Abstract
The ventrolateral division of the periaqueductal gray (vlPAG) and the adjacent deep mesencephalic reticular nucleus have been implicated in the control of sleep. The preoptic hypothalamus, which contains populations of sleep-active neurons, is an important source of afferents to the vlPAG. The perifornical lateral hypothalamus (LH) contains populations of wake-active neurons and also projects strongly to the vlPAG. We examined nonREM and REM sleep-dependent expression of c-Fos protein in preoptic-vlPAG and LH-vlPAG projection neurons identified by retrograde labeling with Fluoro-gold (FG). Separate groups of rats (n=5) were subjected to 3 hours total sleep deprivation (TSD) followed by 1 hour recovery sleep (RS), or to 3 hours of selective REM sleep deprivation (RSD) followed by RS. A third group of rats (n=5) was subjected to TSD without opportunity for RS (awake group). In the median preoptic nucleus (MnPN), the percentage of FG+ neurons that were also Fos+ was higher in TSD-RS animals compared to both RSD-RS rats and awake rats. There were significant correlations between time spent in deep nonREM sleep during the 1-hour prior to sacrifice across groups and the percentage of double-labeled cells in MnPN and ventrolateral preoptic area (VLPO). There were no significant correlations between percentage of double labeled neurons and time spent in REM sleep for any of the preoptic nuclei examined. In the LH, percentage of double-labeled neurons was highest in awake rats, intermediate in TSD-RS rats and lowest in the RSD-RS group. These results suggest that neurons projecting from MnPN and VLPO to the vlPAG are activated during nonREM sleep and support the hypothesis that preoptic neurons provide inhibitory input to vlPAG during sleep. Suppression of excitatory input to the vlPAG from the LH during sleep may have a permissive effect on REM sleep generation.
Keywords: median preoptic nucleus, ventrolateral preoptic area, perifornical lateral hypothalamus, sleep deprivation
1. INTRODUCTION
The preoptic hypothalamus contains populations of sleep-active neurons and is considered an important sleep regulatory site (Szymusiak and McGinty, 2008). The highest densities of sleep-active neurons within the preoptic area are found in the ventrolateral preoptic area (VLPO) and median preoptic nucleus (MnPN). The MnPN and VLPO innervate the ventrolateral subdivision of the periaqueductal gray (vlPAG) (Lu et al., 2006; Uschakov et al., 2006; Yoshida et al., 2005; Saper and Levisohn, 1983), a region implicated in multiple autonomic and behavioral functions, including sleep. The medial preoptic nucleus (MPO) has strong reciprocal connections with the vlPAG (Rizvi et al., 1992; Rizvi et al., 1996; Murphy et al., 1999), as does the bed nucleus of stria terminalis (BST) (Holstege et al., 1985; Gray and Magnuson, 1992; Dong and Swanson, 2006). These projections have been implicated in autonomic and neuroendocrine functions. Projection from the preoptic hypothalamus to the vlPAG may regulate sleep-related changes in physiological variables, such as respiration and heart rate. A subset of the PAG neurons have been shown to exhibit state-dependent activity related to cardiovascular or respiratory cycles, (Ni et al., 1990a,b).
The vlPAG and the area immediately ventral and lateral to it, the deep mesencephalic reticular nucleus (DpMe), have been proposed to play a pivotal role in REM sleep control (Lu et al., 2006; Luppi et al., 2006). Muscimol injections in vlPAG augment REM sleep (Sastre et al., 1996, Sastre et al., 2000; Boissard et al., 2002; Lu et al., 2006; Vanini et al., 2007). Lesions of vlPAG and the adjacent DpMe increase REM sleep (Lu et al., 2006; Kaur et al., 2009). These findings suggest that the vlPAG/DpMe plays an inhibitory role in gating the onset and/or maintenance of REM sleep. Kaur et al (2009) have proposed that sleep-active neurons in preoptic hypothalamus provide inhibition of the vlPAG, allowing REM sleep generating neurons in the pontine reticular formation to become activated.
To investigate patterns of activation in projection neurons from the preoptic area to vlPAG in relation to sleep states, we combined retrograde tracer injection into the vlPAG with c-Fos protein immunohistochemistry in the preoptic area in rats. We examined two conditions designed to cause differential expression of deep nonREM versus REM sleep; 3 hours total sleep deprivation (TSD) followed by 1 hour of recovery sleep and 3 hours selective REM sleep deprivation (RSD) followed by 1 hour of recovery sleep. These conditions were compared to groups of rats subjected to TSD without opportunity for recovery sleep.
In contrast to the preoptic area, neuronal activity in the lateral hypothalamus (LH) is strongly wake-related (Alam et al., 2002; Scammell et al., 2000; Mileykovskiy et al., 2005; Lee et al., 2005). Hypocretin (orexin) neurons in the LH project to the vlPAG (Peyron et al., 1998) and are implicated in the regulation of arousal and suppression of REM sleep (see Sakurai et al., 2010; Bonnavion and de Lecea, 2010 for review). We therefore examined cFos expression as an indicator of neuronal activity in vlPAG projection neurons located in the perifornical LH across the three experimental groups.
2. METHODS
All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. All protocols were reviewed and approved by the Animal Care and Use Committee at the Veterans Administration of the Greater Los Angeles Health Care System. Subjects were male Sprague-Dawley rats weighing between 300–350 grams at the time of surgery.
2.1 Animal surgery
All surgical procedures were performed under deep isoflurane anesthesia. Retrograde tracer, Fluoro-gold (FG; Fluorochrome, Denver, CO), was pressure-injected into the vlPAG stereotaxically (bregma AP −7.4, ML −0.7, DV −6.4 mm) with a Hamilton microsyringe controlled by an infusion pump (KDS310, KD Scientific Inc, Holliston, MA). 10nL of FG solution (4% in dH2O) was infused at a rate of 0.01μL/min, and the injection needle was slowly withdrawn after waiting additional 10 min at the end of infusion. EEG and EMG electrodes were implanted at the time of tracer injection. Small bone screws placed through the skull in contact with the dura at frontal and parietal areas were used to record EEG. EMG was recorded with insulated stainless steel wires placed in the muscles of the dorsal neck. All leads were connected to a plastic connector that was cemented to the skull. Animals were allowed to recover for one week before being subjected to polygraph recording.
2.2 Sleep recording and analysis
A recording cable connected the implanted headplug to a 12-pin commutator (SL12C, Plastics One, Roanoke, VA). EEG and EMG signals were conducted by fully grounded cables, amplified with differential AC amplifiers (Model 1700, A-M Systems, Sequim, WA), bandpassed in 1–30Hz and 10–1000Hz respectively, and digitized with a CED 1401 Plus interface unit (Cambridge Electronic Design, Cambridge, England) sampled in 256Hz before storing into computer hard drive. The polygraphic recording was visually scored with a Spike2 script (Sleepscore v2.02 by Dr. Geoff Horseman) offline.
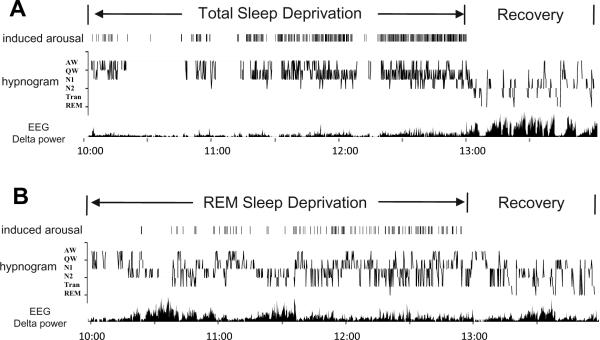
Sleep-wake behaviors were scored in one of the 6 stages (AW, QW, N1, N2, Tran, REM) in 10sec epochs. Active waking (AW) was defined by presence of spontaneous behaviors such as grooming or locomotion, elevated and variable EMG activity in the presence of a desynchronized EEG, while quiet waking (QW) was defined by desynchronized EEG and diminished EMG tonic activity, which typically displayed as an intermediate stage between AW and sleep. Light nonREM sleep (N1) was scored when moderate slow wave activity was present in the EEG, and deep nonREM sleep (N2) was scored when high amplitude slow wave activity occupied more than half of the epoch duration. The transitional stage between NREM and REM (Tran) was defined by high amplitude theta waves intermixed with reduced amplitude slow wave activity in the EEG and low levels of EMG tone. Since Tran almost always precedes the beginning of a fully expressed REM sleep episode, scoring Tran has been shown to improve the analysis of sleep structure (Benington et al, 1994). REM sleep was defined by presence of desynchronized EEG, presence of theta waves in EEG and muscle atonia (minimal EMG activity). Wake represents combined AW and QW, and NREM represents combined N1, N2 and Tran. The EEG power spectrum analysis for delta (1.0–4.0 Hz) was carried out using a Spike2 script provided by CED (SUDSA ver2.2) for each 10sec epoch of artifact-free EEG during deprivation and recovery periods (Figure 1).
Figure 1.
Representative sleep-wake patterns of animals in (A) TSD-RS and (B) RSD-RS groups. For TSD, arousals were induced by tapping on the recording cage at the earliest EEG and behavioral signs of sleep. Experimenter induced arousal are indicated as vertical lines above the hypnogram. For RSD, arousals were induced at signs of nonREM-REM transition (Tran) or electrographic signs of REM sleep onset. The frequency of cage tapping gradually increased as the deprivation progressed, reflecting increasing homeostatic pressure for sleep or REM sleep respectively. Recovery sleep after TSD is dominated by deep nonREM sleep (N2) and elevated EEG delta power, while Tran and REM sleep states are more prevalent in RSD rat.
2.3 Experimental procedures
Animals were housed individually in Plexiglass recording chambers (9 in. × 9 in. × 10 in.) that were placed inside larger incubators (internal dimension: 23 in. × 18 in. × 49 in.). Ambient temperature in the incubator was 23±1°C and a 12/12 light/dark cycle was maintained. Animals were adapted to the recording environment for 9–11 days and to the recording cable for at least 3 days before the experiment. Food and water were continuously available during all adaptation and experimental procedures.
Three experimental groups were used in this study. Separate groups of rats were subjected to either 3-hr total sleep deprivation (TSD) (n=5) or 3-hr selective REM sleep deprivation (RSD) (n=5) starting at the onset of light period (ZT0), followed by 1-hr undisturbed recovery sleep (RS) prior to sacrifice (TSD-RS and RSD-RS, respectively). A third group of rats were subjected to 2-hr TSD without opportunity for recovery sleep prior to the sacrifice (n=5). Two rats in this awake group were sacrificed at ZT4, the same circadian time as TSD-RS and RSD-RS rats. The other 3 rats in the awake group were sacrificed during the dark phase at ZT16. As single- and double labeled cell counts were found to be similar in awake rats sacrificed at ZT4 and ZT16, data from the 5 awake rats were combined for statistical comparison with the TSD-RS and RSD-RS groups.
TSD and RSD were achieved by tapping the Plexiglas recording cage using a stick placed through a port in the incubator that housed the recording cage. This was done whenever animals showed signs of entering either nonREM or REM sleep in the continuous monitoring by polygraph and video camera. The timing of each cage tap was logged by a keystroke at computer keyboard and subjected to analysis as a measure of accumulating sleep pressure (see Figure 1). At the end of recovery period, the incubator was opened and the animal was promptly anesthetized with intraperitoneal pentobarbital injection (100mg/kg).
2.4 Histology and immunohistochemical procedures
Under deep anesthesia of sodium pentobarbital, rats were perfused transcardially with 100mL of 0.1M phosphate buffered isotonic saline (PBS), followed by 500mL of 4% paraformaldehyde (PFA) in 0.1M PB (pH=7.4). The brains were then immersed in same fixative for additional 1 hour before being stored in the 30% sucrose in 0.1M PB in 4°C for at least 2 days. The brains were then rapidly frozen with dry ice and cut in 30-μm coronal sections with a Leica SM2400 microtome. The sections were rinsed and then immersed in cryoprotectant, and stored at −20°C until staining.
Fos protein and FG immunostaining were carried out in sequence on free-floating sections using an ABC method with Nickel-DAB and DAB as chromogens, respectively. According to the information provided by the manufacturer, the anti-c-Fos antibody (PC-38 rabbit polyclonal IgG, Calbiochem, San Diego, CA) was raised against a synthetic peptide corresponding to amino acid 4–17 of human c-Fos, and has shown reactivity in humans, mice & rats. The anti-FG antibody (AB-153 rabbit polyclonal IgG, Millipore Biosciences, Temecula, CA) was raised in rabbits with bovine serum albumin (BSA)-conjugated FG as the immunogen; the specificity has been tested in rats.
Free-floating sections were washed thoroughly in TBS, then incubated in 0.3% H2O2 for 30min, and incubated in the blocking solution consisted of 8% normal goat serum in TBS for 2 hours in room temperature. The sections then were incubated with anti- c-Fos antibody 1:10,000 in TBS with 4% normal goat serum in room temperature with gentle agitation overnight. After rinse, the sections were incubated with the biotinylated goat anti-rabbit secondary antibody 1:250 in TBS with 4% goat serum for 60min and avidin-biotin-HRP solution (Elite kit, Vector Labs, Burlingame, CA) 1:250 for 60min in room temperature. After thorough wash, the section were reacted in TBS containing 0.05% 3,3'-diaminobenzidine-4HCl (DAB, Sigma-Aldrich, St. Louis, MO) and 2.4% nickel ammonium sulfate and 0.0015% H2O2 for approximate 4 min for the visualization of c-Fos immunoreactive neurons. After completion of c-Fos staining, FG staining began with the incubation of 1:4,000 anti-FG antibodies in 8% normal goat serum with 0.2% Triton-X 100 in 4°C with gentle agitation for 3 days. The rest of FG staining was similar to the c-Fos staining except the concentrations of secondary antibody/ABC-HRP solutions were reduced (1:1,000 and 1:500 respectively), and the visualization was achieved with DAB without nickel enhancement. As a result, the nuclei of Fos-immunoreactive neurons were stained in black-purple, and the FG containing neurons were stained in orange-yellow. Neuronal FG staining often had a distinctive granular texture which was readily distinguishable from the background.
2.5 Quantitative analysis of retrograde tracer and c-Fos labeling
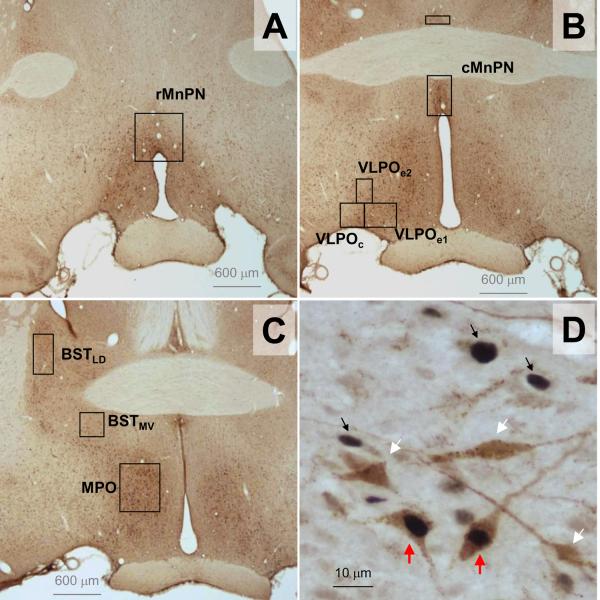
Neurons labeled with c-Fos or Fluoro-gold were quantified with the aid of Neurolucida software (ver.7, MicroBrightField Bioscience Inc, Colchester, VT), conducted with a Nikon Eclipse E600 microscope with a motorized stage and Lucivid system (MicroBrightField), which overlaps lucida markers onto the microscopic field of view. The counts in each region of interest were obtained from the average of 3 representative coronal sections, which were evenly spaced longitudinally and covered the major portion of the anatomical structures of interest. The counting boxes for median preoptic nucleus and ventrolateral preoptic area were the same as described previously (Gong et al., 2000; Gvilia et al., 2006a). Briefly, for the rostral part of the median preoptic nucleus (rMnPN), a square of 600μm × 600μm was centered at the dorsal surface of the third ventricle at approximately 0.22mm posterior to bregma where the two arms of the anterior commissure were separated. For the caudal part of the median preoptic nucleus (cMnPN), a rectangle of 300μm × 600μm was placed above the dorsal surface of the third ventricle at the midline at approximately P0.26mm, where the anterior commissure crossed the midline. The rectangular box split into two parts, above and below the dorsal and ventral border of anterior commissure, respectively, when the distance between the dorsal border of the third ventricle and the ventral border of anterior commissure narrows in the most caudal portion of this structure. For VLPO, three boxes were placed corresponding to the divisions of the core VLPO (VLPOc), the medial extended VLPO (VLPOe1) and the dorsal extended VLPO (VLPOe2) at the level of P0.3 to P0.7mm. The VLPOc box, 300μm × 300μm, was placed along the ventral surface of the brain with its medial border 100μm lateral to the lateral edge of the optic chiasm. The VLPOe1 was placed adjacently medial to VLPOc, 300μm high and 400μm wide. The VLPOe2, centered above the shared border of VLPOc and VLPOe1, was 300μm high and 200μm wide. VLPOe represents the sum of VLPOe1 and VLPOe2 counts in each section. For the medial preoptic nucleus (MPO), a rectangle box of 600μm high and 500μm wide was placed at about 150μm lateral to the edge of third ventricle and centered between the dorsal border of optic chiasm and the ventral border of the anterior commissure at the levels between P0.3 and P0.7mm. For the bed nucleus of stria terminalis (BST), two boxes were used to cover the dorsal part of the lateral BST (BSTLD) and the medial part of the ventral BST (BSTMV) (Paxinos and Watson, 1998) also at the similar level of P0.3 to P0.7mm. For the BSTLD, a box of 500 μm high and 250μm wide was centered between the dorsal border of the anterior commissure and the ventral edge of the lateral ventricle, with its lateral border adjacent to the internal capsule. For the BSTMV, a box of 300μm × 300μm was placed just ventral to the lateral end of anterior commissure, roughly coincided the area assigned as ventral part of BSTam by Swanson (Dong and Swanson, 2006). The positions of these counting boxes can be seen in Figure 5. The counting boxes in perifornical lateral hypothalamus are the same as described previously (Kumar et al., 2008). Briefly, the area of interest centered at the site where most of the hypocretin neurons are distributed as shown by previous studies (bregma −2.5 to −3.5mm) (de Lecea et al, 1998; Yoshida et al, 2006), and was divided into three sub-regions; the perifornical area centered at about 200μm dorsal to the fornix (700μm wide and 1000μm long, Box-1), the medial area (500μm wide and 1000μm long, Box-2) and the lateral area (500μm wide and 1000μm long, Box-3) juxtaposed and shared the vertical borders with Box-1 (Figure 8A).
Figure 5.
Counting boxes used for the quantification of Flurogold+ and c-Fos+ cell counts in the preoptic hypothalamus (A–C). Examples of single Fos+ cells (black arrows) and dual Flurogold-Fos+ neurons (red arrows) shown in panel D.
Figure 8.
(A) Placement of perifornical (1), medial (2) and lateral (3) counting boxes in the lateral hypothalamus. (B) Mean (±SEM) Fluorogold+ cell counts in the lateral hypothalamic counting boxes for TSD-RS (n=3), RSD-RS (n=3) and Awake (n=5) rats. (C) Mean (±SEM) percentages of Fluorogold+ neurons that were also positive for c-Fos protein in the lateral hypothalamus for the three experimental groups. (D) Mean (±SEM) total number of neurons expressing c-Fos in the same areas of these groups. Asterisks indicate the significant difference between paired comparison in Post Hoc tests with Bonferroni correction at p=0.05 level. Scatter plots of % of double labeled neurons versus % time spent in waking (E), nonREM sleep (F) and combined Tran+REM sleep (G) during the 60 minute prior to sacrifice for individual animals in the three experimental groups. Regression lines and correlation coefficients are also shown for each scatter plot.
Statistical analyses were carried out using SPSS (Release 17.0, SPSS Inc., Chicago, IL). Comparison of variables among experimental groups used one-way ANOVA and Post Hoc tests with Bonferroni correction, alpha= 0.05. Bivariable Pearson correlations were conducted with significance noted at p=0.05 level (two-tailed).
3. RESULTS
3.1 Sleep-Wake Amounts
A total of 24 rats were used in this study, but data from 9 are not presented due to unsuitable injections that were outside the target area (n=6) or failed immunohistochemistry (n=3). Fifteen rats were included in the analyses with 5 animals each in the RSD-RS, TSD-RS and awake groups.
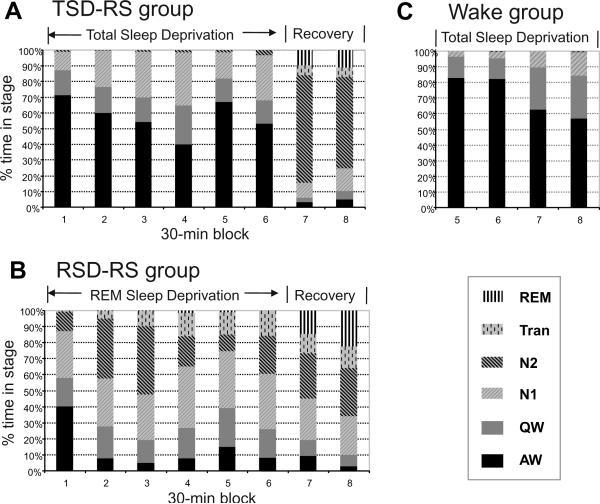
Examples of sleep-wake and EEG data in representative animals from the TSD-RS and RSD-RS groups are shown in Figure 1. Animals displayed increasing propensity for initiating nonREM or REM sleep over the course of 3-hours of TSD or RSD, respectively. Frequent interventions were required to prevent expression of nonREM and REM during the final 70–90 min of deprivation, indicated by increasing frequency of cage tapping-induced arousals marked as a vertical line on the top of the hypnogram (Figure 1). Animals spent 74.6% time awake and 0.0% time in REM sleep in average during the 3-hour TSD, and 32.8% time awake and 0.5% time in REM sleep in the 3-hour RSD. The average distributions of sleep-wake stages during the deprivation and the recovery period are plotted in 30-minute blocks in Figure 2. The gradual increase of the QW and N1 stages during the course of TSD and the increase of the Tran state during the RSD can be viewed as another indication of accumulating nonREM and REM sleep pressure, respectively.
Figure 2.
Distribution of sleep stages, expressed as a percentage of recording time, plotted in 30 min blocks of sleep deprivation and recovery sleep for the 3 experimental groups. Mean values are shown for (A) TSD-RS rats, n=5; (B) RSD-RS rats, n=5; (C) Awake rats, n=5.
Most animals in the TSD-RS group showed sleep onset immediately at the release to recovery sleep, with an average sleep onset latency of 42 sec and an average REM sleep latency of 15 min 36 sec (Table 1). Most of the animals in the RSD-RS group continued to stay asleep at the beginning of recovery period, with an average REM sleep onset latency of 5 min 54 sec (Table 1). N2 comprised 63.3% of time in the recovery period in the TSD-RS group, and only 29.1% in the RSD-RS group. REM sleep occupied 10.3% of the recovery period in TSD-RS rats, and combined REM and Tran sleep accounted for 16.4%. In contrast, REM sleep comprised 18.5% of the recovery period in RSD-RS rats and combined REM and Tran sleep accounted for 31.3%. Hence, the two experimental conditions produced different levels of homeostatic pressure for nonREM and REM sleep during the final hour of deprivation and differential expression of these states during the hour of recovery sleep that preceded sacrifice.
Table 1.
Comparisons of sleep variables during 1-hour recovery sleep period of TSD-RS and RSD-RS rats, and the one hour prior to sacrifice in the awake rats (mean ± S.E.M.).
| Onset latency (sec) | Wake | NREM | REM | |||||
|---|---|---|---|---|---|---|---|---|
| NREM | REM | AW | QW | N1 | N2 | Tran | ||
| TSD-RS (n=5) | 42±28 | 936±192 | 4.1%±0.9% | 4.1%±0.3% | 12.1%±1.2% | 63.3%±2.5% | 6.1%±1.7% | 10.3%±1.2% |
| RSD-RS (n=5) | NA | 354±149 | 6.1%±1.4% | 8.6%±1.4% | 25.1 %±3.0% | 29.1 %±5.2% | 12.8%±2.3% | 18.5%±2.0% |
| Wake (n=5) | N.A. | N.A. | 59.9%±11.9% | 27.0±7.2% | 12.8%±5.0% | 0.3%±0.3% | 0.0%±0.0% | 0.0%±0.0% |
|
| ||||||||
| ANOVA | N.A. | F1,8=5.72 | F2,12= 20.76 | F2,12= 8.08 | F2,12= 4.43 | F2,12= 90.53 | F2,12= 22.33 | F2,12= 29.91 |
| P<0.05 | P<0.001 | P<0.01 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | ||
Data expressed as mean ± SEM of each state expressed as a percentage of recording time. All variables in the table showed significant differences among groups in one-way ANOVA tests at p=0.05 level.
3.2 Injection sites
All rats received 10 nL of retrograde tracer Fluoro-gold (4% in dH2O) via pressure-injection into vlPAG/DpMe area. Only animals that had tracer injections centered in the target area were included in the analyses, though a few of them had some contamination of the tracer at the beginning of the needle tracts over the superior colliculus, or spread of tracer dorsally into lateral or dorsolateral PAG. The camera lucida drawings of the approximate borders of the individual injections are shown at three different rostral-caudal levels in Figure 3. In all of these cases, the vlPAG/DpMe target site received the majority of injected tracer.
Figure 3.
Reconstruction of Flurogold injection sites for all animals in the three experimental groups. The extent of tracer diffusion is indicated by the black outlines for each injection site. All injections were centered at vlPAG and the adjacent DpMe. Numbers indicate distance in mm of each plane of section from bregma (Paxinos and Watson, 1998). CnF= cuneiform nucleus, DR= dorsal raphe nucleus, dmPAG=dorsomedial periaqueductal gray, DpMe= deep mesencephalic nucleus, IC= inferior colliculus, LL= lateral lemniscus, lPAG= lateral periaqueductal gray, MnR= median raphe nucleus, Pa4= paratrochlear nucleus, Pn= pontine nuclei, PnO= pontine reticular nucleus, oral part, PPTg= pedunculopontine tegmental nucleus, py= pyramidal tract, RRF= retrorubral field, SC= superior colliculus, scp= superior cerebellar peduncle, VLTg= ventrolateral tegmental area, VTA= ventral tegmental area.
3.3 Retrogradely labeled neurons
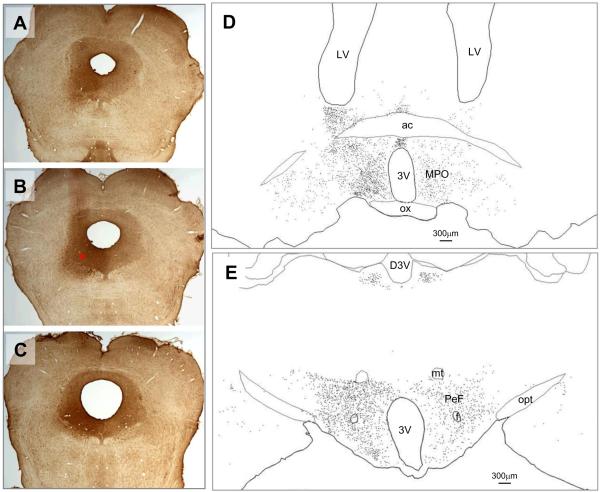
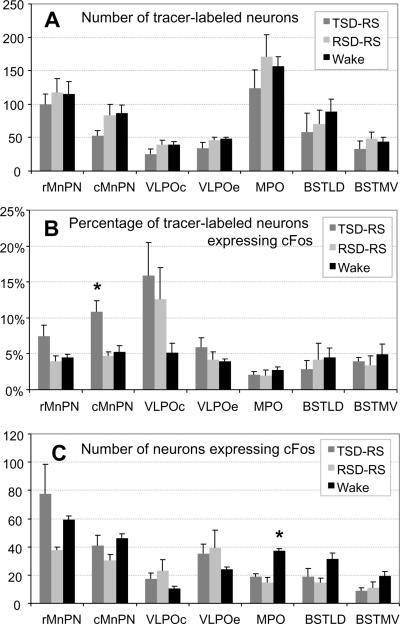
Neurons retrogradely labeled for FG were observed in various structures in the preoptic hypothalamus, mainly in the hemisphere ipsilateral to the injection site, with minor distribution to the contralateral side. The highest numbers of FG-labeled neurons were located in the medial preoptic area (MPO). High numbers of FG-labeled cells were also observed in median preoptic nucleus (MnPN), ventrolateral preoptic area (VLPO), bed nucleus of stria terminalis (BST), lateral preoptic area (LPO, lateral to MPO), and anterior hypothalamic area (AHA, caudal to MPO, not shown in figure). Representative distribution of FG-labeled neurons in the preoptic area for one rat is shown in a camera lucida drawing in Figure 4. Quantification of the distribution of c-Fos positive neurons co-localized with FG was carried out in the MnPN, VLPO, MPO and BST in the preoptic hypothalamus. Figure 5 (A–C) shows the position and dimension of counting boxes that were used to quantify dual- and single labeled neurons in these areas, including rostral and caudal MnPN (rMnPN & cMnPN), core and extended VLPO areas (VLPOc, VLPOe1 and VLPOe2), MPO, dorsal part of lateral division of the BST (BSTLD), and ventral part of the medial division of BST (BSTMV). The MPO had the highest total number FG-labeled cells, followed by the rMnPN. However, after normalization by the size of the counting box, the density (cell count per 104 μm2 per section) of FG-positive cells differed only modestly among the areas studied, with highest density in the MPO, followed by the VLPOe2 and BSTLD (data not shown). There were no statistically significant differences in the number of FG-labeled neurons among 3 experimental groups in any of the preoptic regions examined (one-way ANOVA, F(2,12)=1.204, 1.573, 1.804, 0.859, 0.469, 0.644, p= 0.334, 0.247, 0.207, 0.448, 0.637, 0.542 for MnPN, VLPOc, VLPOe, MPO, BSTLD, BSTMV, respectively (Figure 6A).
Figure 4.
Example of the distribution of retrogradely-labeled neurons in the preoptic area (D) and lateral hypothalamus (E), resulting from the Flurogold injection into the vlPAG shown at three A–P levels in A, B and C. The red asterisk in panel B marks the center of injection. Abbreviations: 3V= third ventricle, ac= anterior commissure, D3V= dorsal third ventricle, f= fornix, mt= mammillothalamic tract, opt= optic tract, ox= optic chiasm, PEF= perifornical area.
Figure 6.
(A) Mean (±SEM) Flurogold+ cell counts in the preoptic counting grids for TSD-RS (n=5), RSD-RS (n=5) and Awake (n=5) rats. (B) Mean (±SEM) values of the percentage of Flurogold+ neurons that were also immunopositive for c-Fos protein. (C) Mean (±SEM) of total number of neurons expressing c-Fos. VLPOe indicates combined data for the two extended VLPO boxes (see Figure 5).
* indicates significantly different from other groups (one-way ANOVA at p=0.05 level).
3.4 c-Fos expression in projection neurons from VLPO
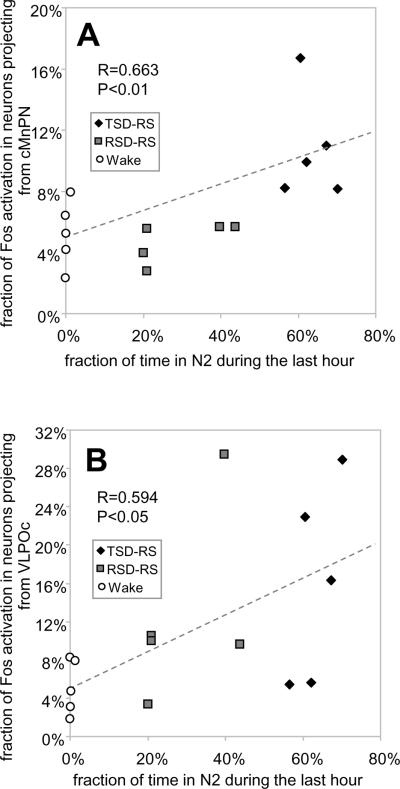
Examples of double labeling for c-Fos and FG are shown in Figure 5-D. Among the areas examined, the VLPOc showed the highest mean percentage of FG-labeled neurons that were immunopositive for c-Fos protein in the recovery sleep conditions, averaging 15.9%±4.6% (mean ±SEM) for TSD-RS and 12.6%±4.4% for RSD-RS (Figure 6B). This percentage was lower in the awake group; 5.2%±1.3%. However, ANOVA indicated no significant effect of experimental condition on Fos expression in VLPOc projection neurons (F(2,12) = 2.10; p=0.17). Nevertheless, there was a significant positive correlation between the percentage of FG labeled neurons immunopositive for Fos in VLPOc and the time spent in N2 during the last hour prior to sacrifice across all groups; r= 0.594, p<0.05, n=15 (Figure 7B). The percentage of FG-labeled neurons that were Fos-positive in VLPO extended areas (dorsal and medial extended divisions combined) also showed a positive correlation with time spent in N2 during the recovery sleep period (r=0.524, p<0.05, n=15). Neither subdivision of VLPO showed significant correlations with REM sleep time (VLPOc, r=0.266; p=0.339 and VLPOe, r=−0.11, p=0.695) or REM+Tran time (VLPOc, r=0.286; p=0.301 and VLPOe, r=−0.044, p=0.875) during the recovery period.
Figure 7.
Scatter plots of the % time spent in deep nonREM sleep (N2) during the 60 min prior to sacrifice versus % of double labeled neurons in the caudal MnPN (A) and the VLPO core (B) for animals in all three experimental groups. Also shown are regression lines and correlation coefficients.
3.5 c-Fos expression in the projection neurons from MnPN
The TSD-RS group showed significantly higher percentage of FG-labeled neurons expressing c-Fos in the caudal MnPN (10.8%±1.6%, mean ±SEM) compared to that in the RSDRS group (4.7%±0.6%) and the awake group (5.2%±1.0%); F(2,12) = 9.197; p<0.01. The same trend was seen for the rostral MnPN, but the difference did not reach statistical significance (Figure 6B). Averaging rostral and caudal MnPN counts, the TSD-RS group exhibited Fos expression in 8.6% ±1.6% of FG-labeled neurons, compared to 4.3% ±0.6 % in RSD-RS rats, and 4.9% ±0.6% in the awake group (ANOVA, F(2,12)=4.674, p<0.05). Significant correlations between the percentage of double-labeled cell counts and time spent in N2 sleep during the hour prior to sacrifice were found in the rostral MnPN (r=0.535, p<0.05) and in the caudal MnPN (r=0.663, p<0.01; Figure 7A). . There were no significant correlations between Fos expression in MnPN FG-labeled neurons and time spent in REM sleep (r=−0.021; p=0.94) or REM+Tran (r=−0.048; p=0.865).
3.6 c-Fos expression in the projection neurons from MPO
The percentage of c-Fos activation in the projection neurons from MPO to vlPAG was similar across TSD-RS, RSD-RS, and awake conditions (Figure 6B). There were no significant correlations between Fos expression in MPO projection neurons and time spent in N2 (r= −0.181, p=0.51), REM sleep (r=−0.407, p=0.132) or REM+Tran (r=−0.332, p=0.227) in the last hour prior to sacrifice.
3.7 c-Fos expression in the projection neurons from BST
There were no significant differences detected in the c-Fos expression in vlPAG projection neurons located in the BST across the 3 experimental conditions. Correlation coefficients between Fos expression in BSTLD and BSTMV projection neurons and time spent in N2 (BSTLD r=−0.129, p=0.648; BSTMV r=−0.229, p=0.411), REM sleep (BSTLD r=−0.166, p=0.55; BSTMV r= −0.081, p=0.77) or REM+Tran (BSTLD r=−0.059, p=0.835; BSTMV r= −0.179, p=0.523) were not significant.
3.8 c-Fos expression in projection neurons from the LH
Results of FG+ and Fos+ cell counts in the LH are shown in Figure 8. LH cell counts were performed in 3 of 5 rats in the TSD-RS and RSD-RS groups and in all 5 of the rats in the waking conditions. Projection neurons from the LH were not included in the analysis at the beginning of the experiments, and sections through the LH were not collected in 2 of 5 rats in the TSD-RS and RSD-RS groups. Figure 8B shows the total number of retrogradely labeled neurons in the LH across experimental groups. There was a significant difference detected in total FG+ cell counts in the Box-1 between TSD-RS and awake groups (ANOVA, F(2,8)=4.592, p<0.05, Post Hoc with Bonferroni correction, p<0.05). Analysis of percentage of retrogradely labeled cells expressing c-Fos demonstrated a consistent pattern in all LH grids, with the highest percentage of FG+ cells expressing c-Fos seen in awake rats, and the lowest percentage observed in RSD-RS rats (Figure 8C). This same pattern was observed across experimental groups for total Fos+ cell counts (Figure 8D). There were significant negative correlations with the percentage of FG+ neurons dual labeled for Fos with percent time spent in N2 and REM+Tran (Figure 8G) and a positive correlation for time spent awake (Figure 8E). For Wake, r=0.825, p<0.01; for NREM, r=−0.764, p<0.01; for REM, r=−0.802, p<0.01; for REM+Tran, r=−0.838, p<0.001; for N2, r=−0.603, p<0.05. Correlation coefficients were calculated for combined cell counts from the 3 grids.
4. DISCUSSION
The present study documents the existence of sleep-active neurons in the preoptic hypothalamus that project to vlPAG/DpMe. MnPN-vlPAG projection neurons were found to exhibit more intense activation during expression of nonREM sleep following TSD, compared to expression of REM sleep following selective RSD. Activation of VLPO-vlPAG projection neurons also displayed a positive correlation with the amount of deep nonREM sleep achieved during the hour prior to sacrifice. These finding are in broad agreement with a previous study describing increased activation of MnPN- and VLPO-vlPAG projection neurons in spontaneously sleeping rats compared to awake rats (Uschakov et al., 2009). Levels of activity, as indicated by cFos immunostaining, in vlPAG projection neurons originating in the MPO and BST did not differ among sleep-wake conditions. The majority of neurons in the vlPAG are active during waking or during both waking and REM sleep, with diminished levels of activity during nonREM sleep (Thakkar et al., 2002; Thankachan et al., 2009). The majority of the sleep-active neurons located in the MnPN and VLPO are GABAergic (Gong et al., 2004). The results of the current study suggest that projections from sleep-active neurons in the MnPN and the VLPO to the vlPAG may contribute to the suppression of vlPAG neuronal activity during sleep.
In contrast to the preoptic hypothalamus, maximum levels of c-Fos expression in LH-vlPAG projection neurons occurred in rats that were awake. This is consistent with findings that the perifornical LH contains populations of wake-active or wake-REM active neurons (Alam et al., 2002; Estabrooke et al, 2001). The lowest level of activity in LH-vlPAG projection neurons occurred in RSD-RS rats. Total Fos+ cell counts in the LH were also lowest in RSD-RS rats. Subsets of wake-active neurons in the perifornical LH exhibit minimal activity during REM sleep (Alam et al., 2002). This discharge pattern is characteristic of identified hypocretin neurons in the LH (Mileykovskiy et al., 2005; Lee et al., 2005). Hypocretin neurons project to the vlPAG (Peyron et al., 1998; Kaur et al., 2009), and vlPAG neurons express hypocretin receptors (Kaur et al., 2009). Lesion and stimulation studies indicate that GABAergic neurons in the vlPAG suppress REM sleep through inhibitory connections with REM sleep generating neurons in the pontine reticular formation (Luppi et al., 2006; Lu et al., 2006). Selective lesions of vlPAG neurons that express hypocretin receptors increase REM sleep amount (Kaur et al., 2009). The findings reported here support the hypothesis that suppression of activity in vlPAG-projecting hypocretin and other wake-active neurons in the LH during sleep, exerts permissive effects for REM sleep generation due to disfacilitation of vlPAG GABAergic neurons (Kaur et al., 2009).
4.1 Technical Limitations
FG has been widely used in neural tract tracing since its introduction over two decades ago (Schmued and Fallon, 1986). It has been shown that FG uptake does not alter the cell survival (Emsley et al., 2001; McClellan et al., 2006). However, it has been reported that FG can reduce c-Fos+ cell counts in populations of retrogradely labeled neurons compared to unlabeled neurons in the equivalent area contralateral to the tracer injection (Franklin and Druhan, 2000). Hence, the actual percentage of dual FG-cFos positive neurons across all experimental groups and in all brain nuclei examined may be higher than the values reported here.
Because we performed relatively short periods of TSD and RSD, it is possible that neurons responsive to more chronic levels of sleep loss were not detected in the study. We have previously documented that 2–3 hours of TSD and RSD is sufficient to alter c-Fos expression in GABAergic neurons in the MnPN and VLPO (Gvilia et al., 2006a, 2006b). In the LH, melanin concentrating hormone (MCH)-containing neurons have been implicated in REM sleep regulation (Peyron et al., 2009), and unit recording studies demonstrate that MCH neurons are maximally active during REM sleep (Hassani et al., 2009). MCH neurons do not express c-Fos during spontaneous sleep or in response to short-term sleep deprivation (Alam et al., 2005, Peyron et al., 2009). However after highly REM sleep enriched recovery sleep that occurs following 72 hours of RSD, c-Fos expression in MCH neurons is high (Verret et al., 2003; Peyron et al., 2009). Thus, a potential REM-active component of the LH-vlPAG projection originating in MCH neurons was probably not detected in this study.
4.2 Sleep propensity versus sleep expression
We carried out 3-hour TSD and RSD procedures in separate groups of animals and created conditions of elevated homeostatic need for nonREM sleep and REM sleep, respectively, in these groups. The one hour of recovery period clearly demonstrated differential sleep propensity for these states in the composition of rebound sleep (Figure 2 and Table 1). The time for sleep-related changes in c-Fos expression has been previously estimated to be 60 to 70 minutes (Shiromani et al., 1995). Other estimates of the timing of peak c-Fos activation in response to stimuli range from 30 min (Giovannelli et al., 1992; Imaki et al., 1992) to 120 min (Miyata et al., 1995; Sim and Morris, 1995). This timing can vary depending on neuronal phenotype (Sim and Morris, 1995). Hence, c-Fos expression quantified in this study may reflect neuronal activity occurring during the latter part of the deprivation period as well as during recovery sleep. It is not possible to distinguish among the effects of sleep pressure accumulating during deprivation versus expression of sleep states during recovery sleep in this study. We have previously shown that MnPN GABAergic neurons can be activated following TSD performed early in the light phase in the absence of recovery sleep, whereas the expression of recovery sleep following TSD is required for increased c-Fos expression in in VLPO GABAergic neurons (Gvilia et al., 2006b).
4.3 Extended ventrolateral preoptic area
It has been reported that lesions of the VLPO core cause primary suppression of nonREM sleep and loss of neurons in the extended VLPO results in comparatively larger deficits in REM sleep, implicating this portion of the VLPO in REM sleep regulation (Lu et al., 2000). Although significant numbers of retrogradely-labeled neurons were seen in the extended VLPO in the present study, the number of double-labeled neurons did not differ between TSD-RS and RSD-RS groups. As in the VLPO core, the percentage of FG-labeled neurons that were immuno-positive for c-Fos in extended VLPO was positively correlated with time spent in deep nonREM sleep, but not significantly correlated with time spent in REM sleep. The failure to see a relationship between activity of extended VLPO neurons and REM sleep may indicate that extended VLPO neurons that project to the vlPAG are not involved in REM sleep control. We did find significant positive correlations between total c-Fos+ cell counts and REM sleep time for both the extended VLPO (r=0.55, p<.05, n=15) and the VLPO core (r=0.60, p<.05n n=15). This is in agreement with a previous study in which RSD was found to have similar effects on c-Fos expression in VLPO core and extended VLPO neurons (Gvilia et al., 2006b).
4.4 Medial preoptic area
The MPO is involved in various components of reproductive physiology and behavior, including copulation, maternal behavior, and release of gonadotropins and prolactin (Simerly and Swanson, 1988). This nucleus is also critical for thermoregulation and body fluid balance (Boulant and Dean, 1986; Simerly and Swanson, 1988). The role of the MPO in sleep regulation is less well-defined. Both sleep-active and wake-active neurons have been identified in this region (McGinty and Szymusiak, 1988), and c-Fos expression in MPO has been reported to be increased during forced wakefulness (Pompeiano et al., 1994; Cirelli et al., 1995). Lesion studies point to a role in sleep regulation; MPO lesions result in loss of both REM and NREM sleep (Asala et al., 1990; Kumar et al., 1993; Schmidt et al., 2000; Vetrivelan et al., 2006; John and Kumar, 1998). Local warming of the MPO is sleep promoting (McGinty et al., 2001). Sleep in mammals is associated with changes in metabolism, activity of thermoregulatory effectors and a fall in body temperature (Szymusiak, 2009). Subsets of warm-sensing neurons in the MPO are also activated during spontaneous sleep and such neurons may function to orchestrate thermoregulatory changes during sleep (Alam et al., 1995b, a). In the present study, the single c-Fos cell counts in the MPO, were higher in the awake group than in the TSD-RS and RSD-RS rats (F(2,12)=22.21, p<0.001; Figure 6C). However, c-Fos staining in MPO-vlPAG projection neurons did not vary across sleeping and awake animals. This finding may reflect the functional heterogeneity of the MPO and of the MPO-vlPAG projection. Sleep-active cell types in the MPO do not appear to be anatomically segregated from wake active neurons as they are in the VLPO and MnPN (Alam et al., 1995a; Szymusiak et al., 1998; Suntsova et al., 2002). Determination that the MPO-vlPAG projection has a sleep active component would require an independent marker (e.g., neurotransmitter phenotype) of sleep-active neurons in the MPO.
In conclusion, these results suggest that neurons projecting from MnPN and VLPO to the vlPAG are activated during nonREM sleep and support the hypothesis that preoptic neurons provide inhibitory input to vlPAG during sleep. Suppression of excitatory input to the vlPAG from the LH during sleep may have a permissive effect on REM sleep generation.
Highlights
Preoptic hypothalamic neurons projecting to the periaqueductal gray are active during sleep.
Lateral hypothalamic neurons projecting to the periaqueductal gray are active during waking.
Hypothalamic inputs modulate sleep/wake-related activity of periaqueductal gray neurons.
Acknowledgements
Supported by the Department of Veterans Affairs and MH63323. The authors thank Ms. Keng-Tee Chow and Mr. Bryan Angara for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol. 1995a;269:R1240–1249. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: thermosensitivity in rapid eye movement sleep. Am J Physiol. 1995b;269:R1250–1257. doi: 10.1152/ajpregu.1995.269.5.R1250. [DOI] [PubMed] [Google Scholar]
- Asala SA, Okano Y, Honda K, Inoue S. Effects of medial preoptic area lesions on sleep and wakefulness in unrestrained rats. Neurosci Lett. 1990;114:300–304. doi: 10.1016/0304-3940(90)90580-3. [DOI] [PubMed] [Google Scholar]
- Benington JH, Kodali SK, Heller HC. Scoring transitions to REM sleep in rats based on the EEG phenomena of pre-REM sleep: an improved analysis of sleep structure. Sleep. 1994;17:28–36. doi: 10.1093/sleep/17.1.28. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Bonnavion P, de Lecea L. Hypocretins in the control of sleep and wakefulness. Curr Neurol Neurosci Rep. 2010;10:174–179. doi: 10.1007/s11910-010-0101-y. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Sleep deprivation and c-fos expression in the rat brain. J Sleep Res. 1995;4:92–106. doi: 10.1111/j.1365-2869.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Lu X, Hagg T. Retrograde tracing techniques influence reported death rates of adult rat nigrostriatal neurons. Exp Neurol. 2001;168:425–433. doi: 10.1006/exnr.2000.7625. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–62. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. The retrograde tracer fluoro-gold interferes with the expression of fos-related antigens. J Neurosci Methods. 2000;98:1–8. doi: 10.1016/s0165-0270(00)00168-0. [DOI] [PubMed] [Google Scholar]
- Giovannelli L, Shiromani PJ, Jirikowski GF, Bloom FE. Expression of c-fos protein by immunohistochemically identified oxytocin neurons in the rat hypothalamus upon osmotic stimulation. Brain Res. 1992;588:41–48. doi: 10.1016/0006-8993(92)91342-c. [DOI] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2079–2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci. 2006a;26:3037–3044. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006b;26:9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Hotta M, Demura H. Early induction of c-fos precedes increased expression of corticotropin-releasing factor messenger ribonucleic acid in the paraventricular nucleus after immobilization stress. Endocrinology. 1992;131:240–246. doi: 10.1210/endo.131.1.1612001. [DOI] [PubMed] [Google Scholar]
- John J, Kumar VM. Effect of NMDA lesion of the medial preoptic neurons on sleep and other functions. Sleep. 1998;21:587–598. doi: 10.1093/sleep/21.6.587. [DOI] [PubMed] [Google Scholar]
- Kaur S, Thankachan S, Begum S, Liu M, Blanco-Centurion C, Shiromani PJ. Hypocretin-2 saporin lesions of the ventrolateral periaquaductal gray (vlPAG) increase REM sleep in hypocretin knockout mice. PLoS One. 2009;4:e6346. doi: 10.1371/journal.pone.0006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Szymusiak R, Bashir T, Suntsova N, Rai S, McGinty D, Alam MN. Inactivation of median preoptic nucleus causes c-Fos expression in hypocretin- and serotonin-containing neurons in anesthetized rat. Brain Res. 2008;1234:66–77. doi: 10.1016/j.brainres.2008.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar VM, Sharma R, Wadhwa S, Manchanda SK. Sleep-inducing function of noradrenergic fibers in the medial preoptic area. Brain Res Bull. 1993;32:153–158. doi: 10.1016/0361-9230(93)90069-n. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical. REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100:271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Zhang L, Palmer R. Fluorogold labeling of descending brain neurons in larval lamprey does not cause cell death. Neurosci Lett. 2006;401:119–124. doi: 10.1016/j.neulet.2006.02.078. [DOI] [PubMed] [Google Scholar]
- McGinty D, Alam MN, Szymusiak R, Nakao M, Yamamoto M. Hypothalamic sleep-promoting mechanisms: coupling to thermoregulation. Arch Ital Biol. 2001;139:63–75. [PubMed] [Google Scholar]
- McGinty D, Szymusiak R. Neuronal unit activity patterns in behaving animals: brainstem and limbic system. Annu Rev Psychol. 1988;39:135–168. doi: 10.1146/annurev.ps.39.020188.001031. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Itoh T, Lin SH, Ishiyama M, Nakashima T, Kiyohara T. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull. 1995;37:391–395. doi: 10.1016/0361-9230(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Rizvi TA, Ennis M, Shipley MT. The organization of preoptic-medullary circuits in the male rat: evidence for interconnectivity of neural structures involved in reproductive behavior, antinociception and cardiovascular regulation. Neuroscience. 1999;91:1103–1116. doi: 10.1016/s0306-4522(98)00677-0. [DOI] [PubMed] [Google Scholar]
- Ni HF, Zhang JX, Harper RM. Cardiovascular-related discharge of periaqueductal gray neurons during sleep-waking states. Brain Res. 1990a;532:242–248. doi: 10.1016/0006-8993(90)91766-a. [DOI] [PubMed] [Google Scholar]
- Ni HF, Zhang JX, Harper RM. Respiratory-related discharge of periaqueductal gray neurons during sleep-waking states. Brain Res. 1990b;511:319–325. doi: 10.1016/0006-8993(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Peyron C, Sapin E, Leger L, Luppi PH, Fort P. Role of the melanin-concentrating hormone neuropeptide in sleep regulation. Peptides. 2009;30:2052–2059. doi: 10.1016/j.peptides.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Cirelli C, Tononi G. Immediate-early genes in spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and protein. J Sleep Res. 1994;3:80–96. doi: 10.1111/j.1365-2869.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J Comp Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Murphy AZ, Ennis M, Behbehani MM, Shipley MT. Medial preoptic area afferents to periaqueductal gray medullo-output neurons: a combined Fos and tract tracing study. J Neurosci. 1996;16:333–344. doi: 10.1523/JNEUROSCI.16-01-00333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–161. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res. 1983;288:21–31. doi: 10.1016/0006-8993(83)90078-1. [DOI] [PubMed] [Google Scholar]
- Sastre JP, Buda C, Kitahama K, Jouvet M. Importance of the ventrolateral region of the periaqueductal gray and adjacent tegmentum in the control of paradoxical sleep as studied by muscimol microinjections in the cat. Neuroscience. 1996;74:415–426. doi: 10.1016/0306-4522(96)00190-x. [DOI] [PubMed] [Google Scholar]
- Sastre JP, Buda C, Lin JS, Jouvet M. Differential c-fos expression in the rhinencephalon and striatum after enhanced sleep-wake states in the cat. Eur J Neurosci. 2000;12:1397–1410. doi: 10.1046/j.1460-9568.2000.00006.x. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MH, Valatx JL, Sakai K, Fort P, Jouvet M. Role of the lateral preoptic area in sleep-related erectile mechanisms and sleep generation in the rat. J Neurosci. 2000;20:6640–6647. doi: 10.1523/JNEUROSCI.20-17-06640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Malik M, Winston S, McCarley RW. Time course of Fos-like immunoreactivity associated with cholinergically induced REM sleep. J Neurosci. 1995;15:3500–3508. doi: 10.1523/JNEUROSCI.15-05-03500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Morris M. Fos activation in cultured tyrosine hydroxylase and oxytocin immunoreactive neurons. Brain Res Bull. 1995;36:399–404. doi: 10.1016/0361-9230(94)00220-u. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R. Thermoregulation during sleep and sleep deprivation. In: Stickgold R, Walker M, editors. The Neuroscience of Sleep. Academic Press; Burlington, MA: 2009. pp. 218–222. [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Phasic but not tonic REM-selective discharge of periaqueductal gray neurons in freely behaving animals: relevance to postulates of GABAergic inhibition of monoaminergic neurons. Brain Res. 2002;945:276–280. doi: 10.1016/s0006-8993(02)02914-1. [DOI] [PubMed] [Google Scholar]
- Thankachan S, Kaur S, Shiromani PJ. Activity of pontine neurons during sleep and cataplexy in hypocretin knock-out mice. J Neurosci. 2009;29:1580–1585. doi: 10.1523/JNEUROSCI.5151-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur J Neurosci. 2006;23:3284–3296. doi: 10.1111/j.1460-9568.2006.04860.x. [DOI] [PubMed] [Google Scholar]
- Uschakov A, McGinty D, Szymusiak R, McKinley MJ. Functional correlates of activity in neurons projecting from the lamina terminalis to the ventrolateral periaqueductal gray. Eur J Neurosci. 2009;30:2347–2355. doi: 10.1111/j.1460-9568.2009.07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Torterolo P, McGregor R, Chase MH, Morales FR. GABAergic processes in the mesencephalic tegmentum modulate the occurrence of active (rapid eye movement) sleep in guinea pigs. Neuroscience. 2007;145:1157–1167. doi: 10.1016/j.neuroscience.2006.12.051. [DOI] [PubMed] [Google Scholar]
- Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, Boissard R, Salin P, Peyron C, Luppi PH. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivelan R, Mallick HN, Kumar VM. Sleep induction and temperature lowering by medial preoptic alpha(1) adrenergic receptors. Physiol Behav. 2006;87:707–713. doi: 10.1016/j.physbeh.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494(5):845–61. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]