Abstract
Background
Infection by Burkholderia cenocepacia in cystic fibrosis (CF) patients is associated with poor clinical prognosis. Previously, we demonstrated that one of the highly transmissible strains, BC7, expresses cable pili and the associated 22 kDa adhesin, both of which contribute to BC7 binding to airway epithelial cells. However, the contribution of these factors to induce inflammation and bacterial persistence in vivo is not known.
Methodology/Principal Findings
Wild-type BC7 stimulated higher IL-8 responses than the BC7 cbl and BC7 adhA mutants in both CF and normal bronchial epithelial cells. To determine the role of cable pili and the associated adhesin, we characterized a mouse model of B. cenocepacia, where BC7 are suspended in Pseudomonas aeruginosa alginate. C57BL/6 mice were infected intratracheally with wild-type BC7 suspended in either alginate or PBS and were monitored for lung bacterial load and inflammation. Mice infected with BC7 suspended in PBS completely cleared the bacteria by 3 days and resolved the inflammation. In contrast, mice infected with BC7 suspended in alginate showed persistence of bacteria and moderate lung inflammation up to 5 days post-infection. Using this model, mice infected with the BC7 cbl and BC7 adhA mutants showed lower bacterial loads and mild inflammation compared to mice infected with wild-type BC7. Complementation of the BC7 cblS mutation in trans restored the capacity of this strain to persist in vivo. Immunolocalization of bacteria revealed wild-type BC7 in both airway lumen and alveoli, while the BC7 cbl and BC7 adhA mutants were found mainly in airway lumen and peribronchiolar region.
Conclusions and Significance
B. cenocepacia suspended in alginate can be used to determine the capacity of bacteria to persist and cause lung inflammation in normal mice. Both cable pili and adhesin contribute to BC7-stimulated IL-8 response in vitro, and BC7 persistence and resultant inflammation in vivo.
Introduction
Burkholderia cenocepacia is an important opportunistic pathogen causing respiratory infections in individuals with cystic fibrosis (CF). It is a member of the Burkholderia cepacia complex (Bcc). The Bcc represents at least 17 phylogenetically closely related yet distinct species of bacteria that are commonly found in the environment and can serve as agents for both plant and human infection [1], [2], [3]. Although most Bcc species have been isolated from CF lungs, the two most common are B. multivorans and B. cenocepacia. Infections with certain strains of B. cenocepacia (in particular, those of the ET12 lineage) are associated with a variable and unpredictable clinical course ranging from asymptomatic carriage to a rapid decline in clinical condition leading to fatal necrotizing pneumonia and septicemia, also known as ‘cepacia syndrome’ [4].
In our earlier studies, we showed that ET12 strains that cause ‘cepacia syndrome’ bind to human respiratory mucins via a pilin-associated 22 kDa adhesin protein [5], [6]. This protein is distributed along the shaft of the large, peritrichous appendages known as cable pili [6]. We also showed that the 22 kDa adhesin mediates the adherence of cable-piliated B. cenocepacia to cytokeratin 13 (CK13), the expression of which is enriched in airway epithelial cells differentiated into the squamous phenotype [7], [8]. CK13 expression is also increased in CF airway epithelial cells, particularly in bronchiolar and respiratory epithelium [9]. This increased CK13 expression is not directly linked to mutation in the CF transmembrane conductance regulator (CFTR), but rather is due to repeated injury of the airway epithelium as observed in the lungs CF patients that can lead to squamous differentiation [10]. Therefore, it is conceivable that B. cenocepacia capable of binding to CK13 may have a greater potential to cause infection, particularly in CF. Consistent with this, we observed that B. cenocepacia strains that express both cable pili and the 22 kDa adhesin bind better to lung sections from CF patients compared to lung sections from normal individuals. Cable pili and 22 kDa adhesin expressing bacteria also showed increased binding to lung sections from CFTR knockout mice compared to sections from wild-type mice [9]. We showed that isogenic mutants of the ET12 lineage strain BC7 lacking either the cable pilus (BC7 cblA or BC7 cblS) or the 22 kDa adhesin (BC7 adhA) were attenuated in binding to and transmigration across squamous differentiated primary airway epithelial cells [11], suggesting that cable pili and the adhesin may be required for causing persistent infection in vivo.
Recently, we and others have shown that the suspension of bacteria in Pseudomonas aeruginosa alginate facilitates persistence of bacteria in both normal and CFTR knockout mice by delaying the initial innate immune responses required for bacterial clearance [12], [13], [14], [15]. Here we have further characterized B. cenocepacia infection model in normal mice and determined the capacity of BC7 cable pili mutants: BC7 cblA, BC7 cblS, and BC7 cblS mutant complemented with cblS in trans, and the BC7 adhA mutant to persist and cause inflammation in vivo. We also determined the capacity of these strains to stimulate IL-8 responses in airway epithelial cells.
Results and Discussion
BC7 cblA, BC7 cblS, and the BC7 adhA mutant show decreased stimulation of an IL-8 response in airway epithelial cells
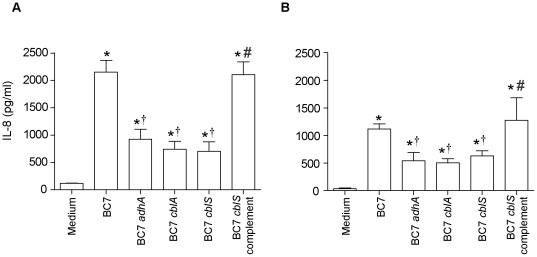
To assess the pro-inflammatory potential of bacteria, we infected IB3 (CF airway) epithelial cells with wild-type BC7, or the BC7 cable pili mutants (BC7 cblA or BC7 cblS) or the BC7 adhA mutant [11] and determined the IL-8 levels (Figure 1A). All strains showed significantly increased IL-8 production in CF cells compared to cells receiving only media. All three mutants stimulated approximately 2.5–3 fold less IL-8, compared to the wild-type BC7 strain.
Figure 1. Stimulation of IL-8 response by B. cenocepacia strains in airway epithelial cells.
IB3 (A) or BEAS2B (B) cells were treated with media or media containing BC7, BC7 adhA, BC7 cblA, BC7 cblS, or the BC7 cblS complemented strain (BC7 cblS complement), and IL-8 was determined by ELISA. Data represents mean ± SEM calculated from three independent experiments carried out in triplicates. (*different from medium control, † different from BC7, #different from BC7 mutants p≤0.05, ANOVA).
To examine whether BC7 cblA, BC7 cblS, and BC7 adhA mutants were similarly attenuated in stimulating IL-8 response in normal airway epithelial cells, BEAS2B cells were infected with wild-type BC7 or the mutants, and IL-8 response was determined. Wild-type BC7 stimulated higher IL-8 production than all three BC7 mutants, although the absolute IL-8 levels were much lower than those observed in the CF airway epithelial cells (Figure 1B) and this may be due to decreased expression of CK13 and TNF receptor I in normal cells compared to CF cells [7], [9].
Previously, we demonstrated that 22 kDa adhesin is associated with cable pili and is required for binding to CK13 in both squamous differentiated cells as well as in undifferentiated normal airway epithelial cells, but the role of this interaction in stimulating IL-8 responses in airway epithelial cells was not investigated [8], [16]. Following this, we demonstrated that interaction of BC7 with TNF receptor 1 partly contributes to BC7-induced IL-8 and this phenomenon was not dependent on the expression of 22 kDa adhesin [7]. Here, using isogenic mutants of cable pili and the adhesin protein, we provide evidence that both cable pili and the 22 kDa adhesin in addition to facilitating binding to CK13, also play a role in BC7 stimulated IL-8 response in airway epithelial cells. These results suggest that BC7-stimulated IL-8 requires interaction of bacteria with both CK13 and TNF receptor I.
Attempts to complement the BC7 cblA and BC7 adhA mutants have been unsuccessful. Similar to our experience, Tomich et al [17] was also unable to complement a cblA mutant. Therefore we compared the BC7 cblS mutant and the mutant complemented with cblS in trans to show that the observed effects are specific at least for cable pili. BC7 cblS is mutated in the gene, cblS, which regulates expression of the cblA gene. BC7 cblS does not express cable pilus similar to the BC7 cblA mutant [11], [18]. The BC7 cblS complemented strain which expresses cable pili [11] stimulated IL-8 response very similar to wild-type BC7, indicating that cable pili is required for this trait. Together, these results suggest both cable pili and the associated 22 kDa adhesin also contributes to BC7 stimulated IL-8 in CF and normal airway epithelial cells.
Characterization of B. cenocepacia infection model in C57BL/6 mice
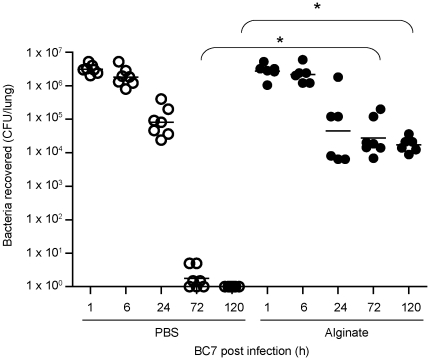
Next, we tested whether the observed differences we observed in vitro between wild-type BC7 and the BC7 cblA, BC7 cblS, and BC7 adhA mutants held true in vivo. In our previous studies, we showed that suspension of bacteria in P. aeruginosa alginate facilitates BC7 persistence in both normal and CFTR knockout mice, but the latter develop severe pneumonic consolidation in the lungs with 30% of the mice dying by 5 days [12]. This high mortality rate makes it difficult to follow bacterial persistence throughout the course of infection, therefore, we used normal mice to determine the capacity of the BC7 cbl and BC7 adhA mutants to persist and cause lung inflammation in vivo. To initiate these studies, we characterized the B. cenocepacia infection model with the wild-type strain BC7 in C57BL/6 mice with regards to bacterial persistence and related lung inflammation. We infected C57BL/6 mice with BC7 suspended in PBS (BC7/PBS) or alginate (BC7/alginate) by the intratracheal route. Mice were sacrificed at 1 h, 6 h, 1, 3 or 5 days post-infection, and examined for lung bacterial load and inflammation. Mice, mock-infected with PBS or alginate alone served as controls. As expected, these mock-infeccted mice did not show bacteria in their lungs. Mice infected with BC7/PBS showed abundant bacteria in their lungs up to 24 h after infection (Figure 2A), which was similar to the bacterial load observed in mice infected with BC7/alginate (Figure 2B). While all mice in the BC7/PBS group cleared bacteria by 3 days after infection, animals in the BC7/alginate group showed bacteria in their lungs up to 5 days, though there was a gradual decrease in this number. The difference between the BC7/PBS and the BC7/alginate groups was not due to initial deposition of bacteria, because both groups of mice showed a similar bacterial load 1 h post-infection as well as 1 day post-infection. These results suggest that alginate facilitates persistence of BC7 in normal mice similar to that observed in CFTR knockout mice [12]. However, unlike CFTR knockout mice, these infected C57BL/6 mice showed no mortality. Consistent with this, alginate has also been shown to increase the persistence of P. aeruginosa in BALB/c mice up to 7 days with no mortality [14].
Figure 2. Lung bacterial load in C57BL/6 mice infected with BC7/PBS or BC7/alginate.
Mice infected with BC7/PBS or BC7/alginate were sacrificed at pre-determined times, and ten-fold serial dilutions of lung homogenates were plated on BCSA to determine bacterial load. Data points represent individual mice and bars represent geometric mean calculated from three independent experiments with a total of 6 - 7 mice per group (*different from BC7/PBS group at respective time points, p≤0.05, ANOVA on Ranks).
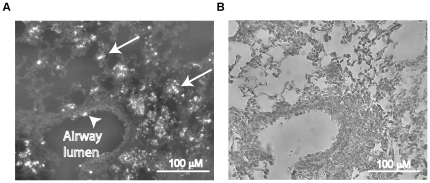
To obtain persistent infection with B. cenocepacia, other investigators have adapted the agarose bead model that was originally developed for P. aeruginosa infection by Cash et al. [19], [20], [21]. Although this method is valuable in establishing chronic infection, it often facilitates infection of conducting airways, likely due to mechanical blocking of bronchioles by the beads [22]. In addition, this damage may mask the inflammation caused by bacteria [22]. In contrast, we and others have shown that the alginate suspension alone does not cause lung inflammation in mice [12]. Further, the mechanical entrapment of bacteria in beads may prevent spreading of bacteria to respiratory zone. This led us to examine the location of bacteria in the lungs of mice infected with BC7 suspended in alginate by immunolocalization. Lung sections immunostained with antibody to the bacteria showed BC7 in the airway lumen as well as in the respiratory zone associated with alveolar septa and inflammatory cells in these mice, suggesting that alginate does not restrict bacteria to conducting airways (Figure 3A and 3B). This distribution was very similar to that observed in CFTR knockout mice infected with B. cenocepacia suspended in alginate [12] as well as that observed in CF patients colonized with B. cenocepacia [23].
Figure 3. Immunolocalization of B. cenocepacia in mice infected with BC7/alginate.
Mice were infected with BC7/alginate and sacrificed one day after infection. Lungs were fixed and embedded in paraffin. Lung sections were deparaffinized and incubated with antibody to B. cenocepacia. Bound antibody was detected by anti-rabbit IgG conjugated with Alexafluor 598 and sections were visualized under fluorescence microscope. A, lung section showing bacteria in both conducting (arrowhead) and respiratory zone (arrow). B, bright field image of A. Images are representative of two independent experiments.
Bacteria suspended in alginate stimulate altered cytokine responses in C57BL/6 mice
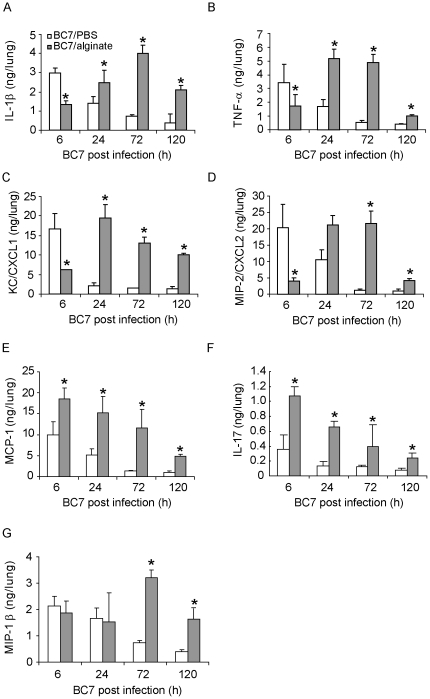
Chemokines and cytokines play an important role in recruiting phagocytes to the site of infection and subsequent clearance of bacteria. To examine whether alginate increases persistence of bacteria by delaying the initial chemokine and cytokine responses in normal mice, we measured the levels of selected chemokines and cytokines in lung homogenates obtained from uninfected control mice, and mice infected with BC7/PBS or BC7/alginate. Mice mock-infected with PBS or alginate alone showed very low or undetectable levels of all the measured cytokines and chemokines at all the time points tested (data not shown). In contrast, mice infected with either BC7/PBS or BC7/alginate showed a significant increase of all cytokines measured compared to the respective sham-infected groups, but with different kinetics (Figure 4A-G). Mice infected with BC7/PBS showed peak induction of KC/CXCL1, MIP-2/CXCL2, IL-1β, TNF-α, as early as 6 h, correlating with the bacterial load in the lung. The levels of these chemokines and cytokines gradually decreased and returned to normal by 3 d post infection. In contrast, mice infected with BC7/alginate showed increased KC/CXCL1, MIP-2/CXCL2, IL-1β and TNF-α at 6 h post infection; these levels were significantly lower than those observed in mice infected with BC7/PBS, despite similar bacterial load. The levels of these cytokines and chemokines in the BC7/alginate group peaked at 24 h post infection and was sustained up to 5 days, although the levels of these chemokines and cytokines decreased by day 5. We observed similar delayed chemokine responses in CFTR knockout mice infected with BC7/alginate, but in these mice the chemokine levels were sustained up to 7 days post infection [12].
Figure 4. Cytokine levels in lungs of mice infected with BC7.
Mice infected with BC7/PBS or BC7/alginate were sacrificed at specified times, and the cytokine levels in lung homogenates were measured by Bio-Plex multiplex immunoassay. A, IL-1β; B, TNF-α; C, KC/CXCL1; D, MIP-2/CXCL2; E, MCP-1; F, IL-17; and G, MIP-1β. Data represents mean and SEM calculated from three independent experiments with a total of 6 - 7 mice per group (*different from BC7/PBS group at respective time points, p≤0.05, ANOVA).
KC/CXCL1 and MIP-2/CXCL2 are potent chemoattractants for phagocytes, and IL-1β and TNF-α, which stimulate the expression of KC/CXCL1 and MIP-2/CXCL2, play a critical role in recruitment of phagocytes to the site of infection and subsequent clearance of bacteria. Therefore, the delay in chemokine response as observed in the BC7/alginate group in normal mice may result in delayed phagocyte recruitment and attenuated bacterial clearance, and thus promote the establishment of bacterial infection. Consistent with our hypothesis, DBA2 mice, which is a susceptible mouse strain, are deficient in bacterial clearance due to an initial delay in phagocyte recruitment [24].
Compared to sham-infected mice, animals infected with either BC7/PBS or BC7/alginate showed significant increases in MCP-1, which recruits monocytes, and IL-17, a cytokine expressed by a subset of CD4 positive T cells. However, at all the time points examined, both MCP-1 and IL-17 were much higher in the BC7/alginate group compared to the BC7/PBS group. In addition, while the levels of MCP-1 and IL-17 returned to almost baseline in the BC7/PBS group by 3 days, mice in the BC7/alginate showed significantly increased levels of these cytokines up to 5 days, compared to sham-infected animals. The level of IL-17 has been shown to be increased in CF airways and is implicated in the increased chemokine responses and development of tissue inflammation [25], [26], [27]. Therefore, it is conceivable that sustained increased levels of IL-17 observed in the BC7/alginate group may contribute to increased chemokine response observed during the late phase of infection (1 to 5 days post infection). We also observed increased levels of MIP-1β/CCL4 at 3 days post infection in the BC7/alginate group compared to the BC7/PBS group. Since MIP-1β also increases the production of IL-1β and TNF-α from leukocytes, it may also contribute to an increased chemokine and cytokine response during the later stage of infection in the BC7/alginate group. Together these results suggest that alginate may facilitate persistence of bacteria by delaying the initial chemokine and cytokine response that is required for recruitment of phagocytes. The increased levels of chemokines that is observed in the BC7/alginate group may be due to both the persistent bacterial load and also exaggerated chemokine response stimulated by the increase in IL-17 and MIP-1β.
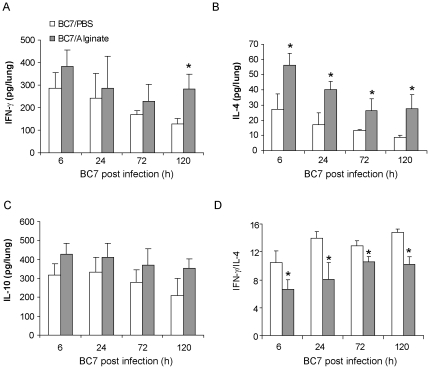
Th1 response following bacterial infection has been shown to be beneficial to the host. CF patients, who are chronically infected with P. aeruginosa, show predominantly a Th2 response that is correlated with a poor prognosis [28]. P. aeruginosa alginate was demonstrated to be refractory to a Th1 immune response [29]. To examine whether alginate protects B. cenocepacia by a similar mechanism, we examined the levels of IFN-γ, IL-4, and IL-10, after infection. There was no significant difference in the levels of IFN-γ (which is Th1 cytokine) and IL-10 (which suppresses expression of pro-inflammatory cytokines) between the BC7/PBS and BC7/alginate groups up to 3 days post-infection (Figure 5). Interestingly, at 5 days post-infection, the BC7/alginate group showed significantly higher levels of IFN-γ than the BC7/PBS group. IL-4 (which is a Th2 cytokine) was also significantly increased in the BC7/alginate group at all the time points compared to the BC7/PBS group. The ratio between IFN-γ to IL-4 is used as an indicator of Th1 versus Th2 responses. Compared to the BC7/PBS group, the BC7/alginate group showed a significantly decreased IFN-γ/IL-4 ratio, suggesting BC7 may tip the balance towards Th2 type of response in the presence of alginate and that this may contribute to persistence of the bacteria.
Figure 5. Th1 and Th2 cytokine levels in mice infected with BC7.
Mice infected with BC7/PBS or BC7/alginate were sacrificed at specified times and levels of IFN-γ (A), IL-4 (B), and IL-10 (C) in lung homogenates were measured by Bio-Plex multiple immunassay. D, ratio of IFN-γ to IL-4. Data represents mean and SEM calculated from three independent experiments with a total of 6 - 7 mice per group (*different from BC7/PBS group at respective time points, p≤ANOVA).
Neutrophil recruitment is delayed by alginate
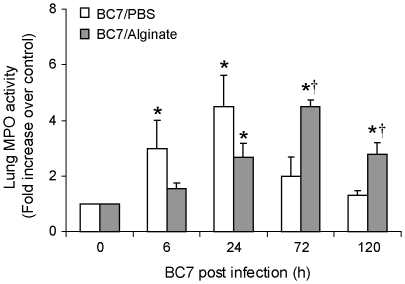
Myeloperoxidase (MPO) is predominantly produced by activated neutrophils and can be used as a surrogate marker for their presence in the lung. Mice mock-infected with either PBS or alginate did not show MPO activity (data not shown). On the other hand, consistent with the cytokine levels, mice infected with BC7/PBS showed increased MPO activity as early as 6 h post-infection, with a peak activity at 24 h post-infection (Figure 6). MPO levels gradually decreased and returned to basal levels by 5 days in these mice. In contrast, mice infected with BC7/alginate did not show increased MPO activity 6 h post-infection, despite similar bacterial loads. This correlated with the attenuated chemokine and cytokine expression at 6 h post infection, observed in the BC7/alginate group. However, at 24 h post infection, the MPO activity increased in the BC7/alginate group reaching a maximum at 3 days corresponding to bacterial load and chemokine levels. Together, these results suggest that the initial delay in the recruitment of phagocytes to the site of infection in the BC7/alginate group prevents early clearance of bacteria thus providing suitable environment for bacteria to establish infection and spread to respiratory zone.
Figure 6. Lung MPO activity in BC7 infected C57BL/6 mice.
Mice infected with BC7/PBS or BC7/alginate were sacrificed at specified times and MPO activity was determined in the lung homogenates. Data represents mean and SEM calculated from three independent experiments with a total of 6-7 mice per group (*different from respective uninfected group p≤0.05, †different from BC7/PBS group at respective time points p≤0.05, ANOVA).
Mice infected with BC7/alginate show persistent lung inflammation
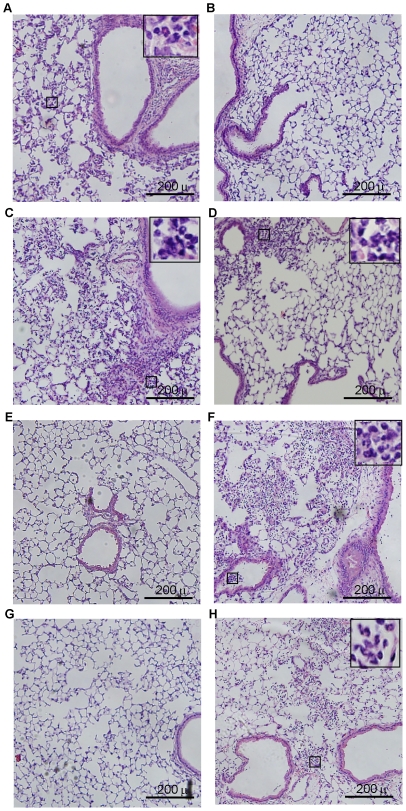
Next we examined the hematoxylin and eosin (H&E) stained lung sections to assess the inflammatory changes in mice infected with BC7/PBS or BC7/alginate. Neither PBS- nor alginate-treated mice showed lung inflammation at any of the time points examined (data not shown). Lung sections from mice infected with BC7/PBS showed mild inflammation at 6 h post-infection, with few neutrophils (Figure 7A). These mice showed moderate neutrophilic inflammation, particularly in peribronchiolar and perivascular areas at 1 d post-infection (Figure 7C), and mild and no inflammation, at 3 and 5 days post-infection, respectively (Figure 7E and 7G). In contrast, BC7/alginate-infected mice showed no inflammation at 6 h post-infection (Figure 7B) and mild neutrophilic inflammation at 24 h post infection (Figure 7D). However, at 3 d post-infection, mice infected with BC7/alginate showed severe widespread inflammation with neutrophil infiltration in both airway lumina and parenchyma (Figure 7F). At 5 d post-infection, these mice still showed inflammation (Figure 7H), but it was less compared to the mice at 3 d post-infection. Taken together these results suggest that alginate facilitates establishment of B. cenocepacia BC7 infection in normal mice similar to that observed in CFTR knockout mice. However, unlike CFTR knockout mice, normal mice show a trend towards clearing bacteria, resolving inflammation and also do not show mortality. Therefore, using this relatively well-characterized B. cenocepacia infection model in normal mice, we examined the capacity of BC7 cbl and BC7 adhA mutants to persist and cause inflammation in vivo.
Figure 7. Lung inflammation in C57BL/6 mice infected with BC7/PBS or BC7/alginate.
Paraffin lung sections from mice infected with BC7/PBS (A, C, E and G) or BC7/alginate (B, D, F and H) were stained with H&E. A and B, 6 h post-infection; C and D, 1 d post-infection; E and F, 3 d post-infection; and G and H, 5 d post-infection, respectively. Insets in A, C, D, F and H represent magnified view of areas marked in respective panels and show infiltrated neutrophils. Images are representative of 3 individual animals at each time point.
BC7 cblA, BC7 cblS, and BC7 adhA mutants are attenuated in their capacity to persist and cause lung inflammation in vivo
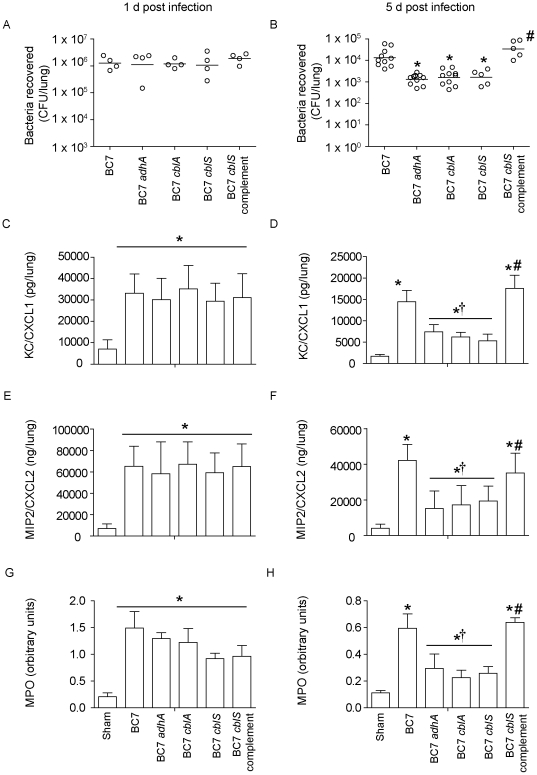
The C57BL/6 mice were infected with BC7 cblA, BC7 cblS, BC7 adhA, the BC7 cblS complemented strain or wild-type BC7; all bacteria were suspended in alginate. Mice were sacrificed 1 or 5 days after infection, and examined for lung bacterial load and levels of inflammatory markers. As observed earlier, mice infected with wild-type BC7 showed persistence of bacteria up to 5 days. On the other hand, mice infected with BC7 cblA, BC7 cblS, or BC7 adhA showed similar bacterial loads at 1 day post infection, but had significantly reduced bacterial loads at 5 day post infection, compared to mice infected with wild-type BC7 (Figure 8A). This is not surprising, because we have previously shown that during the initial phase of infection, alginate protects bacteria from phagocytes by altering bacterial surface properties and this in turn prevents rapid bacterial clearance from the lungs [12]. However at later stages of infection, only bacteria that have the capacity to colonize may persist and the others are cleared. Based on this notion, we believe that BC7 cblA, BC7 cblS, and BC7 adhA are attenuated in their capacity to colonize and persist in the lungs. The BC7 cblS complemented strain on the other hand, persisted in the lungs similar to wild-type BC7 indicating the contribution of cable pili in colonization and persistence. Collectively, these results suggest that both cable pili and adhesin contributes to BC7 persistence in vivo.
Figure 8. Bacterial load and chemokine responses in mice infected with B. cenocepacia strains.
Mice were infected with wild-type BC7, the BC7 adhA, BC7 cblA, BC7 cblS mutants, or the BC7 cblS complement, suspended in alginate, and sacrificed at 1 or 5 d post-infection. Lung homogenates were used to determine bacterial load (A and B), KC (C and D), MIP-2 (E and F) and MPO activity (G and H). Data in represents either median with range (A) or mean with SEM (B–D) determined from two independent experiments with a total of 5 mice per group (*different from wild-type BC7, † different from BC7, #different from BC7 mutants p≤0.05, ANOVA on Ranks or ANOVA).
Next we measured the levels of KC (Figure 8C and 8D), MIP-2 (Figure 8E and 8F) , and MPO activity (Figure 8G and 8H) in mice infected with BC7, BC7 cblA, BC7 cblS, or BC7 adhA, or the BC7 cblS complemented strain, at 1 and 5 days post-infection. Both at 1 and 5 days post infection, mice infected with all strains showed increased levels of KC, MIP-2 and MPO compared to sham-infected animals. However, mice infected with wild-type BC7 showed significantly higher KC, MIP-2 and MPO levels than the mice infected with the BC7 cblA, BC7 cblS, or BC7 adhA at 5 days post infection, but not at 1 day post infection; and this may reflect the bacterial load in the lungs of these mice. Mice infected with the BC7 cblS complemented strain showed chemokine and MPO levels similar to wild-type BC7, indicating that the attenuated response observed with BC7 cblS was due to lack of cable pili expression. The observed decreased levels of chemokines and MPO activity levels in mice infected with the BC7 cable and adhesin mutants may be a combined effect of lower bacteria load and attenuated capacity of these strains to induce inflammation. Together, these results suggest that both cable pili and adhesin play a role in facilitating bacterial persistence and causing inflammation.
BC7 cblA, BC7 cblS, and BC7 adhA mutants are attenuated in spreading infection to respiratory zone
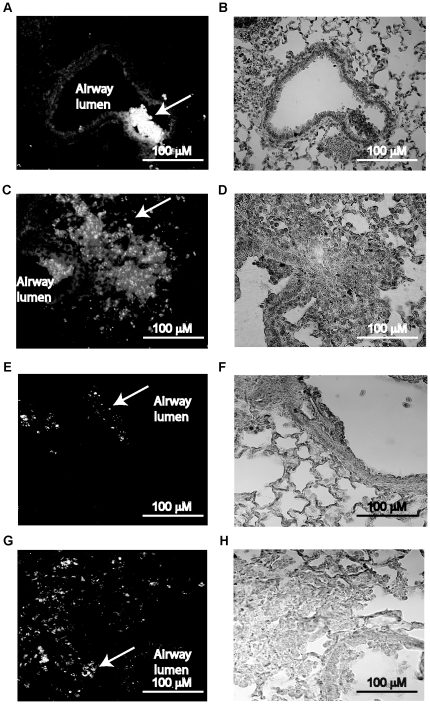
To assess the capacity of the BC7 cblA, BC7 cblS, and BC7 adhA mutants to establish infection beyond the conducting airways, we determined the distribution of bacteria in lungs of mice infected with these mutants using antibody to B. cenocepacia [23]. In contrast to mice infected with wild-type BC7 (Figure 3B), mice infected with BC7 adhA (Figure 9A and 9B), BC7 cblA (Figure 9C and 9D), or BC7 cblS (Figure 9E and 9F), showed bacteria mostly in the airway lumen and in the peribronchiolar area, but not in the respiratory zone associating with alveolar septa. On the other hand, mice infected with the BC7 cblS complemented strain showed bacteria in both airways and in respiratory zone (Figure 9G and 9H) as was observed with wild-type BC7, suggesting that restoring cable pili expression restores capacity of BC7 cblS mutant to spread into peripheral part of the lungs. Previously we have shown that CF patients, who are colonized with B. cenocepacia, often show bacteria in parenchyma [23]. We and others have also shown that B. cenocepacia strains are capable of invading epithelium as well as transmigrating across polarized airway epithelial cells via the paracellular route [23], [30], [31], [32], [33]. Taken together, our results suggest that the spread of infection to respiratory zone may depend on bacterial capacity to invade airway epithelium and to subvert host innate immune mechanisms. These studies also suggest that both cable pili and adhesin are required at least in part to persist and cause inflammation in vivo.
Figure 9. Immunolocalization of bacteria in mice infected with BC7 mutants or BC7 cblS complement.
Mice were infected with the BC7 adhA, BC7 cblA, or BC7 cblS mutants or the BC7 cblS complement suspended in alginate and sacrificed at 1 d post-infection. Paraffin lung sections from these mice were deparaffinized and incubated with antibody to B. cenocepacia. Bound antibody was detected by second antibody conjugated with AlexaFluor 488 and sections were counterstained with hematoxylin. Sections were viewed under fluorescence microscope. A, C, E and G shows bacteria in lung sections from mice infected with BC7 adhA, BC7 cblA, BC7 cblS, and the BC7 cblS complement, respectively. B, D, F and H are bright field images of A, C, E and G respectively. Arrows point to bacteria in the lung. Images are representative of three mice per group.
Conclusions
Our results demonstrated that alginate, a virulence factor produced by mucoid P. aeruginosa, prevents the initial clearance of B. cenocepacia by delaying the early innate immune defense mechanisms. This in turn facilitates persistence of those bacteria that possess the capacity to invade host mucosa and establish infection beyond the conducting airways. Further, we show that both cable pili and the associated 22 kDa adhesin protein are required for persistence and spreading the infection to respiratory zone. Both cable pili and the 22 kDa protein are also required for the stimulation of maximal IL-8 responses in vitro and inflammatory responses in vivo. Therefore, B. cenocepacia ET12 strains expressing both cable pili and the 22 kDa adhesin protein may cause persistent infection leading to severe inflammation in CF patients who are already colonized with P. aeruginosa.
Materials and Methods
Bacteria and growth conditions
B. cenocepacia isolate BC7, the BC7 adhA, BC7 cblA, and BC7 cblS mutants, and the BC7 cblS complemented strain have been described previously [11], [16]. All bacteria were maintained as glycerol stocks at −80°C and subcultured on brain heart infusion agar (BD Diagnostics, Franklin Lakes, NJ) and grown for 24 h at 37°C. BC7 mutant strains were cultured on Luria agar containing 1 mg/ml trimethoprim. The BC7 cblS complemented strain was grown overnight on Luria agar containing 1 mg/ml trimethoprim and 0.3 mg/ml tetracycline. A single colony was transferred to 10 ml tryptic soy broth (TSB, BD Diagnostics), TSB containing trimethoprim, or TSB containing trimethoprim and tetracycline, as appropriate, and grown overnight in a shaking incubator. Bacteria were harvested by centrifugation at 5,000×g, washed once with endotoxin-free PBS (Sigma-Aldrich, St. Louis, MO), and finally suspended in PBS or purified Pseudomonas alginate (prepared as described below) to the appropriate concentration based on OD600 (1 O.D. unit is equivalent to 1×109 CFU/ml). The actual concentration of bacteria in a suspension was determined by plating.
Cell cultures and infection
IB3 cells are immortalized CF airway epithelial cells and were obtained from Dr. P. Zeitlin (Johns Hopkins University School of Medicine, Baltimore) and were cultured in LHC-8 media (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum and L-glutamine. BEAS2B cells (American Type Culture Collection, Manassas, VA) are immortalized normal bronchial epithelial cells and cultured in bronchial epithelial cell growth media (Lonza, Walkersville, MD). For bacterial infection assays, cells were grown in 24-well cell culture dishes until 80% confluent. Cells were serum-starved overnight, infected with bacteria (at a multiplicity of infection (MOI) = 1.0), and the media was collected at 6 h post-infection, for determination of cytokines by ELISA (R & D Systems, Minneapolis, MN).
Isolation and purification of alginate
P. aeruginosa alginate was isolated as described previously from a clinical strain of mucoid P. aeruginosa, isolate NH57388A (kindly provided by Dr. N. Hoffmann (University of Copenhagen, Copenhagen, Denmark)) [12], [14], [15]. For infection of mice, bacteria were suspended in 1.8 mg/ml alginate and a 50 µl of this suspension (90 µg of alginate per mouse) was used.
Infection of animals
Ten to twelve week-old C57BL/6 mice were purchased from Charles River (Wilmington, MA) and maintained in a specific pathogen-free barrier facility in micro-isolator cages throughout the experiment. Mice were acclimatized for two weeks before infection. All the procedures performed on the mice were approved by the Animal Care and Use Committee of the University of Michigan (Approval Identification number 09527). Mice were anesthetized by intraperitoneal injection of xylazine (1 mg/kg body weight) and ketamine (50 mg/kg body weight) and infected with 50 µl of PBS, alginate (sham), or bacteria (5×106 CFU) suspended in either PBS (BC7/PBS) or alginate (BC7/alginate) by the intratracheal route, as described previously [14], [15]. For experiments with other strains, bacteria were suspended in alginate. Mice were sacrificed at predetermined times, as indicated, by intraperitoneal injection of 0.3 ml of 30% pentobarbital.
Lung bacterial load
Lungs were harvested aseptically and homogenized in sterile PBS. Ten-fold serial dilutions of lung homogenates were plated on B. cepacia isolation agar (BCSA) [34].
Analysis of lung cytokines and myeloperoxidase activity
Lung homogenates were prepared in the presence of protease inhibitors, centrifuged and supernatants were used in cytokine analysis by Bio-Plex multiplex immunoassay (Biorad, Hercules, CA). Lung myeloperoxidase (MPO) activity was quantified, as described previously [35].
Histopathology and immunolocalization of bacteria
Lungs were inflation fixed via the trachea with 10% formalin overnight and embedded in paraffin. Lung sections (5 µm thick) were stained with hematoxylin and eosin (H&E). Immunolocalization of bacteria in the lung sections was performed as described previously using antibody to B. cenocepacia, R418 [23].
Statistical Analysis
Results are expressed as mean ± SEM or a range of data with geometric mean. Data were analyzed by using SigmaStat statistical software (Systat Software, Inc., San Jose, CA). To compare groups, one-way analysis of variance (ANOVA) with Student's t test or Tukey-Kramer post-hoc analysis, or ANOVA on Ranks with Dunn's post-hoc analysis was performed, as appropriate. A ‘p’ value ≤0.05 was considered significant.
Acknowledgments
We thank Marisa Linn and Adam Goldsmith for their assistance in processing lung tissues for histology and Bio-Plex ELISA's, respectively.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by both the Cystic Fibrosis Foundation and the National Institute of Health: Cystic Fibrosis Foundation (USAJJAN08G0 to USS and GOLDBE07G0 to JBG; National Institutes of Health (AI072679 to JBG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mahenthiralingam E, Baldwin A, Dowson CG. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol. 2008;104:1539–1551. doi: 10.1111/j.1365-2672.2007.03706.x. [DOI] [PubMed] [Google Scholar]
- 2.Vanlaere E, Lipuma JJ, Baldwin A, Henry D, De Brandt E, et al. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int J Syst Evol Microbiol. 2008;58:1580–1590. doi: 10.1099/ijs.0.65634-0. [DOI] [PubMed] [Google Scholar]
- 3.Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3:144–156. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- 4.Isles A, Maclusky I, Corey M, Gold R, Prober C, et al. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 5.Sajjan US, Corey M, Karmali MA, Forstner JF. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992;89:648–656. doi: 10.1172/JCI115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sajjan US, Forstner JF. Role of a 22-kilodalton pilin protein in binding of Pseudomonas cepacia to buccal epithelial cells. Infect Immun. 1993;61:3157–3163. doi: 10.1128/iai.61.8.3157-3163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sajjan US, Hershenson MB, Forstner JF, LiPuma JJ. Burkholderia cenocepacia ET12 strain activates TNFR1 signalling in cystic fibrosis airway epithelial cells. Cell Microbiol. 2008;10:188–201. doi: 10.1111/j.1462-5822.2007.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajjan US, Sylvester FA, Forstner JF. Cable-piliated Burkholderia cepacia binds to cytokeratin 13 of epithelial cells. Infect Immun. 2000;68:1787–1795. doi: 10.1128/iai.68.4.1787-1795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sajjan U, Wu Y, Kent G, Forstner J. Preferential adherence of cable-piliated Burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. J Med Microbiol. 2000;49:875–885. doi: 10.1099/0022-1317-49-10-875. [DOI] [PubMed] [Google Scholar]
- 10.Roger P, Puchelle E, Bajolet-Laudinat O, Tournier JM, Debordeaux C, et al. Fibronectin and alpha5beta1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur Respir J. 1999;13:1301–1309. [PubMed] [Google Scholar]
- 11.Urban TA, Goldberg JB, Forstner JF, Sajjan US. Cable pili and the 22-kilodalton adhesin are required for Burkholderia cenocepacia binding to and transmigration across the squamous epithelium. Infect Immun. 2005;73:5426–5437. doi: 10.1128/IAI.73.9.5426-5437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chattoraj SS, Murthy R, Ganesan S, Goldberg JB, Zhao Y, et al. Pseudomonas aeruginosa alginate promotes Burkholderia cenocepacia persistence in cystic fibrosis transmembrane conductance regulator knockout mice. Infect Immun. 2010;78:984–993. doi: 10.1128/IAI.01192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, et al. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(-/-) mice. Antimicrob Agents Chemother. 2007;51:3677–3687. doi: 10.1128/AAC.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann N, Rasmussen TB, Jensen PO, Stub C, Hentzer M, et al. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun. 2005;73:2504–2514. doi: 10.1128/IAI.73.4.2504-2514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai WC, Hershenson MB, Zhou Y, Sajjan U. Azithromycin increases survival and reduces lung inflammation in cystic fibrosis mice. Inflamm Res. 2009;58:491–501. doi: 10.1007/s00011-009-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sajjan U, Ackerley C, Forstner J. Interaction of cblA/adhesin-positive Burkholderia cepacia with squamous epithelium. Cell Microbiol. 2002;4:73–86. doi: 10.1046/j.1462-5822.2002.00171.x. [DOI] [PubMed] [Google Scholar]
- 17.Tomich M, Mohr CD. Adherence and autoaggregation phenotypes of a Burkholderia cenocepacia cable pilus mutant. FEMS Microbiol Lett. 2003;228:287–297. doi: 10.1016/S0378-1097(03)00785-7. [DOI] [PubMed] [Google Scholar]
- 18.Tomich M, Mohr CD. Genetic characterization of a multicomponent signal transduction system controlling the expression of cable pili in Burkholderia cenocepacia. J Bacteriol. 2004;186:3826–3836. doi: 10.1128/JB.186.12.3826-3836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernier SP, Silo-Suh L, Woods DE, Ohman DE, Sokol PA. Comparative analysis of plant and animal models for characterization of Burkholderia cepacia virulence. Infect Immun. 2003;71:5306–5313. doi: 10.1128/IAI.71.9.5306-5313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cash HA, Woods DE, McCullough B, Johanson WG, Bass JA. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119:453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- 21.Cieri MV, Mayer-Hamblett N, Griffith A, Burns JL. Correlation between an in vitro invasion assay and a murine model of Burkholderia cepacia lung infection. Infect Immun. 2002;70:1081–1086. doi: 10.1128/IAI.70.3.1081-1086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Song Z, Hentzer M, Andersen JB, Heydorn A, et al. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology. 2000;146:2481–2493. doi: 10.1099/00221287-146-10-2481. [DOI] [PubMed] [Google Scholar]
- 23.Sajjan U, Corey M, Humar A, Tullis E, Cutz E, et al. Immunolocalisation of Burkholderia cepacia in the lungs of cystic fibrosis patients. J Med Microbiol. 2001;50:535–546. doi: 10.1099/0022-1317-50-6-535. [DOI] [PubMed] [Google Scholar]
- 24.Morissette C, Francoeur C, Darmond-Zwaig C, Gervais F. Lung phagocyte bactericidal function in strains of mice resistant and susceptible to Pseudomonas aeruginosa. Infect Immun. 1996;64:4984–4992. doi: 10.1128/iai.64.12.4984-4992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Park H, Li Z, Yang XO, Chang SH, Nurieva R, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, et al. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. Apmis. 2000;108:329–335. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 29.Song Z, Wu H, Ciofu O, Kong KF, Hoiby N, et al. Pseudomonas aeruginosa alginate is refractory to Th1 immune response and impedes host immune clearance in a mouse model of acute lung infection. J Med Microbiol. 2003;52:731–740. doi: 10.1099/jmm.0.05122-0. [DOI] [PubMed] [Google Scholar]
- 30.Martin DW, Mohr CD. Invasion and intracellular survival of Burkholderia cepacia. Infect Immun. 2000;68:24–29. doi: 10.1128/iai.68.1.24-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sajjan SU, Carmody LA, Gonzalez CF, LiPuma JJ. A type IV secretion system contributes to intracellular survival and replication of Burkholderia cenocepacia. Infect Immun. 2008;76:5447–5455. doi: 10.1128/IAI.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajjan US, Yang JH, Hershenson MB, LiPuma JJ. Intracellular trafficking and replication of Burkholderia cenocepacia in human cystic fibrosis airway epithelial cells. Cell Microbiol. 2006;8:1456–1466. doi: 10.1111/j.1462-5822.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 33.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 34.Henry DA, Campbell ME, LiPuma JJ, Speert DP. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J Clin Microbiol. 1997;35:614–619. doi: 10.1128/jcm.35.3.614-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai WC, Strieter RM, Wilkowski JM, Bucknell KA, Burdick MD, et al. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol. 1998;161:2435–2440. [PubMed] [Google Scholar]