcata FX, Duarte JC. Molecular characterisation of a versatile peroxidase from a Bjerkandera strain. J Biotechnol. 2005;118:339–352. doi: 10.1016/j.jbiotec.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Neilands JB. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- Nüske J, Scheibner K, Dornberger U, Ullrich R, Hofrichter M. Large scale production of manganese-peroxidase using agaric white-rot fungi. Enz Microb Technol. 2002;30:556–561. doi: 10.1016/S0141-0229(02)00013-3. [DOI] [Google Scholar]
- Payne SM. Detection, isolation, and characterization of siderophores. Meth Enzymol. 1994;235:329–344. doi: 10.1016/0076-6879(94)35151-1. [DOI] [PubMed] [Google Scholar]
- Raaska L, Mattila-Sandholm T. Effects of iron level on the antagonistic action of siderophores from non-pathogenic Staphylococcus spp. J Ind Microbiol. 1995;15:480–485. doi: 10.1007/BF01570018. [DOI] [Google Scholar]
- Reddy CA, D’Souza TM. Physiology and molecular biology of the lignin peroxidases of Phanerochaete chrysosporium. FEMS Microbiol Rev. 1994;13:137–152. doi: 10.1111/j.1574-6976.1994.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Renshaw JC, Robson GD, Trinci APJ, Wiebe MG, Livens FR, Collison D, Taylor RJ. Fungal siderophores: structures, functions and applications. Mycol Res. 2002;106:1123–1142. doi: 10.1017/S0953756202006548. [DOI] [Google Scholar]
- Rodakiewicz-Nowak J, Jarosz-Wilkołazka A, Luterek J. Catalytic activity of versatile peroxidase from Bjerkandera fumosa in aqueous solution of water-miscible organic solvents. Appl Cat A General. 2006;308:56–61. doi: 10.1016/j.apcata.2006.04.009. [DOI] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1997;160:45–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shah V, Nerud F. Lignin degrading system of white-rot fungi and its exploitation for dye decolorization. Can J Microbiol. 2002;48:857–870. doi: 10.1139/w02-090. [DOI] [PubMed] [Google Scholar]
- Shimada M, Akamatsu Y, Tokimatsu T, Mii K, Hattori T. Possible biochemical roles of oxalic acid as a low molecular weight compound involved in brown-rot and white-rot wood decays. J Biotechnol. 1997;53:103–113. doi: 10.1016/S0168-1656(97)01679-9. [DOI] [Google Scholar]
- Takao S. Organic acid production by Basidiomycetes. Appl Microbiol. 1965;13:732–737. doi: 10.1128/am.13.5.732-737.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzua U, Kersten P, Vicuna R. Manganese peroxidase-dependent oxidation of glyoxylic and oxalic acids synthesized by Ceriporiopsis subvermispora produces extracellular hydrogen peroxide. Appl Environ Microbiol. 1998;64:68–73. doi: 10.1128/aem.64.1.68-73.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wariishi H, Valli K, Gold MH. Manganese (II) oxidation by manganese peroxidase from basidiomycetes Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J Biol Chem. 1992;267:23688–23695. [PubMed] [Google Scholar]
- Winkelmann G. Ecology of siderophores with special reference to the fungi. Biometals. 2007;20:379–392. doi: 10.1007/s10534-006-9076-1. [DOI] [PubMed] [Google Scholar]


