Dear Editor
Disruption of endoplasmic reticulum (ER) function is associated with numerous human diseases.1,2 It leads to the initiation of a stress response known as the unfolded protein response, whose initial goal is to resolve the ensuing stress; however, when unable to do so, it induces cell death.3,4 ER stress-induced cell death has been shown to proceed primarily through apoptosis. It remained unclear whether ER stress is also associated with other forms of cell death. Using bax−/−bak−/− or Bcl-xL-overexpressing cells that are defective in apoptosis; here, we show that prolonged ER stress still results in cell death in a fashion resembling necrosis. This necrosis-like cell death is associated with autophagy. In response to ER stress, autophagy is induced in both wild-type and bax−/−bak−/− cells to a similar extent. Overexpression of wild-type Bax or Bak, as well as ER-targeted Bak does not induce autophagy, indicating that the multi-domain proapoptotic Bcl-2 proteins do not affect autophagy. Inhibition of autophagy results in enhanced cell death in apoptosis-competent cells, but reduced cell death in apoptosis-deficient cells. Thus, in response to ER stress, while autophagy serves as a survival response to delay apoptosis, it promotes cell death by necrosis in cells with impaired apoptosis.
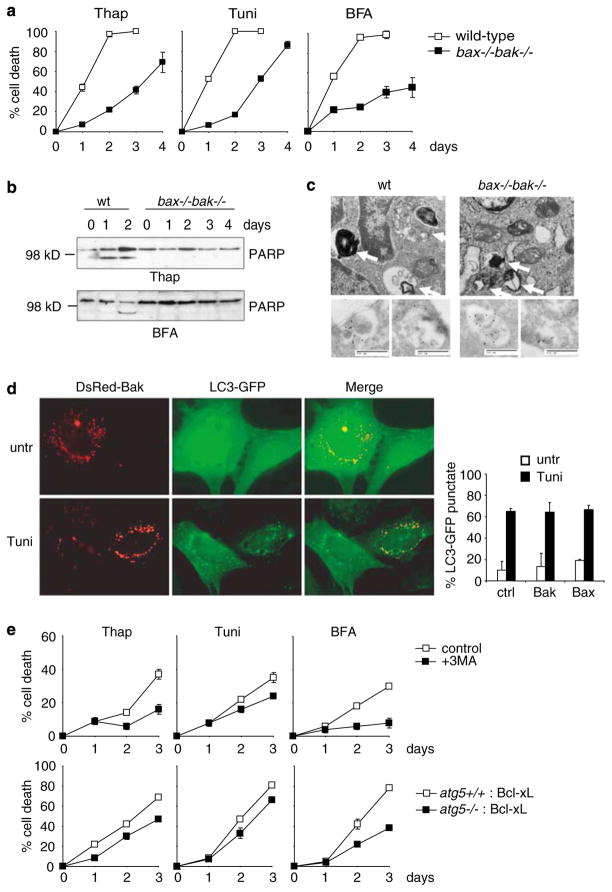
It has been previously shown that cells deficient in both Bax and Bak are resistant to apoptosis induced by ER stress.5 As ER stress is often sustained for extended periods of time under physiological conditions,3,4 we sought to investigate cell survival in response to prolonged ER-stress treatment. Mouse embryonic fibroblasts (MEFs) isolated from wild-type and bax−/−bak−/− mice were treated with ER stressors thapsigargin, tunicamycin, and brefeldin A. Consistent with previous reports, almost all wild-type MEFs died after 1–2 days of ER stress treatment, while Bax/Bak doubly deficient cells were well protected. Interestingly, when treated with ER stress for a prolonged period of time, apoptosis-deficient bax−/−bak−/− MEFs progressively died (Figure 1a). Similar results were observed in MEFs ectopically expressing the antiapoptotic protein Bcl-xL (Supplementary Figure S1A). Thus, ER stress can cause two phases of cell death, the first of which is Bax/Bak-dependent apoptosis; the other is Bax/Bak-independent or Bcl-xL uninhibitable cell death that occurs at a slower rate.
Figure 1.
(a) ER stress can induce non-apoptotic cell death. Wild-type and bax−/− bak−/− MEFs were treated with thapsigargin (0.1 μM), tunicamycin (0.5 μg/ml), or brefeldin A (0.5 μg/ml). At the indicated time points, cells were collected and cell death was measured by PI exclusion. Data are averages of triplicate treatments ± S.E.M. (P<0.01 for all time points). (b) Caspase-3-dependent PARP cleavage does not occur in bax−/− bak−/− MEFs. Wild-type and bax−/− bak−/− cells were treated with either brefeldin A or thapsigargin for the indicated times, cell lysates were prepared followed by immunoblotting for PARP. (c) ER stress induces autophagosome formation. Wild-type and bax−/− bak−/− MEFs stably expressing GFP-conjugated LC3 were treated with tunicamycin (0.5 μg/ml) for 12 h. Cells were then fixed and observed with an electron microscope and representative images are shown. Electron microscopy immunohistochemistry was also performed using an anti-GFP antibody.20 Note the presence of gold particles indicative of autophagosomes in both wild-type and bax−/− bak−/− cells. Bar = 500 nm. (d) Multi-domain proapoptotic Bcl-2 proteins do not affect autophagy. bax−/− bak−/− MEFs were transfected with LC3-GFP together with DsRed-tagged Bak or Bax. Twenty-four hours later, cells were left untreated or treated with tunicamycin (0.5 μg/ml) for 8 h. Cells were then observed under a Zeiss Axiovert fluorescence microscope. Note that DsRed-Bak did not induce autophagy, nor did it alter tunicamycin-induced autophagy. Quantitation of autophagic cells in control cells and cells expressing DsRed-Bax or Bak. (e) Inhibition of autophagy protects apoptosis-deficient MEFs from endoplasmic reticulum (ER) stress-mediated cell death, while promoting death in apoptosis-competent cells. atg5−/− and atg5 +/+ MEFs were infected with a retroviral construct expressing Bcl-xL. Cells were treated for the indicated times with ER stress and cell death was measured by PI exclusion and indicated as an average of triplicate samples ± S.E.M. (P<0.01 for all time points). Note that atg5−/− cells died faster than atg5 +/+ cells, whereas when Bcl-xL was expressed, atg5−/− cells died slower than atg5 +/+ cells
Upon further examination using phase-contrast microscopy the dead bax−/−bak−/− cells displayed morphological features such as plasma membrane dilation and disruption that resemble necrosis (Supplementary Figure S1B). To characterize this more, we examined the cleavage of poly(ADP-ribose) polymerase (PARP) from its full-length 114 kDa form to generate an 89-kDa fragment, a characteristic feature of apoptotic cells. The caspase-3-specific 89 kDa fragment was observed in wild-type cells 1–2 days after ER-stress treatment, but not in bax−/−bak−/− cells even after 3–4 days, at which time the amount of cell death was comparable to that of wild-type cells treated for 1–2 days (Figure 1b). Furthermore, the release of HMGB1 into the extracellular environment, an indicator of necrosis,6 was observed to coincide with the timing of ER stress-induced bax−/−bak−/− cell death, but not wild-type cell death (Supplementary Figure S1C). These findings indicate that while apoptosis is blocked in bax−/−bak−/− cells, prolonged treatment with ER stress can induce caspase-independent, necrotic cell death.
When observed with an electron microscope, wild-type and bax−/−bak−/− MEFs exposed to ER stress for a short period of time (12 h) displayed an increase in multi- or double-layered membrane structures enclosing electron-dense materials, which are characteristic of autophagosomes (Figure 1c). The induction of autophagy was verified further by the conversion of microtubule-associated protein 1 (MAP1) light chain (LC3) from its cytosolic form (LC3-I) to a membrane-bound form (LC3-II) (Figure 1c, Supplementary Figure S2C and D), a key event in autophagy.7 3-Methyladenine (3MA), a pharmacological inhibitor of autophagy, blocked the induction of LC3-GFP puncta formation. In contrast, chloroquine, a chemical that blocks lysosome function by increasing lysosomal pH, increased the formation of GFP-LC3 puncta (Supplementary Figure S2C and D). These findings indicate that ER stress may induce autophagic response in both wild-type and bax−/−bak−/− cells. We noticed that after tunicamycin treatment for 48 h wild-type cells appeared apoptotic, characterized by condensed chromatin, whereas bax−/−bak−/− MEFs showed no signs of apoptosis yet drastic accumulation of autophagosomes (Supplementary Figure S2A and B). This suggests that after longer exposure to ER stress, wild-type cells die by apoptosis if the damage is not relieved, whereas autophagy keep accumulating in bax−/−bak−/− cells due to their deficiency in apoptosis. The prolonged accumulation of autophagy may eventually lead to cell damage and demise independent of Bax and Bak.
Interestingly, it was noted that autophagy was induced in both wild-type and bax−/−bak−/− cells in response to ER stress. The balance between the levels of the antiapoptotic and proapoptotic Bcl-2 family of proteins plays a critical role in determining the fate of a cell to survive or to die by apoptosis. The BH1 and BH2 domains of Bcl-2 are required for Bcl-2–Bax interaction and inhibition of apoptosis.8 It has been recently reported that the antiapoptotic Bcl-2 family members can regulate autophagy,9–11 possibly by interacting with Beclin 1.9,10 Beclin 1 is essential for autophagy induction,12,13 and was found to interact with Bcl-2 via the BH1 and BH2 domains of Bcl-2.10 In light of these reports, there exists the possibility that the proapoptotic proteins Bax and Bak may affect the induction of autophagy via their interaction with Bcl-2. To address this issue, we quantitated the formation of autophagosomes in wild-type and bax−/−bak−/− MEFs treated with ER stress. Both cell types showed equivalent amounts of LC3-GFP puncta (Supplementary Figure S3A). In addition, both wild-type and bax−/−bak−/− MEFs treated with ER stressors showed clear conversion of LC3-I to LC3-II, with no apparent difference, judged by immunoblotting of LC3 (Supplementary Figure S3B). The lysosomal protease inhibitors E-64-D and pepstatin A14 enhanced the accumulation of LC3-II to a similar degree in wild-type and bax−/−bak−/− cells (Supplementary Figure S3C and d). These results indicate that deficiency in Bax and Bak has no significant effect on ER stress-induced autophagy.
Conversely, we tested whether overexpression of Bak or Bax could affect the induction of autophagy. DsRed-targeted Bak or Bax were co-transfected with LC3-GFP into bax−/−bak−/− MEFs. Bak was predominantly localized to mitochondria indicated by its punctate localization. Expression of DsRed-Bak did not induce autophagy, nor did it affect tunicamycin-induced autophagy (Figure 1d). Bax was predominantly localized in the cytosol. In response to ER stress, it translocated from the cytosol to mitochondria. Similar to Bak, expression of DsRed-Bax failed to induce autophagy and did not alter ER stress-induced autophagy (Figure 1d and Supplementary Figure S4A). An ER-targeted Bak mutant also failed to affect on autophagy induction (Supplementary Figure S4C). This mutant has been previously shown to localize to the ER and cause ER Ca2+ release and apoptosis.15 Taken together, our findings indicate that unlike the antiapoptotic Bcl-2 family proteins, the multi-domain proapoptotic Bcl-2 proteins Bax and Bak do not affect autophagy, nor do they affect Bcl-2’s ability to regulate autophagy.
Autophagy has been shown to promote either cell survival or death. Given that autophagy can be induced by ER stress, we evaluated whether autophagy plays a role in bax−/−bak−/− cell death resulting from prolonged ER stress. Cells treated with ER stress in the presence of the autophagy inhibitor 3MA appeared to be more adherent to the culture plates, and maintained relatively normal fibroblast appearance (Supplementary Figure S5A). In addition, when measured by plasma membrane permeability, 3MA significantly enhanced the viability of bax−/−bak−/− cells in response to ER stress (Figure 1e). These results indicate that autophagy can enhance cell death in bax−/−bak−/− cells. In contrast, 3MA in combination with ER stress-enhanced cell death in apoptosis-competent wild-type cells (Supplementary Figure S5B). These findings suggest that autophagy may have an opposite effect in determining cell fate in response to ER stress in apoptosis-competent and deficient cells. To determine more specifically whether autophagy plays a role in Bax/Bak-independent cell death in response to ER stress, the essential autophagy factor Atg5 was knocked down by short hairpin RNA technique in bax−/−bak−/−MEFs. Similar to 3MA, inhibition of autophagy by Atg5 knockdown resulted in significant protection against thapsigargin and brefeldin A (Supplementary Figure S5C).
The above results suggest that autophagy can promote cell death in apoptosis-deficient cells. To determine whether this is a peculiar affect that can only be observed in bax−/−bak−/− cells, Bcl-xL was ectopically expressed at similar levels in atg5+/+ and atg5−/− cells to block apoptosis. When treated with ER stress, atg5−/− cells died faster than atg5+/+ cells (Supplementary Figure S5D), consistent with recent reports that autophagy acts as a survival mechanism to escape apoptosis in response to ER stress.16,17 As anticipated, Bcl-xL protected both atg5+/+ and atg5−/− cells from acute apoptosis. In response to prolonged ER stress the Bcl-xL-overexpressing cells progressively died. Interestingly, Bcl-xL-overexpressing atg5−/− MEFs survived better than Bcl-xL-overexpressing atg5+/+ MEFs (Figure 1e). Taken together, our findings indicate that while autophagy serves as a survival mechanism in apoptosis-competent cells, those with defects in apoptosis can utilize autophagy as a means to promote non-apoptotic cell death in response to ER stress.
Our results show that cells deficient in apoptosis, due to either the lack of both Bax and Bak, or expression of Bcl-xL, can die in response to prolonged ER stress. This Bax/Bak-independent and Bcl-xL unblockable cell death is necrotic, as revealed by morphological and biochemical criteria. This may be pathologically significant especially in cells with impaired apoptosis, such as cancer cells. Additionally, we find that ER stress-induced necrosis is associated with autophagy. While autophagy was originally described as a cell survival mechanism, it recently has been proposed that under certain circumstances it can be a mechanism of cell death.9,18 With respect to ER stress, it is suggested that autophagy helps the cell to adapt the stress. Here, we show that while autophagy can be induced to similar levels in wild-type and bax−/−bak−/− cells in response to ER stress, the resulting outcome of this response appears to be much different. While inhibition of autophagy using 3MA or by Atg5 knockdown was able to delay cell death in bax−/−bak−/− cells, inhibition of autophagy made apoptosis-competent cells more sensitive to ER stress. Thus in wild-type cells, autophagy apparently confers protection from apoptosis resulting from excessive ER stress, possibly by promoting protein degradation to resolve the stressing conditions.16–19 In contrast, in apoptosis-deficient cells prolonged autophagy provokes cell death, and its absence imparts protection. This may be because in these cells where excessive ER stress fails to induce apoptosis, the stressing situation keeps accumulating to a point where autophagy is massively induced subsequently leading to cell damage and necrosis. It is interesting to note, however, inhibition of autophagy did not completely abrogate this Bax/Bak-independent cell death, nor did it promote clonological survival (data not shown). This indicates that cell death in response to ER stress may involve complex cell death pathways as observed in other cell systems. Autophagy, rather than acting as a distinguished form of cell death, instead contributes to the cell fate determination by differentially affecting apoptosis and necrosis.
Materials and Methods
Cell culture and drug treatment
Wild-type and bax−/−bak−/− murine embryonic fibroblasts (MEFs) were generated as previously described (37). atg5−/− and atg5+/+ cells were a kind gift from Dr. Noboru Mizushima at Tokyo Medical and Dental University, Japan. MEF and 293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were treated with tunicamycin (0.5 μg/ml), thapsigargin (0.1 μM) or brefeldin A (0.5 μg/ml) alone or in combination with either 3-methyladenine (5 mM), chloroquine (10 μM), or E-64-D (10 μg/ml) and pepstatin A (10 μg/ml) for the indicated times.
Expression vectors and cell transfection/infection
The retroviral LPC-LC3-GFP was generated using the EGFP-tagged LC3 excised from pEGFP-C1-LC3 expression vector, a kind gift from Dr. Tamotsu Yoshimori at the National Institute of Genetics, Japan. 293T packaging cells were transfected with LPC-LC3-GFP using the calcium phosphate precipitation method. Twenty-four to 72 hours after transfection viral supernatant was collected, filtered through a 0.45-μm filter and subsequently used to infect MEFs. Stable cell lines expressing LC3-GFP were generated using limited dilution to select for GFP positive clones. Bcl-xL overexpression in Bax/Bak wild-type, atg5−/−, and atg5+/+ MEFs was performed in a similar manner using a retroviral plasmid expressing Bcl-xL. pcDNA3-DsRed-Bak, pcDNA3-DsRed-Bax, and pcDNA3-DsRed-Bak-cb5 (28) were transfected into MEFs using Lipofectamine2000 (Invitrogen).
Atg5 knockdown by shRNA
For the generation of Atg5 short hairpin (shAtg5), a PCR reaction was performed using a forward oligonucleotide with the sequence from an RNA polymerase III-specific U6 promoter (CAGTGGAAAGACGCGCAGGCA) in combination with reverse primer (AAAAAACAACTTGCTTTACTCTCTATCACCTCGAGCTGATAGAGAGTAAAGCAAGTTGGGTGTTTCGTCCTTTCCACAA) that contains a hairpin for atg5 (38), in addition to a sequence from the U6 promoter. This construct were subsequently cloned into a pBabe-puro-based retroviral vector. The retroviral vector was transfected into 293T packaging cells by calcium phosphate transfection. Virus-containing supernatant was collected 24–72 hours post transfection, and was used to infect MEFs. Stable shRNA knockdown of Atg5 was verified by immunoblotting.
Electron microscopy
Samples used for transmission electron microscopy (TEM) were processed using standard techniques. Briefly, samples were collected and fixed with 2.0% paraformaldehyde/2.5% EM grade glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) at 37°C. After fixation, samples were placed in 2% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.4), dehydrated in a graded series of ethyl alcohol and embedded in Durcupan resin. Ultrathin sections of 80 nm were cut with a Reichert-Jung UltracutE ultramicrotome and placed on formvar coated slot copper grids. Sections were then counterstained with uranyl acetate and lead citrate and viewed with a FEI Tecnai12 BioTwinG2 electron microscope. Digital images were acquired with an AMT XR-60 CCD Digital Camera System.
For electron microscopy immunohistochemistry, samples were fixed in 0.1% glutaraldehyde in PBS for 1hr on ice, washed in PBS and then quenched with 0.05M ammonium chloride. Samples were then dehydrated in a graded series of ethyl alcohol, embedded in LR White (hard grade) resin (Electron Microscopy Sciences, Fort Washington, PA.), and polymerized in a 50°C oven overnight. Pale gold ultrathin sections were cut on a Reichert-Jung UltraCut E ultramicrotome and placed on formvar coated slot grids. Sections were blocked with 1%BSA in PBS, incubated in a polyclonal anti-GFP antibody at 1:1000, washed and then placed in a secondary IgG antibody conjugated to 15nm gold particles at a dilution of 1:25. Sections were then counterstained with uranyl acetate and viewed.
Immunoblotting analysis
Cells were lysed in RIPA buffer (1% sodium deoxycholine, 0.1% SDS, 1% Triton X-100, 10 mM Tris at pH 8.0, 0.14 M NaCl) with protease inhibitor complex (Roche). Thirty micrograms of protein was resolved on SDS-PAGE gels and then transferred to PVDF membranes, and subsequently blotted with each primary antibody. The following antibodies were used: PARP (BD PharMingen), LC3 (a kind gift from Dr. Tamotsu Yoshimori), HMGB1 (BD PharMingen), CHOP (Santa Cruz), β-tubulin (Sigma), Atg5 (ABGENT), and Bcl-xL (clone 13.6).
Measurement of cell death
For assessment of cell death, cells were harvested and resuspended in DMEM with propidium iodide (PI, 1 μg/ml). Cell viability was determined by PI exclusion via flow cytometry using a FACs caliber. Alternatively trypan blue staining was also used as a measure of cell death. After treating cells for the indicated times cells were collected, harvested and stained with trypan blue (0.2%), and counted under a phase contrast light microscope. Pictures were taken with a Zeiss AxioPlan 2 microscope using an x32 objective. Images were captured using a Spot camera (Diagnostic Instruments).
Observation and quantification of the LC3-GFP puncta formation
Cells were fixed in 4% paraformaldehyde for 10 min then washed with phosphate buffered saline (PBS). LC3-GFP transfectants were imaged and pictures taken with the Zeiss Axiovert Fluorescence microscope, using the x63 oil objective. To quantify the percent of autophagic cells, 50 cells were randomly counted and those containing more than 8 green puncta and less nuclear LC3-GFP were considered autophagic and expressed as a percent of the total cells counted.
Statistical analysis
Data from cell death assays or autophagosome counting are presented as mean +/− S.E.M. (standard error of the mean). Comparisons between two groups were made using a Student’s t-test. Statistical analyses were performed using Microsoft Excel.
Supplementary Material
Acknowledgments
We thank Drs. Tamotsu Yoshimori and Noboru Mizushima for providing reagents, Susan Van Horn (Central Microscopy Imaging Center at Stony Brook University) for assistance on electron microscopy. We also thank Drs. Michael Frohman, Nancy Reich, Howard Crawford, Patrick Hearing, and Janet Hearing for critical readings. WXZ is supported by the NCI Howard Temin Award and the Carol Baldwin Breast Cancer Research Foundation at Stony Brook.
The abbreviations used are
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- MEF
murine embryonic fibroblast
- LC3
microtubule-associated protein 1 (MAP1) light chain 3
- 3MA
3-methyl adenine
- CQ
chloroquine
- HMGB1
high-mobility group protein B1
- MNNG
N-methyl-N’-nitro-N-nitrosoguanidine
References
- 1.Schroder M, et al. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 2.Marciniak SJ, et al. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 3.Rao RV, et al. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 4.Boyce M, et al. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 5.Wei MC, et al. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaffidi P, et al. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 7.Kabeya Y, et al. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin XM, et al. Nat Cell Biol. 1994;369:321–323. [Google Scholar]
- 9.Shimizu S, et al. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 10.Pattingre S, et al. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Hoyer-Hansen M, et al. Mol Cell. 2007;25:193–205. [Google Scholar]
- 12.Liang XH, et al. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 13.Yue Z, et al. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanida I, et al. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 15.Zong WX, et al. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogata M, et al. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouroku Y, et al. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, et al. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 19.Ding WX, et al. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- 20.Wang QJ, et al. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.