Abstract
The evolution of gastric carcinogenesis remains largely unknown. We established two gastric carcinogenesis models in New-World nonhuman primates. In the first model, ACP03 gastric cancer cell line was inoculated in 18 animals. In the second model, we treated 6 animals with N-methyl-nitrosourea (MNU). Animals with gastric cancer were also treated with Canova immunomodulator. Clinical, hematologic, and biochemical, including C-reactive protein, folic acid, and homocysteine, analyses were performed in this study. MYC expression and copy number was also evaluated. We observed that all animals inoculated with ACP03 developed gastric cancer on the 9th day though on the 14th day presented total tumor remission. In the second model, all animals developed pre-neoplastic lesions and five died of drug intoxication before the development of cancer. The last surviving MNU-treated animal developed intestinal-type gastric adenocarcinoma observed by endoscopy on the 940th day. The level of C-reactive protein level and homocysteine concentration increased while the level of folic acid decreased with the presence of tumors in ACP03-inoculated animals and MNU treatment. ACP03 inoculation also led to anemia and leukocytosis. The hematologic and biochemical results corroborate those observed in patients with gastric cancer, supporting that our in vivo models are potentially useful to study this neoplasia. In cell line inoculated animals, we detected MYC immunoreactivity, mRNA overexpression, and amplification, as previously observed in vitro. In MNU-treated animals, mRNA expression and MYC copy number increased during the sequential steps of intestinal-type gastric carcinogenesis and immunoreactivity was only observed in intestinal metaplasia and gastric cancer. Thus, MYC deregulation supports the gastric carcinogenesis process. Canova immunomodulator restored several hematologic measurements and therefore, can be applied during/after chemotherapy to increase the tolerability and duration of anticancer treatments.
Introduction
Gastric cancer is the fourth most frequent cancer type and the second highest cause of cancer mortality worldwide. Gastric cancer prevalence is influenced by geographic, ethnic, and cultural factors [1]. In addition, adenocarcinoma is the most common digestive tract neoplasia [2].
Nonhuman primates offer a useful model for carcinogenesis studies. Nonhuman primates present close phylogenic relationship to humans and greater similarities with regard to anatomy, physiology, biochemistry, and organ systems, as compared to rodents. They also present a relatively large organ size which enables repeated diagnostic procedures, such as endoscopic examination, blood sample collection and biopsy, on the same animal over a long period of time [3]. Although nonhuman primate models are not common and are expensive compared to rodent models, the long life span observed in nonhuman primates allows for long-term carcinogenic studies.
Chemical carcinogens cause genetic and epigenetic changes that lead to neoplastic transformation. N-methyl-nitrosourea (MNU) is a well-known direct carcinogen, which does not need metabolic activation to exert carcinogenicity. MNU leads to the production of O6-methylguanine adducts, resulting in premutagenic lesions and DNA strand breaks. MNU is a nitrosation product of creatinine metabolism that is formed in the presence of nitrites in the acidic gastric environment. MNU production is associated with the ingestion of meat products, cured meats, and seafood [4]. Moreover, it is possible that many species, including humans, are exposed to carcinogenic MNU, generated in their alimentary tract [5]. Thus, tumorigenesis induced by MNU is an interesting model to study gastric cancer.
Canova may be a potential anticancer treatment in patients with gastric carcinoma. It is a complex homeopathic immunomodulator indicated for patients whose immune system is depressed. Canova activates macrophages both in vivo and in vitro and indirectly induces lymphocyte proliferation [6]. Since innate and adaptive immune responses play a role in tumor surveillance and clearance [7], enhancing the ability to trigger a specific immunologic response against malignant cells is an important anticancer approach.
In the present study, we aimed to establish a gastric carcinogenesis model in Cebus apella, a nonhuman primate. We induced stomach tumors by gastric cancer cell line inoculation as well as MNU treatment for the duration of approximately 2.5 years. We evaluated body weight, serum biochemistry values and hematological parameters, as well as MYC proto-oncogene expression and copy number, in these in vivo models. In these models, we also assessed if Canova immunomodulator through the enhancement of immunity can contribute to a reduction in adverse effects of anticancer treatment.
Methods
2.1 Nonhuman Primates
36 adult Cebus apella (6–7 years old) were evaluated (2.7–3.6 kg). Animals were identified with microchips and were individually housed in Centro Nacional de Primatas, Pará State, Brazil. The animals were fed a healthy balanced diet not enriched with sodium chloride and were weighed daily. In this study, the details of animal welfare and steps taken to ameliorate suffering were in accordance with the recommendations of the Weatherall report, “The use of non-human primates in research”. This study was approved by the Ethics Committee of Universidade Federal do Pará (PARECER MED002-10).
According to a basic veterinary examination, all animals were considered healthy at the time of first blood sampling, endoscopy, and ultrasound. This was confirmed by the animals' behavior as judged by the veterinary check.
2.2 Experimental Design
36 animals were randomly separated in six groups and included in 2 studied models:
1o model: cell line inoculation
Negative Control (NC): 6 control C. apellas that received saline solution injections instead of Canova or cell line inoculation.
-
-
Canova group (CA): 6 C. apellas treated with 7 µl/g of Canova during 14 days. These animals did not receive cell line inoculation.
-
-
Cell line group (CL): 6 C. apellas inoculated with gastric cancer cell line and that received saline solution injections instead of Canova
-
-
Cell line plus Canova during 10 days (CLCA1): 6 C. apellas inoculated with gastric cancer cell line and after 5 days were treated with 7 µl/g of Canova during 10 days.
-
-
Cell line plus Canova during 14 days (CLCA2): 6 C. apellas that received gastric cancer cell line inoculation and 7 µl/g of Canova during 14 days (since day 0).
2o model: MNU treatment
6 C. apellas treated with MNU. After tumorigenesis, one animal received Canova treatment (MNU group).
2.3 Cell line inoculation
One week before the cell line inoculation, the C. apellas of CL, CLCA1 and CLCA2 groups were immunosuppressed by a single dose of 50 mg/kg of cyclophosphamide.
Four gastric cancer cell lines were tested: ACP02, ACP03, AGP01 and PG100. The first 3 cell lines were established by our research group from tumor samples of individuals from Northern Brazil [8]. The PG100 a cell line established from a primary gastric adenocarcinoma was obtained from Rio de Janeiro Cell Bank, Brazil (BCRJ). Only the ACP03 cell line, that was establish from an intestinal-type gastric cancer, was inoculated in the animals included in the present study, since it was the only one that was able to start a tumorigenesis process in C. apella.
One week after immunosupression, animals of CL, CLCA1 and CLCA2 groups received percutaneous inoculation of 1010 cells of ACP03 at the 85th passage between the mucosal and submucosal layers of antral stomach region. Ultrasonography was used to visualize the stomach tissues during cell line inoculation.
2.4 Canova treatment
Canova is standardized and authorized by competent agencies for medicinal application. Experiments were performed with commercial Canova donated by ‘Canova do Brasil’, a Brazilian company, which holds the international patent of this medicine (www.canovadobrasil.com.br).
Animals of CA, CLCA1 and CLCA2 groups, as well as one animal of MNU group, were treated with Canova. These animals received 7 µl/g of Canova daily. Canova concentration was determined according the study of Sato et al. in mice model [9]. Dose distributions were calculated at the time of treatment. Canova solution was succussed before treatment and injected by slow infusion in the right femoral vein of C. apella in a single dose.
2.5 MNU treatment
The animals of the second model received oral fresh doses of MNU (N1517 Sigma-Aldrich, USA) daily for 940 days at a dosage of 16 mg/kg body weight. The animals also received drink water containing MNU in light-shielded bottles daily. Water was restricted during MNU treatment and given ad libitum during Canova treatment.
2.6 Animal evaluation
Blood samples of all animals were collected for the determination of hematimetric and leukocytic parameters, evaluation of hepatic and renal functions, and serum measurement of C-reactive protein (CRP), folic acid, and homocysteine on day 0 (baseline). During the treatment periods, the animals were inspected daily and their clinical symptoms were recorded. Body weight was determined and peripheral blood from the left femoral vein was collected for all serum analyses.
Chemistry analysis included testing of levels of glucose, urea nitrogen, creatinine, total protein, albumin, globulin, total bilirubin, cholesterol, triglyceride, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, lactate dehydrogenase, creatine kinase, amylase, calcium, inorganic phosphorus, sodium, potassium, and chloride. Clinical hematology included red blood cell count, hemoglobin, hematocrit, platelet count, white blood cell (WBC) and differential (segmented neutrophil, lymphocyte, monocyte, eosinophil and basophile) counts. Methods and reference values for male adult animal were previously described [10].
CRP was measured by turbidimetric assay as previously described by Price et al. [11]. Serum level of folic acid was measured using chemiluminescent microparticle immunoassay (CMIA) (Abbott System, USA). Serum homocysteine levels were measured with high pressure liquid chromatography (HPLC) (Betamed, Agilent 1100 series, Chromosystems Reagent Kit).
For the first studied model, our results focus mainly in the blood analyses of days 0 and 14. The analysis of folic acid and of homocysteine concentration was also presented on the 9th day. For the second carcinogenesis model, our results focus mainly in the blood analyses of the days 0, 90, 120, 300, 940 and 960.
2.7 Tissue samples and gastric mucosa examination
Biopsy samples of gastric normal and non-normal (e.g. non-atrophic and atrophic gastritis, metaplasia, neoplasia) gastric mucosa were collected by endoscopy. Lymphadenectomy was performed to collect axillary and inguinal lymphonode samples.
Gastric mucosa alterations and tumor growth was followed by endoscopy examination and ultrasonography. A pachymeter was used to measure the tumor biopsies.
Histologic analysis of gastric mucosa and axillary and inguinal lymphonode biopsies of Cebus apella were embedded into paraffin, cut in 5 µm sections, and stained by hematoxylin and eosin.
2.8 MYC expression and copy number analyses
The MYC proto-oncogene has been described as a key in the gastric carcinogenic process [12] and, thus, it was select to confirm the presence of a gastric carcinogenesis process. For the first studied model, gastric biopsies of tumorfactions observed on the 9th day after cell line inoculation were used to evaluate the MYC expression and copy number, because only fibrotic lesions were observed in the studied animals on the 14th day. For the second studied model, C. apella gastric samples at days 0, 90, 120, 300, 940 and 960 were used to evaluate the MYC expression and copy number.
Fluorescent in situ Hybridization (FISH) was performed to determine MYC gene copy number according to the protocol of Pinkel et al. [13] with modifications introduced from Calcagno et al. [14], [15]. Cells were hybridized with digoxigenin-labeled probe (ONPON0824, Bioagency Biotechnology, Brazil) for MYC gene region (8q24) and nuclei were counterstained with 4′,6-diamidino-2-phenylindole antifade. Positive MYC gene signals appeared as red spots in nuclei and were scored using the criteria of Hopman et al. [16].
Quantitative TaqMan Copy Number Variation (CNV) assays (Applied Biosystems, USA) using real-time quantitative PCR (RT-qPCR) were applied as a confirmation to FISH analysis. RT-qPCR was performed using the FAM/MGB-labeled TaqMan probe for MYC gene (Hs01764918_cn) and VIC/TAMRA-labeled TaqMan CNV RNAse P (#4403326) for the internal control. RT-qPCR reactions were performed in quadruplicate with genomic DNA (gDNA) according to the manufacturer's protocol (Applied Biosystems, USA). A known human gDNA (Promega, USA) was used for calibration.
MYC mRNA expression was evaluated RT-qPCR. First, complementary DNA was synthesized using High-Capacity cDNA Archive kit according to the manufacturer's protocol (Applied Biosystems, Poland). All RT-qPCR reactions were performed in triplicate for both target gene (MYC -Hs00153408_m1) and internal control (GAPDH - NM_002046.3). Relative quantification (RQ) of the gene expression was calculated according to Livak and Schmittgen [17]. In the present study, the NC group was designated as a calibrator of the first model where as the baseline values (from day 0) of the animals were used to calibrate the second study model.
Immunohistochemical analyses for MYC protein were performed on formalin-fixed, paraffin-embedded sections. Immunohistochemical staining was performed on the paraffin sections according to Calcagno et al. [15] with primary mouse monoclonal antibody against MYC (dilution 1∶50; DBS, USA). Positive protein expression was defined as clear nuclear imunostaining in more than 10% of the cells.
2.9 Data Analysis
In the first model (cell line inoculation), we first evaluated the normal distribution of all data using the Shapiro-Wilk normality test to determine subsequent use of appropriate tests for statistical comparison. Data that were not normally distributed were transformed (z-score transformation) for analysis such that they followed a normal distribution. Analysis of variance in body weight, serum biochemistry values, hematological parameters, MYC expression, and copy number were performed by univariate General Linear Model (GLM) followed by Bonferroni post-hoc test. The effect size for GLM analyses was based on Eta Squared (η2), in which 0.15 and below was determined as a small effect size; 0.16–0.40, medium effect size; and above 0.40, large effect size. Chi-square test was performed to compare MYC immunostaining among groups. In these analyses, the confidence interval was 95% and p values less than 0.05 were considered significant.
In the second model (MNU treatment), only non-parametric tests to repeated measures were used due to the small number of samples. The Friedman test followed by Wilcoxon analysis with Bonferroni's adjustment were performed to analysis of variance in body weight, serum biochemistry values, hematological parameters, MYC expression, and copy number at days 0 (baseline), 90 and 120. In these statistical analyses, one animal was excluded due to its early death. In these analyses, p<0.016 was considered statistically significant. Also, due to small numbers of animals, the statistical data analysis concerning MNU treatment among days 300–940 and Canova treatment among days 940–960, was not possible and results are presented in a descriptive format.
Results
3.1 First model – cell line inoculation
Before this study, several methods of cell line inoculation were tested including intraperitoneal, subcutaneous, gavage, orthopic implantation and percutaneous (data not shown). The percutaneous inoculation was the only via that resulted in the development of a tumorigenic process. We also previously detected that 1010 cells was the cell number needed to increase the total tumorous percentage compared to small number of cells (108 e 109). However, the high number of cells did not increase the total tumorous percentage (data not shown).
In the first model, eighteen C. apella received percutaneous inoculation of 1010 of ACP03 cells. On the 9th day after cell line inoculation, these animals presented tumorfactions in the antral region of the stomach (Figure 1A). The tumor volumes of CL, CLCA1, and CLCA2 groups were similar and all animals were able to eliminate the tumors. On the 14th day, they presented only an inflammatory zone and fibrotic lesions in the region.
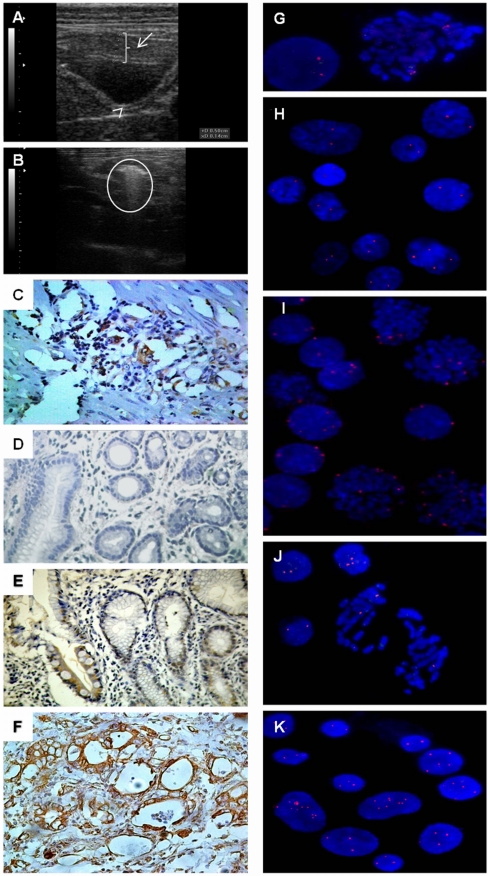
Figure 1. Ultrasonography, immunohistochemistry and FISH analysis in C. apella gastric carcinogenesis models.
A) ultrasound image showing a “space” between the stomach wall where ACP03 cell line was inoculated and developed a tumor (2.5 cm); B) ultrasound image showing a tumor mass in a MNU-treated animal on the 940th day (5 cm); C); MYC immunoreactivity in a tumor sample of a CLCA1 animal (400×); D); lack of MYC immunoreactivity in non-atrophic gastritis sample in a MNU-treated animal (400×); E) MYC immunoreactivity in intestinal metaplasia sample of a MNU-treated animal (400×); F) MYC immunoreactivity in a tumor sample of a MNU-treated animal (400×); G) lymphocytes of a healthy C. apella showing two signals for MYC probe (1000×); H) normal gastric mucosa cells of NC animal presenting two MYC signals (1000×); I) neoplastic gastric mucosa of CL animal showing MYC amplification (1000×); J) intestinal metaplasia sample of MNU-treated animal presenting 1, 2 and 3 MYC signals (1000×); K) tumor sample of MNU-treated animal showing MYC amplification (1000×). Arrow indicates the space with gastric cancer cell line; arrowhead indicates a normal gastric wall thickness; circle indicates the proliferative process.
Among the cell inoculation methods, we also evaluated the intraperitoneal inoculation. The intraperitoneal ACP03 inoculation induced lymphatic congestion in C. apella. After 48 h of cell line inoculation, the animals presented auxiliary and inguinal lymph node enlargement. Lymphadenectomy was performed and the histopathologic analysis showed only reactive lymphoid hyperplasia.
During this study, the animals in the NC group presented normal levels of the biochemical and hematologic evaluated parameters according to Riviello et al. study in C. apella [10]. To our knowledge, there are no CRP, homocysteine, and folic acid reference ranges determined for C. apella. However, the non-treated animals presented homocysteine and folic acid levels similar to those described for healthy Macaca fascicularis [18].
One week before the cell line inoculation, the C. apellas of CL, CLCA1 and CLCA2 were immunosuppressed by a single dose of 50 mg/kg of cyclophosphamide. However, on the day of cell line inoculation, no significant difference was observed among these groups, nor between NC and CA groups, regarding the animal's baseline weight, biochemical, hematologic, folic acid, and homocysteine measurements.
Concerning the biochemical analysis, significant changes in triglycerides (F4,25 = 335.695, p<0.001, by GLM test; η2 = 0.982), urea nitrogen (F4,25 = 33.537, p<0.001; η2 = 0.843) and CRP (F4,25 = 20.135, p<0.001; η2 = 0.763) levels were observed among the studied groups on the 14th day (Figure 2A–C, Table S1). The Bonferroni post-hoc analyses demonstrated a significant increase of triglycerides, urea nitrogen, and CRP level in CL, CLCA1 and CLCA2 compared to NC and CA groups (p<0.001, for all pair wise comparisons). No significant difference in biochemical measurements was observed between NC and CA groups. The increase of triglyceride level in the cell-line inoculated animals was inside the normal reference level according to Riviello et al. [10]. On the other hand, abnormal levels of the other biochemical parameter were observed in the cell-line inoculated animals.
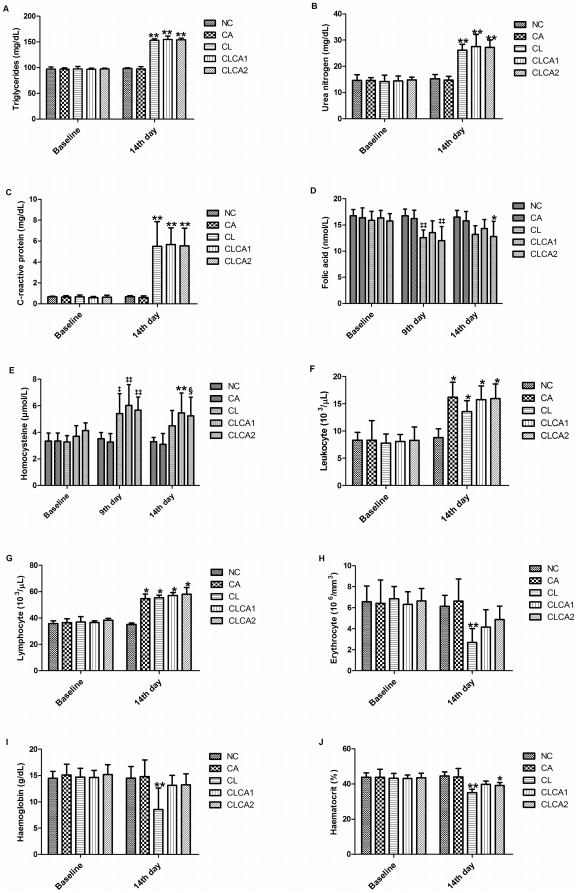
Figure 2. Abnormal biochemical and hematologic measurements in animals of the first carcinogenesis model.
A) triglycerides; B) urea nitrogen; C) C-reactive protein; D) leukocyte; E) lymphocyte; F) erythrocyte; G) haemoglobin; H) haematocrit; I) folic acid; J) homocysteine. NC: negative control; CA: Canova group; CL: animals inoculated with ACP03 cell line; CLCA1: animals inoculated with ACP03 cell line and treated with Canova during 10 days; CLCA2: animals inoculated with ACP03 cell line and treated with Canova during 14 days. N = 6/group. * Significantly different from NC group (p<0.05) on the 14th day. ** Significantly different from NC and CA groups (p<0.05) on the 14th day. § Significantly different from NC group (p<0.05) on the 14th day. ‡ Significantly different from CA group (p<0.05) on the 9th day. ‡‡ Significantly different from NC and CA groups (p<0.05) on the 9th day.
To our knowledge, no previous study reported the normal level of CRP in healthy C. apella. In the present study, we observed that the range of CRP levels were between 0.34–0.99 mg/dL (n = 36, on day 0). The serum CRP level increased 5.7–13.6 folds due to cell line inoculation.
We observed that the levels of folic acid changed significantly among the studied groups on the 9th (F4,25 = 7.446, p<0.001, by GLM test; η2 = 0.544) and on the 14th day (F4,25 = 4.056, p = 0.011; η2 = 0.394). Bonferroni post-hoc analyses demonstrated a significant reduction of folic acid in CL and CLCA2 group than NC (p = 0.008 and p = 0.003, respectively) and CA (p = 0.03 and p = 0.009, respectively) on the 9th day and in animals from CLCA2 group compared to NC group (p = 0.031) on the 14th day, which suggests an effect of cell line inoculation (Figure 2D, Table S1). In the present study, we observed that the range of folic acid level was 13.29–18.84 nmol/dL in healthy C. apella (n = 36, on day 0). However, on the 9th day, 4 animals of CL, 4 of CLCA2 and 1 of CLCA1 presented lower levels of folic acid (less than 13 nmol/L) as well as 3 animals of CL, 3 of CLCA2, and 1 of CLCA1 on the 14th day.
We observed that homocysteine levels changed significantly among the studied groups on the 9th day (F4,25 = 7.887, p<0.001, by GLM test; η2 = 0.558) and on the 14th day (F4,25 = 5.6, p = 0.002; η2 = 0.473). On the 9th day, CLCA1 and CLCA2 groups presented higher homocysteine levels than animals from NC (p = 0.007 and p = 0.028, respectively, by Bonferroni analyses) and CA (p = 0.003 and p = 0.011, respectively). The CL group also presented higher homocysteine levels than animals from CA group (p = 0.028). However, on the 14th day, only animals from CLCA1 group presented higher homocysteine levels than animals from NC (p = 0.026) and CLCA1 and CLCA2 groups presented higher levels than CA (p = 0.013 and p = 0.29, respectively) group, which suggests some effects of cell line inoculation and of Canova treatment (Figure 2E, Table S1). In the present study, we observed that the range of homocysteine was between 2.5–5.21 µmol/L in healthy C. apella (n = 36, on day 0). In addition, we observed that 2 animals of CL, 4 of CLCA1 and 3 of CLCA2 presented homocysteine levels higher than 5.21 µmol/L on the 9th and 14th days.
Concerning hematologic analyses, we observed a significant alteration in leukocyte (F4,25 = 10.506, p<0.001, by GLM test; η2 = 0.627), lymphocyte (F4,25 = 55.213, p<0.001; η2 = 0.898), erythrocyte (F4,25 = 6.405, p = 0.001; η2 = 0.506), haemoglobin (F4,25 = 4.798, p = 0.005; η2 = 0.434), and haematocrit (F4,25 = 12.028, p<0.001; η2 = 0.658) counts among the studied groups on the 14th day (Figure 2F–J, Table S1). The Bonferroni post-hoc analyses demonstrated a significant increase of leukocyte and lymphocyte count in CA (p<0.001 and p<0.001, respectively), CL (p = 0.017 and p<0.001, respectively), CLCA1 (p<0.001 and p<0.001, respectively) and CLCA2 (p<0.001 and p<0.001, respectively) as compared to the NC group, which suggests that Canova and cell line inoculation affect leukocyte and lymphocyte levels. Although CA group presented abnormally high lymphocyte count according to Riviello et al. [10], these animals were clinically healthy.
We also detected that CL group presented a significant reduction of erythrocyte (p = 0.006 and p = 0.001, respectively by Bonferroni analyses) and haemoglobin count (p = 0.011 and p = 0.007, respectively) as compared to NC and CA groups, which suggests a cell line inoculation effect that is improved by Canova treatment. According to Riviello et al. [10], all animals of the CL group were anemic. We also observed a significant reduction of haematocrit count in CL and CLCA2 groups as compared to NC group (p<0.001 and p = 0.022, respectively). The CL group also presented reduced haematocrit count as compared to the CA group (p<0.001). According to Riviello et al. [10], 5 animals of the CL and 1 of the CLCA1 group presented abnormal haematocrit level. The hematocrit count difference among groups again suggests a cell line inoculation effect that is in part improved by Canova treatment.
3.2 Second model – MNU treatment
We treated six C. apella with MNU for a duration of approximately 2.5 years. All animals developed pre-neoplastic lesions and five died of drug intoxication before the development of gastric cancer. All animals presented non-atrophic gastritis on the 90th day. On the 110th day, one animal died from drug intoxication and the other five animas presented atrophic gastristis on the 120th day. On the 134th, 140th and 290th days, three more animals died due to drug intoxication. On the 300th day, the two surviving C. apella presented intestinal metaplasia in gastric mucosa. One animal died with symptoms of drug intoxication on the 520th day and the last surviving animal developed intestinal-type adenocarcinoma in the antral region of stomach. This tumor was observed by ultrasonography (Figure 1B) and endoscopy on the 940th day and this finding was confirmed by histopathologic analysis. None of the animals developed any other tumor types.
The deceased animals showed the typical symptoms of intoxication: mydriasis, confusion, sleepiness, giddiness, loss of balance, tremor, hyperthermia, low food consumption, nonspecific gastrointestinal symptoms (diarrhea and vomiting), urinary retention, cutaneous eruptions, and caustic and ulcerative oral lesions. They also presented renal, hepatic and respiratory failure, hypokalemia, chronic cholecystitis and steatosis.
In the second model of carcinogenesis, we observed that the MNU treatment led to significant changes in triglycerides (χ2 = 7.6, df = 2, p = 0.0224, by Friedman test), urea nitrogen (χ2 = 10, df = 2, p = 0.0067), phosphorus (χ2 = 10, df = 2, p = 0.0067), alanine aminotransferase (χ2 = 10, df = 2, p = 0.0067), total bilirubin (χ2 = 8.4, df = 2, p = 0.015), creatinine (χ2 = 8.4, df = 2, p = 0.015), CRP (χ2 = 8.4, df = 2, p = 0.015), folic acid (χ2 = 8.4, df = 2, p = 0.015) and homocysteine (χ2 = 10, df = 2, p = 0.0067) levels (Figure 3A–I, Table S2). However, no significant difference was confirmed by Wilcoxon test with Bonferroni correction. The largest fold chance was observed in the CRP levels. After 90 days of MNU treatment, the CRP levels increased 5.3–13 folds compared to baseline level. The folic acid concentration was reduced more than 2-fold change and the homocysteine concentration increased almost 5-fold on the 120th day as compared to baseline levels (Figure 3).
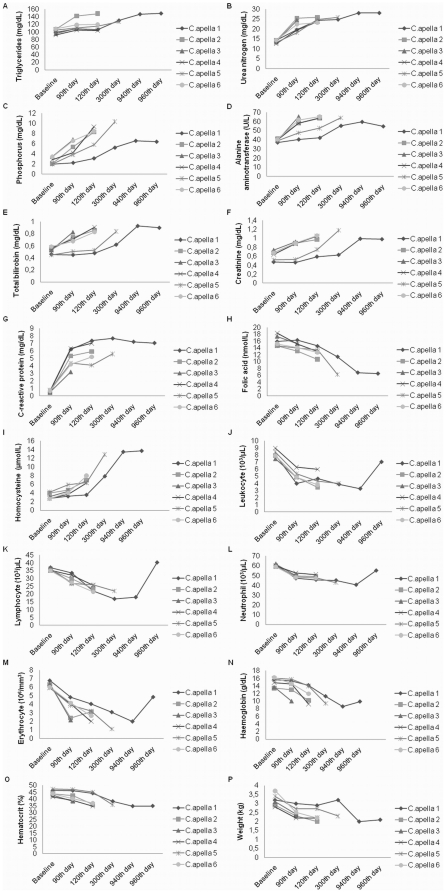
Figure 3. Abnormal biochemical and hematologic measurements in animals MNU-treated and Canova-treated.
A) triglycerides; B) urea nitrogen; C) phosphorus; D) alanine aminotransferase; E) total bilirubin; F) creatinine; G) C-reactive protein; H) leukocyte; I) lymphocyte; J) neutrophil; K) erythrocyte; L) haemoglobin; M) haematocrit; N) folic acid; O) homocysteine; P) weight. N = 6 on the 0–90th days (non-atrophic gastritis); N = 5 on the 120th day (atrophic gastritis); N = 2 on the 300th day (intestinal metaplasia); N = 1 on the 940th (gastric cancer development) and 960th (Canova treatment effect).
Concerning hematologic analyses, we observed a significant alteration of leukocyte (χ2 = 8.4, df = 2, p = 0.015), lymphocyte (χ2 = 10, df = 2, p = 0.0067), neutrophil (χ2 = 10, df = 2, p = 0.0067), erythrocyte (χ2 = 8.4, df = 2, p = 0.015), haemoglobin (χ2 = 10, df = 2, p = 0.0067) and haematocrit (χ2 = 10, df = 2, p = 0.0067) counts during MNU treatment (Figure 3J–O, Table S2). Although no significant difference was confirmed by Wilcoxon test with Bonferroni correction, all animals reached abnormal levels of these hematologic parameters following MNU treatment, consistent with Riviello et al. [10].
The analysis of Canova treatment was based on the observation of its effects in only one animal that developed gastric cancer. After 20 days of Canova treatment, the tumor volume did not change (about 1 cm3). In addition, no change (less than 1.2 fold-change) was observed between the 940th and 960th day concerning the biochemical, including folic acid and homocysteine, measurements in the surviving animal. However, we observed that Canova acted mainly on the hematologic measurements. The surviving animal presented more than 2-fold increase in leukocyte, lymphocyte, and erythrocyte counts after Canova treatment as well as a 1.4-fold increase in the neutrophil count. Canova treatment restored normal count of leukocyte, lymphocyte and neutrophil according Riviello et al. [10].
On the 960th day, the survinving C. apella was submitted for surgical removal of the tumor. This animal was clinically monitored for one year after the end of the experiment and he did not show any complications resulting from the treatments.
3.3 MYC copy number
The MYC probe for FISH analysis was first tested in the lymphocytes of a healthy C. apella. In C. apella (Figure 1G), the MYC probe had similar efficiency as that observed in our previous results with human cells [19].
Table 1 shows the mean and standard deviation of the MYC copy number by FISH and qRT-PCR of the groups included in the first carcinogenesis model. By FISH assay, the number of cells presenting 2 (F4,25 = 2578.912, p<0.001, by GLM test; η2 = 0.998), 3 (F4,25 = 150.51, p<0.001; η2 = 0.960), 4 (F4,25 = 590.872, p<0.001; η2 = 0.99) and 5 or more MYC signals (F4,25 = 117.013, p<0.001; η2 = 0.949) as well as high MYC amplification (F4,25 = 22.973, p<0.001; η2 = 0.786) was significantly different among the studied groups (Figure 1H–I). By qRT-PCR, we also observed that the number of MYC copies were significantly different among the studied groups (F4,25 = 95.986, p<0.001, by GLM test; η2 = 0.939). The Bonferroni post-hoc analyses of FISH results also showed that the number of cells presenting 3, 4, 5 or more MYC signals and high amplification was significantly higher in CL, CLCA1 and CLCA2 groups than in NC and CA groups (p<0.001, for all pair wise comparisons), confirming RT-qPCR results. These MYC signal number alterations were observed in the biopsies of all CL, CLCA1 and CLCA2 animals. No significant difference was observed between CL and CLCA groups, suggesting no Canova effect in the MYC copy number.
Table 1. Immunohistochemistry, relative quantitation of mRNA MYC expression and MYC gene copy number variation by Taqman and fluorescence in situ hybridization in tumor biopsies of animals included in the first carcinogenesis model on the 9th day of treatments.
| Group | IHQ | mRNA expression(mean±SD) | CNV(number [mean ± SD]) | Nuclei exhibiting MYC signals (mean±SD) | |||||
| 1 signal | 2 signals | 3 signals | 4 signals | ≥5 signals | HA | ||||
| NC | Negative | - | 2 (2.03±0.04) | 3.17±1.17 | 196.83±1.17 | - | - | - | - |
| CA | Negative | −0.51±0.22 | 2 (2.04±0.56) | 3.33±1.21 | 196.33±1.37 | 0.33±0.52 | - | - | - |
| CL | Positivea | 6.45±0.24b | 5 (4.65±0.43)a | 5.83±1.94 | 19.83±5.98a | 49.17±10.57a | 69.00±4.29a | 37.33±6.35a | 18.33±7.78a |
| CLCA1 | Positivea | 6.24±0.29b | 5 (4.6±0.34)a | 3.5±1.76 | 17.17±6.34a | 45.33±4.32a | 70.17±6.911a | 39.17±7.68a | 24.67±6.4a |
| CLCA2 | Positivea | 6.01±0.35b | 4 (4.24±0.52)a | 4.33±2.34 | 17.67±5.65a | 47.5±1.64a | 72.67±3.14a | 38.83±3.82a | 19±8.69a |
IHC: immunohistochemistry; CNV: copy number variation; HA: high amplification; NC: negative control; CA: Canova group; CL: animals inoculated with ACP03 cell line; CLCA1: animals inoculated with ACP03 cell line and treated with Canova during 5 days; CLCA2: animals inoculated with ACP03 cell line and treated with Canova during 9 days.
Significantly different from NC and CA groups (p<0.05).
Significantly different from CA group (p<0.05).
Table 2 shows the median and interquartile range of MYC copy number by FISH and qRT-PCR in samples from animals treated with MNU (second model of carcinogenesis). Using the FISH and RT-qPCR assays, no significant difference was observed among biopsies of day 0, 90 and 120 after Wilcoxon test with Bonferroni correction. On the 300th day, intestinal metaplasia was observed in the two surviving animals. They had approximately 30% of cells with 3 MYC signals and about 9.5% of cells with 4 MYC signals as determined by FISH and almost 3 copies by qRT-PCR (Figure 1J). The animal that survived the end of the MNU treatment showed a continuous increase in the number of cells with MYC amplification during gastric carcinogenesis. On the 940th day, the animal developed intestinal-type gastric cancer and had 46% of cells with 3 or more MYC copies, including 5% of cells with high amplification as determined by FISH and 3 MYC copies by qRT-PCR (Figure 1K). In the second carcinogenesis model, Canova treatment during 20 days did not appear to change the number of MYC copies.
Table 2. Immunohistochemistry, relative quantitation of mRNA MYC expression and MYC gene copy number variation by Taqman and fluorescence in situ hybridization in biopsies of MNU-treated animals.
| Treatment | IHQ | mRNA expression(median ± interquartile range) | CNV(number [median ± interquartile range]) | Nuclei exhibiting MYC signals (median ± interquartile range) | |||||
| 1 signal | 2 signals | 3 signals | 4 signals | ≥5 signals | HA | ||||
| Baseline | Negative | - | 2 (2.04±0.32) | 3±1.5 | 196±3 | 1±1 | - | - | - |
| MNU/90th day | Negative | 1.52±1.06 | 2 (1.87±0.55) | 3±1.5 | 194±3 | 2.5±1.75 | - | - | - |
| MNU/120th daya | Negative | 2.24±1.73 | 2 (1.92±0.74) | 4±0.5 | 188±5 | 5±4 | 2±2 | - | - |
| MNU/300th dayb | Positive | 3.35±0.09 | 3 (2.85±0.11) | 3.5±0.5 | 115.5±0.5 | 59.5±1.5 | 19±3 | 2.5±2.5 | - |
| MNU/940th dayc | Positive | 4.75 | 3 (3.12) | 3 | 105 | 61 | 16 | 5 | 10 |
| Canova/960th dayc | Positive | 5.04 | 3 (3.04) | 4 | 109 | 52 | 18 | 9 | 8 |
IHC: immunohistochemistry; CNV: copy number variation; HA: high amplification.
Five animals;
Two animals;
One animal.
3.4 MYC expression
Table 1 shows the mean and standard deviation of MYC mRNA expression and its immunoreactivity in biopsy samples of animals of the first carcinogenesis model. All normal gastric samples (NC and CA groups) showed standard staining for MYC protein expression. We also observed an association between MYC immunoreactivity and cell line inoculation (χ2 = 12, df = 1, p = 0.001). All the tumor biopsies of animals inoculated with ACP03 (CL, CLCA1 and CLCA2 groups) presented MYC protein overexpression (Figure 1C). We did not observe an increase in MYC mRNA expression in gastric mucosa of CA group compared to NC group. The MYC expression differed among CA, CL, CLCA1 and CLCA2 groups (F3,20 = 885.646, p<0.001, by GLM test; η2 = 0.993). CL, CLCA1 and CLCA2 groups presented a higher MYC expression than the CA group (p<0.001). A six-fold increase in mRNA expression was observed in tumor samples of CL, CLCA1 and CLCA2 groups relative to the NC group. No difference in MYC expression was observed among CL, CLCA1 and CLCA2, which suggests Canova does not effect MYC expression.
Table 2 shows the median and interquartile range of MYC mRNA expression and its immunoreactivity in samples from animals treated with MNU (second model of carcinogenesis). By qRT-PCR, we observed a continuous increase in MYC expression during MNU-induced gastric carcinogenesis. Although negative MYC immunoreactivity was observed (Figure 1D), the mRNA expression was about 2-fold higher on 120th day (atrophic gastritis) compared to baseline. On the 300th day, we observed an about 3-fold increase of MYC mRNA expression in the metaplasia lesions of the two surviving animals in this period relatively to their baseline level. On the 940th day, the surviving animal presented about 5-fold increase in MYC mRNA expression relative to its baseline level. MYC nuclear immunoreactivity was observed only in the intestinal metaplasia and gastric cancer biopsies (Figure 1E–F). No change in MYC expression was observed in the tumor biopsy of this animal after 20 days of Canova treatment.
Discussion
4.1 C. apella gastric carcinogenesis
Spontaneous tumors have been reported in nonhuman primates, usually due to the aging process [3]. Nonhuman primates offer a useful model for cancer research and other basic research into genetic and immunopathogenesis mechanisms as well as for the development and validation of new therapies for several diseases. Cebus apella, a New World monkey, is a convenient model for biomedical studies because they can be easily housed in Primate Research Centers due to their flexibility, opportunism, adaptability, and small size. To our knowledge, this is the first study that established a gastric carcinogenesis model in Cebus apella.
Gastric adenocarcinoma is divided mainly into intestinal and diffuse types according to Laurén classification [20]. The intestinal-type gastric cancer progresses through a number of sequential steps beginning with atrophic gastritis followed by intestinal metaplasia, intraepithelial neoplasia, and carcinoma [21]. On the other hand, diffuse-type gastric cancer generally does not evolve from precancerous lesions [2]. Here, we induced intestinal-type gastric adenocarcinoma in C. apella by treatment with MNU carcinogen and by human cancer cell line (ACP03) inoculation.
Cell lines derived from human cancers are useful to understand the chromosomal alterations and other molecular alterations in the carcinogenesis process. Cell lines are also an important tool for the study of anticancer treatments in in vitro and in animal xenograft models. For the induction of gastric cancer by cell line inoculation in C. apella, we initially tested four different gastric cancer cell lines. ACP02, AGP01 and PG100 cell lines did not induce tumors in C. apella despite the method of inoculation. Only the ACP03 cell line was able to induce gastric cancer in the animals. The tumorigenic potential of a cell line is attributed to the presence of a subset of cells called cancer stem cells, which have capability to recapitulate the development of the original tumors in vivo. The study of human cancer stem cells largely relies on models of xenograft transplantation into immunodeficient mice [22]. Probably, only ACP03 cell line has cancer stem cell proprieties.
One week before ACP03 inoculation, animals of CL, CLCA1 and CLCA2 groups received a single dose of 50 mg/kg of cyclophosphamide. Our group previously observed that white blood cell count decrease after 4 days of cyclophosphamide treatment (unpublished observation). However, total tumor remission was observed after 14 days of ACP03 inoculation probably due to immune system activation. We did not give a second cyclophosphamide dose, because in a previous study we observed that it was lethal to 50% of C. apella (unpublished observation). Furthermore, the ACP03 cell line may have low tumorigenic potential and therefore, did not present the same proliferation and metastatic patterns of the original human tumor in these animals. This model can be applied to other gastric cancer cell lines, especially with high tumorigenic potential, in C. apellas after their immunosupression. This may be useful for the identification of hematological and biochemical markers that can help to guide immunotherapy cancer treatments.
The presence of gastric tumor due to ACP03 inoculation was confirmed by MYC deregulation. The MYC proto-oncogene has been described as a key in the gastric carcinogenic process [12]. Groups of genes involved in cell cycle regulation, metabolism, ribosome biogenesis, protein synthesis, and mitochondrial function are over-represented in the MYC target gene network. MYC also consistently represses genes involved in cell growth arrest and cell adhesion and also has a direct role in the control of DNA replication [23]. MYC amplification has been observed in gastric cancer cell lines and primary stomach tumors [8], [14], [15], [19], [24], [25], [26], [27], [28], [29], [30].
In the first carcinogenesis model, we observed MYC immunoreactivity, amplification (more than 3 MYC copies) and mRNA overexpression in the tumor biopsies. Four MYC copies was the most frequent copy number alteration in the tumor biopsies supporting our FISH findings in the ACP03 cells culture at the 85th passage [31].
In the second studied model, we induced gastric carcinogenesis by MNU treatment. MNU accumulation leads to the development of several types of tumors in the digestive tract, i.g. in oral cavity, larynx, pharynx and mainly esophagus and stomach of nonhuman primates [3], [32], [33]. MNU induces pre-neoplastic lesions before the development of intestinal type gastric adenocarcinoma [34], usually in antral stomach region of treated animals [35]. In the present study, all MNU-treated C. apellas presented pre-neoplastic lesions: non-atrophic gastritis (6 animals), atrophic gastritis (5 animals) and intestinal metaplasia (2 animals). We also observed the development of intestinal-type gastric adenocarcinoma in the antral stomach region of one animal. MNU induced intestinal-type gastric carcinogenesis in C. apella, presented the sequential steps similar to those described for humans [21]. Therefore, this model allows for the study of the evolution of intestinal-type gastric cancer, the identification of genes evolved in the early steps of the carcinogenesis process, and the determination of specific targets of gastric neoplastic transformation.
The multistep process of intestinal-type carcinogenesis was also supported by the detection of an increased of mRNA expression and MYC copy number during the sequential steps, which begins with atrophic gastritis and is followed by intestinal metaplasia and carcinoma. FISH assay and CNV analysis by qRT-PCR showed that normal mucosa, non-atrophic gastritis, and atrophic gastritis samples of MNU-treated C. apellas presented mainly cells with 2 MYC copies. Intestinal metaplasia presented cells with 3 MYC copies as a clonal alteration (30% of cells) and this gene amplification was observed in about 40% of cells. In gastric cancer samples induced by MNU, almost 50% of cells presented MYC amplification, including 5% of cells with MYC high amplification. The findings in C. apella gastric cancer corroborate our observations in human gastric carcinogenesis, in which the presence of MYC amplification, including high amplification, was detected in all human intestinal-type gastric cancer [14], [15], [25], [26], [27], [28] and a significant increase of MYC copy number was seen with the evolution of human carcinogenesis process: normal mucosa, intestinal metaplasia, and gastric cancer [27].
MYC immunoreactivity was only observed in the intestinal metaplasia and cancer samples, corroborating our previous study with human samples [27]. MYC protein overexpression was previously detected in intestinal metaplasia and neoplastic tissue from all patients with intestinal type gastric cancer [15], [27], [28]. Immunohistochemistry results demonstrated that clonal MYC amplification is necessary to induce its protein immunoreactivity.
To our knowledge, this is the third study to describe gastric adenocarcinoma in experimental model in monkeys. Takayama et al. reported 2 nonhuman primates of the Old Word with gastric adenocarcinoma after 10 years of continuous MNU treatment (10 mg/kg) [3]. The small size of C. apella, a New World monkey, may contribute to the faster development of gastric adenocarcinoma compared to the Old-World monkeys treated with MNU. Another previous study in literature reported intraepithelial neoplasia induced by ethyl-nitro-nitrosoguanidine treatment in combination with H. pylori infection during 5 years in 3 rhesus monkeys [36]. Moreover, the carcinogenesis model described in this study did not infect the animals with H. pylori, as frequently described in Mongolian gerbil gastric carcinogenesis model (for review, see [35]).
4.2 Biochemical and hematologic measurements in gastric carcinogenesis models
Chronic inflammation is involved with malignant change in several neoplasias. In both studied models, we observed that CRP levels increased significantly with cell line inoculation and MNU treatment. CRP is a representative marker for inflammatory conditions and it has been reported that the risk of cancer is increased when pre-diagnostic CRP levels are high [37]. Elevated CRP has been associated with progressive disease or an advanced stage and a worse survival rate for gastric cancer patients [38]. Chang et al. suggested that although serum CRP is not a specific biomarker for gastric cancer, it might be a potential prognostic biomarker and a promising therapeutic target for gastric cancer patients [39]. In the first carcinogenesis model, elevated CRP may be due to an inflammatory process in the local of cell line inoculation in addition to the tumor cell proliferation. In the second model, we observed that CRP increased 5.3–13 folds on the 90th day of MNU treatment compared to baseline level. The inflammatory process was confirmed by detection of non-atrophic gastritis in all treated C. apella. During MNU treatment, the CRP level continually increased until the presence of intestinal metaplasia was elevated in the surviving animal that developed gastric cancer, confirming that CRP is not a specific gastric cancer biomarker.
In both studied models, we observed a reduction of folic acid associated with gastric carcinogenesis. Folic acid maintains genomic stability by regulating DNA biosynthesis, repair and methylation. It has been reported that folic acid deficiency induces and accelerates carcinogenesis by the induction of DNA strand breaks, chromosomal and genomic instability, uracil misincorporation, and impaired DNA repair [40], [41]. Our results in the experimental model of gastric carcinogenesis showed an inverse correlation between folic acid concentration and the risk of gastric cancer development as reported in several experimental and epidemiologic studies with colorectum, esophagus, stomach, pancreas, lungs, cervix, ovary, neuroblastoma cancers, and leukemia [42].
In the first model, homocysteine concentration increased with the presence of gastric cancer on the 9th day. In the second studied model, we observed a continuous increase in homocysteine concentration with cancer development. Serum homocysteine concentration has been suggested as a tumor marker for monitoring cancer patients during anticancer treatment. Elevated circulating total homocysteine has been observed in cancer patients due to cancer cell proliferation and a decline of the high concentration of homocysteine is observed with the death of cancer cells [43]. Hyperhomocysteinemia may induce oxidative stress and DNA hypomethylation, leading to an increase in the risk of cancer, including gastric cancer [44], which corroborate our findings in both carcinogenesis models. In the present study, we did not observe a significant difference between the CL and NC groups on the 14th day of the first studied model, suggesting that homocysteine concentration began to decrease with the tumor regression. Thus, homocysteine concentration can be used for monitoring the efficiency of anticancer treatment.
Concerning hematologic measurements, cell line inoculation leads to anemia and leukocytosis in the first studied model. Anemia is a common complication in patients with inflammatory diseases of many kinds, including cancer. The mechanisms include cytokine-mediated changes in both the production of and the response to erythropoietin, as well as alterations in iron metabolism and increase in the leukocyte production [45]. The increase of white blood cell counts, in agreement with the elevated CRP levels, may be due to an inflammatory process and may have a role in the response against human malignant cells and the tumor remission after 14 days of cell line inoculation.
In the second carcinogenesis model, we observed that urea nitrogen, phosphorus, alanine aminotransferase, total bilirubin and creatinine levels increased with MNU treatment, which may be associated to typical symptoms of drug intoxication presented by 5 Cebus apella at the time before their death. We also observed that leukocyte, lymphocyte, neutrophil, erythrocyte, haemoglobinhemoglobin, and haematocrit were significantly reduced with MNU treatment, in agreement with the MNU effects in a broad spectrum of target organs, including particularly the lympho-hematopoietic system [5].
4.3 Canova effect in C. apella gastric carcinogenesis
In the present study, we observed that Canova acted mainly in hematopoietic system. In the first studied model, Canova induced an increase in leukocyte and lymphocyte and protected ACP03-inoculated animals to present anemia. Although only one animal was evaluated, the Canova treatment seems to restore the normal counts of leukocyte, lymphocyte, and neutrophil and also induced an increase in erythrocyte count that was abnormal after MNU treatment in the second carcinogenesis model.
Abud et al. described that the number of macrophages increased in cultures of bone marrow cells treated with Canova [46], corroborating previous in vivo and in vitro studies which showed macrophage activation by Canova treatment [47], [48], [49], [50], [51], [52]. According to Abud et al., Canova-active macrophages induce the production of lymphocytes and erythrocytes [46]. Our group also previously observed that Canova induces macrophage activation and indirectly leads to human lymphocyte proliferation in vitro [47]. Therefore, these findings are in agreement with those observed in Canova-treated C. apella.
In the first studied model, we observed some combinatory effects of cell line inoculation and Canova treatment in homocysteine level on the 14th day, when we observed total tumor regression. However, no conclusion can be made since the normal reference range of homocysteine in C. apella is still unknown.
In the second studied model, the tumor volume did not change after 20 days of Canova treatment. We also did not observe differences in the tumor volume among CL, CLCA1 and CLCA2 groups. In addition, MYC expression and copy number did not change with Canova treatment in both studied models. These findings suggest that Canova treatment did not lead to tumor regression in C. apella gastric carcinogenesis. However, Sato et al. reported that sarcoma 180 tumor size was significantly smaller in mice treated with Canova (20 days) compared to untreated animals and that 30% of Canova-treated animals presented total tumor regression [9]. These authors also described that all animals of Canova-treated group survived.
Although we did not observed that Canova has a role in gastric tumor regression, the ability of Canova immunomodulator to increase leukocyte count supports a human therapeutic applications such as restoring the hematopoietic system during/after chemotherapy and, thus, increasing the tolerability and duration of anticancer treatments.
Supporting Information
Abnormal biochemical and hematologic measurements in animals included in the first carcinogenesis model.
(DOT)
Abnormal biochemical and hematologic measurements in MNU-treated animals and Canova-treated animal of the second studied model.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (www.cnpq.br) grant number 550885/2007-2, 302774/2009-2 to RRB and 301609/2007-1 to MdACS and by the Fundação de Amparo à Pesquisa do Estado de São Paulo (www.fapesp.br) grant number 2007/02470-3 to MFL and 2010/11174-1 to DQC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takayama S, Thorgeirsson UP, Adamson RH. Chemical carcinogenesis studies in nonhuman primates. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84:176–188. doi: 10.2183/pjab/84.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prater MR, Zimmerman KL, Ward DL, Holladay SD. Reduced birth defects caused by maternal immune stimulation in methylnitrosourea-exposed mice: association with placental improvement. Birth Defects Res A Clin Mol Teratol. 2004;70:862–869. doi: 10.1002/bdra.20082. [DOI] [PubMed] [Google Scholar]
- 5.Uwagawa S, Tsuda H, Inoue T, Tagawa Y, Aoki T, et al. Enhancing potential of 6 different carcinogens on multi-organ tumorigenesis after initial treatment with N-methyl-N-nitrosourea in rats. Jpn J Cancer Res. 1991;82:1397–1405. doi: 10.1111/j.1349-7006.1991.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burbano RR, Leal MF, da Costa JB, Bahia Mde O, de Lima PD, et al. Lymphocyte proliferation stimulated by activated human macrophages treated with Canova. Homeopathy. 2009;98:45–48. doi: 10.1016/j.homp.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Bergman PJ. Cancer immunotherapy. Top Companion Anim Med. 2009;24:130–136. doi: 10.1053/j.tcam.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Leal MF, Martins do Nascimento JL, da Silva CE, Vita Lamarao MF, Calcagno DQ, et al. Establishment and conventional cytogenetic characterization of three gastric cancer cell lines. Cancer Genet Cytogenet. 2009;195:85–91. doi: 10.1016/j.cancergencyto.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Sato DY, Wal R, de Oliveira CC, Cattaneo RI, Malvezzi M, et al. Histopathological and immunophenotyping studies on normal and sarcoma 180-bearing mice treated with a complex homeopathic medication. Homeopathy. 2005;94:26–32. doi: 10.1016/j.homp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Riviello MC, Wirz A. Haematology and blood chemistry of Cebus apella in relation to sex and age. J Med Primatol. 2001;30:308–312. doi: 10.1034/j.1600-0684.2001.300604.x. [DOI] [PubMed] [Google Scholar]
- 11.Price CP, Trull AK, Berry D, Gorman EG. Development and validation of a particle-enhanced turbidimetric immunoassay for C-reactive protein. J Immunol Methods. 1987;99:205–211. doi: 10.1016/0022-1759(87)90129-3. [DOI] [PubMed] [Google Scholar]
- 12.Calcagno DQ, Leal MF, Assumpcao PP, Smith MA, Burbano RR. MYC and gastric adenocarcinoma carcinogenesis. World J Gastroenterol. 2008;14:5962–5968. doi: 10.3748/wjg.14.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986;83:2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calcagno DQ, Leal MF, Taken SS, Assumpcao PP, Demachki S, et al. Aneuploidy of chromosome 8 and C-MYC amplification in individuals from northern Brazil with gastric adenocarcinoma. Anticancer Res. 2005;25:4069–4074. [PubMed] [Google Scholar]
- 15.Calcagno DQ, Leal MF, Seabra AD, Khayat AS, Chen ES, et al. Interrelationship between chromosome 8 aneuploidy, C-MYC amplification and increased expression in individuals from northern Brazil with gastric adenocarcinoma. World J Gastroenterol. 2006;12:6207–6211. doi: 10.3748/wjg.v12.i38.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopman AH, Ramaekers FC, Raap AK, Beck JL, Devilee P, et al. In situ hybridization as a tool to study numerical chromosome aberrations in solid bladder tumors. Histochemistry. 1988;89:307–316. doi: 10.1007/BF00500631. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Lentz SR, Sobey CG, Piegors DJ, Bhopatkar MY, Faraci FM, et al. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest. 1996;98:24–29. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calcagno DQ, Guimaraes AC, Leal MF, Seabra AD, Khayat AS, et al. MYC insertions in diffuse-type gastric adenocarcinoma. Anticancer Res. 2009;29:2479–2483. [PubMed] [Google Scholar]
- 20.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. an Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 21.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 22.Baiocchi M, Biffoni M, Ricci-Vitiani L, Pilozzi E, De Maria R. New models for cancer research: human cancer stem cell xenografts. Curr Opin Pharmacol. 2010;10:380–384. doi: 10.1016/j.coph.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Dang CV, O'Donnell KA, Zeller KI, Nguyen T, Osthus RC, et al. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Lima EM, Rissino JD, Harada ML, Assumpcao PP, Demachki S, et al. Conventional cytogenetic characterization of a new cell line, ACP01, established from a primary human gastric tumor. Braz J Med Biol Res. 2004;37:1831–1838. doi: 10.1590/s0100-879x2004001200008. [DOI] [PubMed] [Google Scholar]
- 25.Assumpcao PP, Ishak G, Chen ES, Takeno SS, Leal MF, et al. Numerical aberrations of chromosome 8 detected by conventional cytogenetics and fluorescence in situ hybridization in individuals from northern Brazil with gastric adenocarcinoma. Cancer Genet Cytogenet. 2006;169:45–49. doi: 10.1016/j.cancergencyto.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Burbano RR, Assumpcao PP, Leal MF, Calcagno DQ, Guimaraes AC, et al. C-MYC locus amplification as metastasis predictor in intestinal-type gastric adenocarcinomas: CGH study in Brazil. Anticancer Res. 2006;26:2909–2914. [PubMed] [Google Scholar]
- 27.Calcagno DQ, Leal MF, Demachki S, Araujo MT, Freitas FW, et al. MYC in gastric carcinoma and intestinal metaplasia of young adults. Cancer Genet Cytogenet. 2010;202:63–66. doi: 10.1016/j.cancergencyto.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Costa Raiol LC, Figueira Silva EC, Mendes da Fonseca D, Leal MF, Guimaraes AC, et al. Interrelationship between MYC gene numerical aberrations and protein expression in individuals from northern Brazil with early gastric adenocarcinoma. Cancer Genet Cytogenet. 2008;181:31–35. doi: 10.1016/j.cancergencyto.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro HF, Alcantara DF, Matos LA, Sousa JM, Leal MF, et al. Cytogenetic characterization and evaluation of c-MYC gene amplification in PG100, a new Brazilian gastric cancer cell line. Braz J Med Biol Res. 2010;43:717–721. doi: 10.1590/s0100-879x2010007500068. [DOI] [PubMed] [Google Scholar]
- 30.Costa Guimaraes A, Goncalves Quintana L, Ferreira Leal M, Satomi Takeno S, Pimentel Assumpcao P, et al. Aneuploidy of chromosome 8 detected by fluorescence in situ hybridisation in ACP01 cell line gastric adenocarcinoma. Clin Exp Med. 2006;6:129–133. doi: 10.1007/s10238-006-0108-5. [DOI] [PubMed] [Google Scholar]
- 31.Leal MF, Calcagno DQ, Costa JFFB, Silva TCR, Khayat AS, et al. MYC, TP53 and chromosome 17 copy number alterations in multiple gastric cancer cell lines and in their parental primary tumors. Journal of Biomedicine and Biotechnology 2011. 2011 doi: 10.1155/2011/631268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adamson RH, Krollkowski FJ, Correa P, Sieber SM, Dalgard DW. Carcinogenecity of 1-methyl-1-nitrosourea in nonhuman primates. Journal of the National Cancer Institute. 1977;59:8. [PubMed] [Google Scholar]
- 33.Thorgeirsson UP, Dalgard DW, Reeves J, Adamson RH. Tumor incidence in a chemical carcinogenesis study of nonhuman primates. Regul Toxicol Pharmacol. 1994;19:130–151. doi: 10.1006/rtph.1994.1013. [DOI] [PubMed] [Google Scholar]
- 34.Tsukamoto T, Mizoshita T, Tatematsu M. Animal models of stomach carcinogenesis. Toxicol Pathol. 2007;35:636–648. doi: 10.1080/01926230701420632. [DOI] [PubMed] [Google Scholar]
- 35.Kodama M, Murakami K, Sato R, Okimoto T, Nishizono A, et al. Helicobacter pylori-infected animal models are extremely suitable for the investigation of gastric carcinogenesis. World J Gastroenterol. 2005;11:7063–7071. doi: 10.3748/wjg.v11.i45.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, Merrell DS, Semino-Mora C, Goldman M, Rahman A, et al. Diet synergistically affects helicobacter pylori-induced gastric carcinogenesis in nonhuman primates. Gastroenterology. 2009;137:e1361–1366. doi: 10.1053/j.gastro.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. Jama. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 38.Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer. 2009;9:155. doi: 10.1186/1471-2407-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang CC, Sun CF, Pai HJ, Wang WK, Hsieh CC, et al. Preoperative serum C-reactive protein and gastric cancer; clinical-pathological correlation and prognostic significance. Chang Gung Med J. 2010;33:301–312. [PubMed] [Google Scholar]
- 40.Duthie SJ. Folate and cancer: how DNA damage, repair and methylation impact on colon carcinogenesis. J Inherit Metab Dis. 2011 doi: 10.1007/s10545-010-9128-0. [DOI] [PubMed] [Google Scholar]
- 41.Fenech M. Nutrition and genome health. Forum Nutr. 2007;60:49–65. doi: 10.1159/000107067. [DOI] [PubMed] [Google Scholar]
- 42.Kim YI. Folate and colorectal cancer: an evidence-based critical review. Mol Nutr Food Res. 2007;51:267–292. doi: 10.1002/mnfr.200600191. [DOI] [PubMed] [Google Scholar]
- 43.Wu LL, Wu JT. Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin Chim Acta. 2002;322:21–28. doi: 10.1016/s0009-8981(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 44.Zacho J, Yazdanyar S, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Hyperhomocysteinemia, methylenetetrahydrofolate reductase c.677C>T polymorphism and risk of cancer: cross-sectional and prospective studies and meta-analyses of 75,000 cases and 93,000 controls. Int J Cancer. 2011;128:644–652. doi: 10.1002/ijc.25375. [DOI] [PubMed] [Google Scholar]
- 45.Adamson JW. The anemia of inflammation/malignancy: mechanisms and management. Hematology Am Soc Hematol Educ Program. 2008:159–165. doi: 10.1182/asheducation-2008.1.159. [DOI] [PubMed] [Google Scholar]
- 46.Abud AP, Cesar B, Cavazzani LF, de Oliveira CC, Gabardo J, et al. Activation of bone marrow cells treated with Canova in vitro. Cell Biol Int. 2006;30:808–816. doi: 10.1016/j.cellbi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Takeno SS, Leal MF, Lisboa LC, Lipay MV, Khayat AS, et al. Genomic alterations in diffuse-type gastric cancer as shown by high-resolution comparative genomic hybridization. Cancer Genet Cytogenet. 2009;190:1–7. doi: 10.1016/j.cancergencyto.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Cesar B, Abud AP, de Oliveira CC, Cardoso F, Gremski W, et al. Activation of mononuclear bone marrow cells treated in vitro with a complex homeopathic medication. Micron. 2008;39:461–470. doi: 10.1016/j.micron.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Pereira WK, Lonardoni MV, Grespan R, Caparroz-Assef SM, Cuman RK, et al. Immunomodulatory effect of Canova medication on experimental Leishmania amazonensis infection. J Infect. 2005;51:157–164. doi: 10.1016/j.jinf.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira CC, de Oliveira SM, Godoy LM, Gabardo J, Buchi Dde F. Canova, a Brazilian medical formulation, alters oxidative metabolism of mice macrophages. J Infect. 2006;52:420–432. doi: 10.1016/j.jinf.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Lopes L, Godoy LM, de Oliveira CC, Gabardo J, Schadeck RJ, et al. Phagocytosis, endosomal/lysosomal system and other cellularaspects of macrophage activation by Canova medication. Micron. 2006;37:277–287. doi: 10.1016/j.micron.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Da Rocha Piemonte M, De Freitas Buchi D. Analysis of IL-2, IFN-gamma and TNF-alpha production, alpha5 beta1 integrins and actin filaments distribution in peritoneal mouse macrophages treated with homeopathic medicament. J Submicrosc Cytol Pathol. 2002;34:255–263. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abnormal biochemical and hematologic measurements in animals included in the first carcinogenesis model.
(DOT)
Abnormal biochemical and hematologic measurements in MNU-treated animals and Canova-treated animal of the second studied model.
(DOC)