Abstract
Human endothelial cells derived from umbilical cord blood (hCB-ECs) represent a promising cell source for endothelialization of tissue engineered blood vessels. hCB-ECs cultured directly above human aortic smooth muscle cells (SMCs), which models native and tissue engineered blood vessels, produce a confluent endothelium that responds to flow like normal human aortic endothelial cells (HAECs). The objective of this study was to quantify the elastic modulus of hCB-ECs cocultured with SMCs under static and flow conditions using atomic force microscopy (AFM). Cytoskeleton structures were assessed by AFM cell surface imaging and immunofluorescence of F-actin. The elastic moduli of hCB-ECs and HAECs were similar and significantly smaller than the value for SMCs in monoculture under static conditions (p<0.05). In coculture, hCB-ECs and HAECs became significantly stiffer with moduli 160 – 180% larger than their corresponding values in monoculture. While the moduli of hCB-ECs and HAECs almost doubled in monoculture and flow condition, their corresponding values in coculture declined after exposure to flow. Both the number and diameter of cortical stress fiber per cell width increased in coculture and/or flow conditions, whereas the subcortical stress fiber density throughout the cell interior increased by a smaller amount. These findings indicate that changes to biomechanical properties in coculture and/or exposure to flow are correlated with changes in the cortical stress fiber density. For ECs, fluid shear stress appeared to have greater effect on the elastic modulus than the presence of SMCs and changes to the elastic modulus in coculture may be due to EC-SMC communication.
Keywords: endothelial progenitor cells, smooth muscle cells, mechanics, atomic force microscopy, cytoskeleton, stress fiber
1. Introduction
Endothelial progenitor cells (EPCs), which can be easily isolated from peripheral or umbilical cord blood and expanded (Ingram et al., 2004), represent a promising source of endothelial cells (ECs) for vascular regeneration and tissue engineering (Kaushal et al., 2001; Schmidt et al., 2004; Melero-Martin et al., 2008). ECs derived from late outgrowth umbilical cord blood EPCs (hCB-ECs) have a higher proliferative potential than peripheral blood-derived EPCs and early outgrowth cord blood-derived EPCs (Ingram et al., 2004; Yoder et al., 2007). hCB-ECs express EC-specific markers such as platelet endothelial cell adhesion molecule (PECAM), von Willebrand Factor and VE-cadherin (Brown et al., 2009). Unlike early outgrowth EPCs, hCB-ECs do not express cell surface markers found on blood monocytes and macrophages (Yoder et al., 2007; Brown et al., 2009).
We found that following exposure to 15 dyne/cm2 for 48 hours, hCB-ECs oriented and elongated in the direction of flow, and expressed similar numbers of α5β1 and αvβ3 integrins as well as antithrombotic and anti-inflammatory genes compared to human aortic ECs (HAECs) (Brown et al., 2009). When injected intravenously in immune compromised mice, hCB-ECs accelerated vein graft reendothelialization and prevented vein graft thrombosis (Brown et al., 2010).
In native blood vessels, the biomechanical environment of ECs and smooth muscle cells (SMCs) influences their biological responses and likely regulates key functions of engineered blood vessels (Davies, 1995; Nerem, 2003; Li et al., 2005; Haga et al., 2007; Kliche et al., 2011). ECs convert the shear stress resulting from blood flow into intracellular signals that affect gene expression and cellular function such as proliferation, apoptosis, migration, permeability, cell alignment and mechanical properties. ECs’ mechanical properties depend on the cytoskeletal structures, vary over different regions of cells and are increased after exposure to flow (Sato et al., 2000; Mathur et al., 2001). Using a direct coculture of ECs on SMCs in which ECs or EPCs form a confluent monolayer on extracellular matrix produced by quiescent smooth muscle cells (SMCs) (Lavender et al., 2005; Wallace et al., 2007a; Brown et al., 2009), ECs lacked focal adhesions and had a reduced spreading rate compared with ECs adhered to polyacrylamide gels of similar stiffness as the underlying SMCs (Wallace et al., 2007b). Further, interactions between α5β1-integrin and fibrillar FN on the surface of SMCs induced fibrillar adhesions rich in tensin and led to a reduction in focal adhesion protein expression (Wallace et al., 2007b).
Given the role of cell stiffness on focal adhesion formation (Na et al., 2008) and the absence of focal adhesions by ECs cultured on SMCs, we used hCB-ECs and HAECs to test the hypothesis that, relative to cells grown in monoculture under static conditions, the mechanical properties of ECs are affected by coculture with SMCs under static and flow conditions, and that changes to the cell elastic modulus are due to alterations in stress fibers in ECs.
2. Materials and methods (Expanded Methods are provided in the Supplementary Materials)
2.1 Cell culture and flow experiments
hCB-ECs were isolated from late outgrowth EPCs in umbilical cord blood samples (Ingram et al., 2004) (Carolina Cord Blood Bank, Duke University) per protocols approved by the Duke University Institutional Review Board. Cocultures were formed by plating HAECs or hCB-ECs on quiescent SMCs at a density of 80,000 – 100,000 cells/cm2 and incubating with coculture medium (EBM-2 supplemented with 3.3% of FBS, Lonza) for 2 days before testing (Brown et al., 2009). EC confluence of was verified using PECAM (Invitrogen) staining of EC junctions (Figure 1 a&b).
Figure 1.
Photographs of immunofluorescence of PECAM show the confluent layer of hCB-ECs (a) or HAECs (c) in coculture with SMCs. Light microscopy images illustrating examples of AFM testing of hCB-EC (b) and HAEC (d) in coculture. The boundaries of the cells (as outlined) can be identified by moving images during the experiment. The AFM cantilever is also shown. Scale bars = 50 µm.
The slides with cells in monoculture or coculture were carefully assembled into a parallel plate flow chamber and exposed to a steady wall shear stress of 15 dyne/cm2 for 48 hours before AFM mechanical testing or immunofluoresence. At the same time, control samples were maintained under static conditions in the incubator for 48 hours. All static and flow experiments were performed with flow medium (DMEM/F12 with 15 mM HEPES, 3.3% FBS, and 1× ITS). hCB-ECs were used at passages 4–11, and HAECs and SMCs were used at passages 8–11 for all experiments.
2.2 AFM indentation testing, cell surface imaging and cortical stress fiber quantification
All biomechanical testing and cell surface imaging were performed at room temperature and atmospheric CO2 using atomic force microscopy (AFM; MFP-3D, Asylum Research, Santa Barbara, CA) under contact mode (Figure 1 c&d). The cells were maintained in flow medium which contains 15 mM HEPES to keep a constant pH during the AFM experiment.
2.3 PECAM and F-actin immunofluorescence and quantification
After labeling for PECAM and F-actin as described in the Supplementary Materials, images were obtained using a confocal laser scanning microscope (LSM 510, Carl Zeiss Inc., Thornwood, NY). The stress fiber density and colocalization with PECAM were quantified by a custom MATLAB program.
2.5 Statistical analysis
The variations of measurements among different groups were tested by one-factor or two-factor analysis of variance (ANOVA and post hoc Tukey test) using R Statistics (version 2.10.0) with statistical significance reported at the 95% confidence level. Data were presented as mean ± SEM.
2 Results
When cultured alone on plastic under static conditions, the elastic moduli of hCB-ECs and HAECs were 0.96±0.04 KPa (Mean ± SEM, n=55) and 1.27±0.08 KPa (n=57), respectively, significantly lower than the modulus of SMCs (2.17±0.14 KPa, n=48, Figure 2a, p<0.05). In coculture, the moduli of hCB-ECs and HAECs increased to more than 160% – 180% of the corresponding values in monoculture (p<0.00001). After exposure to physiological shear stress for 48 hours, both HAECs and hCB-ECs elongated and oriented in the direction of flow in a similar manner for both monoculture and coculture conditions (Supplementary Figure 1). Further, the elastic modulus of hCB-ECs and HAECs in monoculture almost doubled compared to corresponding values under static conditions (Figure 2b, p<0.00001). In contrast, the moduli of the ECs in coculture were lower after exposure to flow relative to values under static conditions (p<0.01). No difference was found for either cell type between monoculture and coculture groups under flow condition (p>0.05). Comparing hCB-ECs with HAECs, the modulus of hCB-ECs was ~ 25% lower than that of HAECs under monoculture and static condition (p<0.05), and coculture and static or flow condition (p<0.05), but only ~ 10% lower under monoculture and flow condition (p>0.05).
Figure 2.
Elastic moduli of hCB-ECs and HAECs in monoculture or coculture exposed to static (a) or flow (b) conditions. The moduli of hCB-ECs and HAECs in coculture are significantly higher than their corresponding values in monoculture under static conditions (^p<0.00001, ANOVA and post hoc Tukey test). In monoculture, SMCs are stiffer than hCB-ECs and HAECs (#p<0.005). The moduli of hCB-ECs and HAECs in monoculture under flow condition were doubled compared to values under static condition (& p<0.00001). Interestingly, the moduli of both cell types in coculture after exposure to 15 dyne/cm2 for 48 hours were lower than those under static conditions (*p<0.01). The elastic moduli of hCB-ECs were lower than those of HAECs in both monoculture and coculture under static or flow conditions (†p<0.05, ‡p<0.05).
The normalized frequency histograms of elastic modulus values were well fit by a lognormal distribution (Figure 3). Compared to ECs in monoculture under static conditions, the peak modulus (or mean of the lognormal distribution) for ECs in coculture shifted towards higher values (Figure 3, a&b). The distribution of the modulus of ECs in co-culture was much broader than in monoculture and this was reflected in an increase in the variance of the lognormal distribution. Relative to static conditions, flow caused some broadening of the distribution for cells in monoculture and some decrease in the variability of the distribution for ECs in coculture. The distributions of EC in monoculture and coculture under flow conditions were similar (Figure 3, c&d).
Figure 3.
Normalized frequency histograms of elastic modulus with superposed lognormal distribution curves for each cell population. The fitted parameters (μ, mean; σ, standard deviation) on a logarithmized scale for each curve are also listed. Modulus distributions of hCB-ECs (a&c) and HAECs (b&d) showed a shift towards higher peak values from monoculture to coculture under static condition (a&b), but not seen under flow condition (c&d). Both cell types exhibited narrow distributions for the moduli in monoculture and static condition, but broader distributions in coculture and/or flow conditions.
AFM deflection images revealed cortical actin filaments on the cell apical surface of hCB-ECs and HAECs in confluent monoculture and coculture exposed to static or flow conditions (Figure 4). ECs displayed complex organized networks, showing long stress fibers and short branching filaments. The number of cortical stress fibers per cell width increased from monoculture to coculture under static condition for both types of ECs (Figure 5, p<0.005), and after exposure to flow (p<0.05). The diameter of cortical stress fibers was ~ 60% greater for ECs in coculture relative to monoculture under static condition (p<0.00001). Exposure of ECs to flow for 48 hours reduced the diameter ~ 20% under flow conditions (p<0.001). HAECs and hCB-ECs showed similar trends in the quantitative measurements of number of cortical stress fibers per cell width and size and distribution of cortical stress fibers. (Table 1)
Figure 4.
Representative AFM deflection images of living cells showing abundant cortical stress fibers on the cell apical surface under different conditions for hCB-ECs and HAECs. Increased number and diameter of cortical stress fibers were seen for cells in coculture a nd static condition and all flow conditions. Arrows represent flow direction.
Figure 5.
Quantification of number of cortical stress fiber per cell width and its projected diameter from AFM deflection images (a–c), showing significant actin cytoskeleton changes on the cell surface under coculture and/or flow condition (Table 1). The number of cortical stress fiber per cell width increased 30 – 65% for cells in coculture and static condition, compared to cells in monoculture and static condition, and 30 – 50% more after exposure to flow. The diameter of cortical stress fibers also increased ~ 60% in coculture and static condition, yet was reduced under flow conditions for ECs in coculture.
Table 1.
Number of cortical stress fiber per cell width and its diameter for endothelial cells in monoculture and coculture after exposure to static or flow condition
| Static |
Flow |
|||||||
|---|---|---|---|---|---|---|---|---|
| Monoculture |
Coculture |
Monoculture |
Coculture |
|||||
| hCB-EC | HAEC | hCB-EC | HAEC | hCB-EC | HAEC | hCB-EC | HAEC | |
| Number of cortical stress fiber/cell width |
8.9±0.6 (n=8) |
11.4±0.6 (n=9) |
14.9±1.0a (n=8) |
14.6±0.5a (n=12) |
19.9±1.2a,c (n=7) |
18.5±1.0a,c (n=6) |
18.2±0.5b,d (n=6) |
18.2±0.8b,c (n=10) |
| Cortical stress fiber diameter (µm) |
0.33±0.03 | 0.35±0.02 | 0.54±0.05b | 0.51±0.04b | 0.46±0.03b,c | 0.44±0.05b,d | 0.45±0.03b,c | 0.47±0.03b |
Entries are mean ± SEM. n, number of cells analyzed.
ap<0.005 and bp<0.00001, compared to corresponding cells in monoculture and static condition, ANOVA and post hoc Tukey test.
cp<0.001 and dp<0.05, compared to corresponding cells in coculture and static condition.
Imaging actin filaments in the apical regions of the cell by confocal microscopy (not shown) suggested that actin represents the cortical stress fibers observed with AFM, as noted previously (Pesen and Hoh, 2005). Cytoskeletal structures in the deeper, subcortical region of the hCB-ECs were revealed by confocal microscopy of F-actin and PECAM (Figure 6), showing alignment of actin filaments with the direction of flow after exposure to 48-hour steady laminar flow, which was consistent with cell alignment (Supplementary Figure 1). Results with HAECs were similar.
Figure 6.
Immunofluorescent images of F-actin in the interior of hCB-ECs showing increased stress fiber density in coculture (b&d) or under flow conditions (c&d), compared to cells in monoculture and static condition (a). More stress fibers were seen across the cell body in coculture and/or flow conditions while most of them located at the periphery of the cell in monoculture and static condition. F-actin alignment to the flow direction was also observed. Arrows represent flow directions. Scale bars = 25 µm.
The stress fiber area fraction of hCB-ECs and HAECs was 10 – 15% greater in coculture or after exposure to flow than that in monoculture and static conditions (Figure 7a, p<0.005 for hCB-ECs; p<0.05 for HAECs). The F-actin colocalized with PECAM in these groups was 20% less (Figure 6b, p<0.0001 for hCB-ECs; p<0.05 for HAECs), indicating increased stress fibers were located across the cell body instead of cell periphery. Cell spreading area decreased ~ 20% in coculture, compared to that in monoculture (Figure 7c, p<0.0001), yet no difference was found when comparing static to flow conditions. Overall, EC height decreased with flow to a similar extent as observed previously (Barbee et al., 1995); however, this change in height was less than the cell area change (AFM height images not shown).
Figure 7.
(a) Stress fiber area fraction for both hCB-ECs and HAECs increased 10 – 15% in coculture and/or flow conditions, compared to cells in monoculture and static condition. (b) F-actin staining co-localized with PECAM in monoculture and static conditions was ~ 20% higher than that in coculture and/or flow condition, indicating increased stress fibers across the cell body. (c) Cell areas outlined by PECAM staining decreased in coculture for both hCB-ECs and HAECs, indicating less cell spreading in coculture environment, yet no difference was seen when comparing static to flow conditions. ^p<0.0001, #p<0.005, *p<0.05, ANOVA and post hoc Tukey test. Data are presented as Mean ± SEM, n=15 – 19 images per group for (a&b) and n=80 – 110 cells per group for (c).
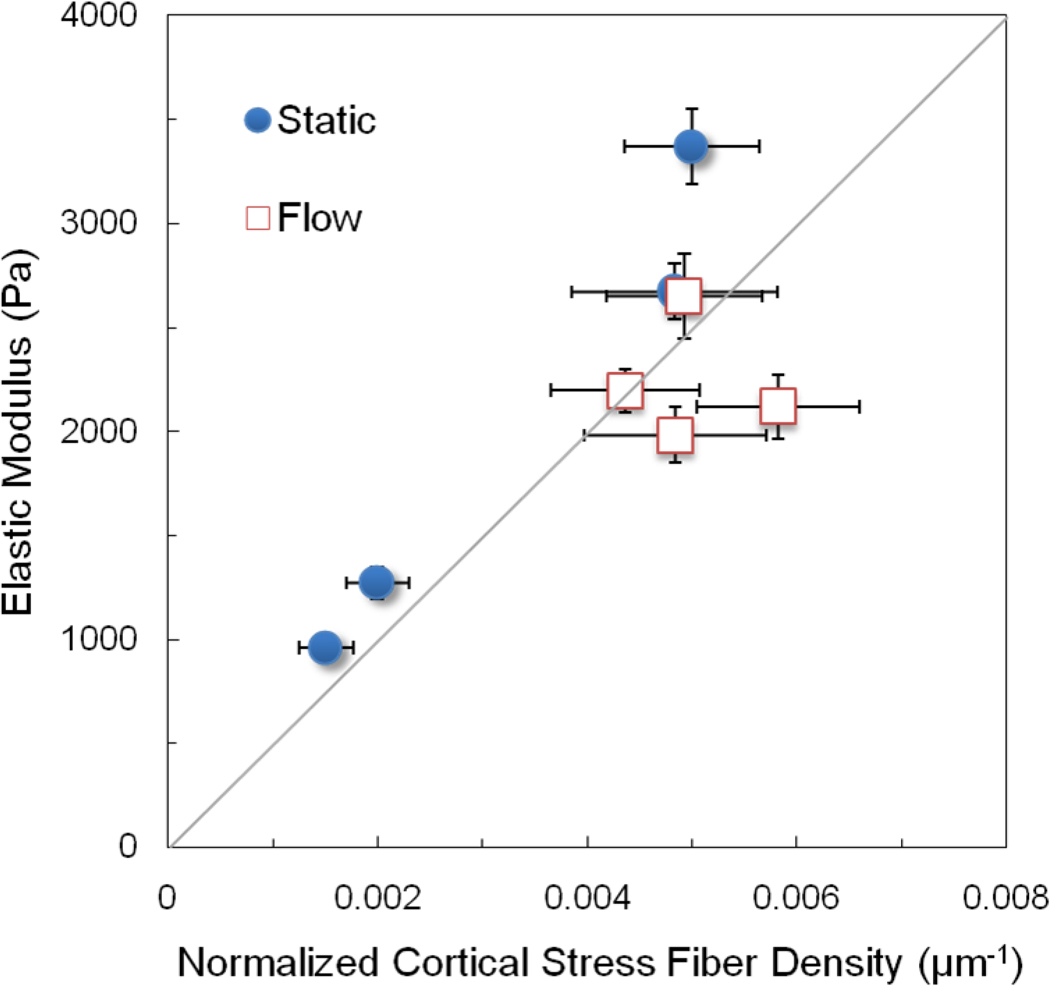
To further assess the relative contribution of cortical actin filaments to changes in the modulus with coculture and flow, the elastic modulus data in Figure 2 was plotted versus the normalized cortical stress fiber density - the number of cortical stress fibers per cell width times the fiber diameter and divided by the cell area from Figure 7, which normalizes for differences in cell size among the various conditions. The data fall into two clusters (Figure 8). ECs in monoculture under static conditions have a lower elastic modulus and fewer and thinner fibers. Flow and coculture produce an increase in the normalized cortical stress fiber density in proportion to the change in elastic modulus as denoted by the line at 45 degrees in Figure 8. Such a result suggests that the change in elastic modulus due to coculture and flow is due to changes in the density of cortical actin.
Figure 8.
The elastic modulus is plotted against the normalized cortical stress fiber density, which equals the product of the number of cortical stress fibers per cell width times the stress fiber diameter and divided by the projected cell area. Values for the elastic modulus are reported in Figure 1, the number of cortical stress fibers per cell width and the stress fiber diameter are reported in Figure 5 and the projected cell area is reported in Figure 7. The line represents a direct proportionality between the elastic modulus and normalized cortical stress fiber density.
3 Discussion
The findings of this study provide the first quantitative data on the elastic modulus of ECs when cultured directly on quiescent smooth muscle cells. The average elastic modulus of hCB-ECs was slightly lower than values for HAECs and SMCs in monoculture and static condition. Surprisingly, hCB-ECs and HAECs became stiffer when cocultured on the SMC layer (Figures 2 and 3). Many studies have shown that cell stiffness matches the soft substrate stiffness within a range of stiffness spanning that of soft tissues (Solon et al., 2007; Tee et al., 2011). Although ECs on the passive substrate in monoculture do secrete and remodel an extracellular matrix, their mechanical properties are largely influenced by the substrate (Byfield et al., 2009). SMCs are much softer than the plastic substrate in monoculture; therefore we expected that the elastic modulus of hCB-ECs and HAECs in coculture would be softer than when cultured on plastic or similar to the SMC modulus. The new findings here suggest that the biomechanical responses of ECs cultured on SMCs are quite different from ECs cultured on passive soft substrates, consistent with our previous study (Wallace et al., 2007b) in which we found that EC spreading and focal adhesion formation on SMCs differed significantly from ECs on polyacrylamide gels of the small elastic modulus.
After exposure to 48 hour laminar flow, the moduli of hCB-ECs and HAECs in monoculture increased, as expected (Mathur et al., 2007; Sato et al., 2007). Interestingly, the modulus of the ECs in coculture under flow was similar to the value of ECs on plastic after exposure to flow. This result suggests that the effect of flow is the dominant biomechanical factor affecting ECs cocultured with SMCs. Further, human ECs cocultured with SMCs form fibrillar adhesions instead of focal adhesions (Wallace et al., 2007a) and, after exposure to flow, focal adhesions are not observed on ECs cultured on SMCs (Wallace, 2008). Fibrillar adhesions are rich in tensin which binds to the ends of actin filaments. When localized to fibrillar adhesions, tensin links actin to fibrillar fibronectin in the matrix permitting force transmission (Pankov et al., 2000). The current results suggest that fibrillar adhesions do not limit cortical stress fiber formation or cell elastic modulus (Figures 6 and 7) and can adapt so that cell orientation can respond dynamically to flow like ECs that form focal adhesions on rigid substrates.
Actin cytoskeleton is one of the major contributors to the cell’s mechanical properties (Janmey and McCulloch, 2007; Fletcher and Mullins, 2010). The average number of cortical stress fiber per cell width for hCB-ECs and HAECs (Figure 5) is similar to what was previously reported (Frame and Sarelius, 2000). In response to shear stress, ECs rapidly adapt their F-actin networks (Franke et al., 1984; Satcher et al., 1997). Flow caused increased the number and diameter of cortical stress fiber per cell width (Figure 5) in both monoculture and coculture. The product of the number and size of cortical stress fibers per cell area changed in proportion to changes in the elastic modulus (Figure 8). The absence of significant changes in the incremental modulus with indentation depth (Supplementary Figure 2) and small, but statistically significant redistribution of subcortical stress fibers (Figure 7) support a primary role for cortical actin.
Flow or coculture alone or together leads to more variability in the distribution of the elastic modulus among the cell populations. The heterogeneous distribution of elastic modulus after exposure to flow may arise from the shape of the ECs, with the nucleus protruding into the flow stream producing a nonuniform distribution of shear stress over the cell (Barbee et al., 1995). In coculture, the SMC substrate is not flat like the rigid plastic substrate. Under static conditions, ECs align with the local orientation of the SMCs (Wallace et al., 2007a). Increased heterogeneity in the value of the modulus may reflect a combination of the variability of the modulus of individual SMCs, the variation in SMC orientation and height, and the local positioning of the ECs relative to individual SMCs.
Coculture alone or with shear stress elicited changes on the apical surface and throughout the cell interior, possibly due to close cell-cell and cell-matrix interaction between two cell types. ECs release vasodilators such as nitric oxide and prostacyclin, and vasoconstrictors such as endothelin which act on SMCs to control smooth muscle tone and SMC growth (Little et al., 2008; Mitchell et al., 2008). SMCs are also able to respond to the ECs by altering gene expression or collagen synthesis of the extracellular matrix proteins, such as collagen types I and IV and fibronectin (Powell et al., 1997). In direct contact coculture, the confluent and quiescent SMCs remain a nonproliferating and more contractile phenotype with increased calponin expression (Lavender et al., 2005; Wallace et al., 2007a). Therefore, EC–SMC interactions lead to reorganization of the extracellular matrix between the ECs and SMCs and may influence EC signaling mechanisms leading to cytoskeletal structure changes. Candidate signaling pathways include prostacylcin release by ECs (Fetalvero et al., 2006) and rapamycin (mTOR) activity by SMCs (Martin et al., 2004). In monoculture, shear stresses upregulate mTOR pathway in ECs but not SMCs whereas SMCs mTOR signaling is not enhanced by flow, but is upregulated by growth factors (Balcells et al., 2010). However, in coculture, mTOR on ECs or SMCs is not activated which may promote the differentiated state of SMCs and influence RhoA activity thereby influencing stress fiber formation.
The phenotypic state of the ECs may affect their biomechanical behavior. When ECs are cocultured with proliferative SMCs on the opposite side of a porous membrane, the coculture of SMC under static condition causes upregulation of EC proinflammatory gene expressions (Chiu et al., 2005). Application of shear stress largely inhibits this inflammatory effect. TNF-α treatment of human pulmonary microvascular ECs caused rearrangement of the actin cytoskeleton and a reduction in the mechanical properties of the ECs (Kang et al., 2008) suggesting that the elastic modulus is sensitive to the inflammatory state of ECs. In our direct contact coculture model, instead, quiescent SMCs reduce the TNF-α-mediated EC inflammatory response (Wallace and Truskey, 2010) and ECs exhibit a number of antithrombotic qualities such as increased gene expression of nitric oxide synthase, prostacyclin and ET-1 (Brown et al., 2009). No upregulation of ICAM-1, VCAM-1 or E-selectin is observed in coculture with quiescent SMCs (Brown et al., 2009).
Mechanical properties of ECs directly impact biological responses (Janmey and McCulloch, 2007; Fletcher and Mullins, 2010; Kliche et al., 2011). For example, in the presence of aldosterone, plasma sodium in the high physiological range increases EC stiffness 20% to 40% and the EC stiffening counteracts the endothelial formation of nitric oxide and thus undermines the ability of ECs to trigger vasodilation (Oberleithner et al., 2007), while an acute increase of potassium in the physiological range does the opposite (Oberleithner et al., 2009). One recent study also showed that C-reactive protein, an inflammatory marker of cardiovascular diseases, enhances the effect of aldosterone on the EC stiffness, highlighting the important role of EC stiffening in the inflammatory and vascular disease (Kusche-Vihrog et al., 2011).
Finally, hCB-ECs and HAECs had similar projected areas, patterns of PECAM and F-actin staining, alignment to flow direction during monoculture and coculture, as well as similar trend of elastic modulus changes in response to coculture and/or flow, pointing to large similarity between the two cell types. However, hCB-ECs were 10 – 25% softer than HAECs under all conditions in our study. One study has shown that an increase of C-reactive protein concentration from physiological level to inflammatory level leads to ~ 30% of EC stiffness increase (Kusche-Vihrog et al., 2011). This may indicate some functional differences between hCB-ECs and HAECs.
In conclusion, our findings show that coculture and/or flow have significant effects on biomechanical behavior of ECs. These findings provide new insight in cell-cell and cell-matrix interactions in models that mimics native or engineered blood vessels under physiological shear stress.
Research Highlights.
Examined effect of smooth muscle coculture on endothelial cell (EC) elastic modulus.
SMC coculture increased EC elastic moduli, which declined after exposure to flow.
Elastic moduli in coculture or exposure to flow correlated with actin fiber density.
Fluid shear stress had greater effect on EC elastic modulus than presence of SMCs.
Biomechanical response differs for ECs cultured on SMCs or synthetic substrates.
Supplementary Material
Acknowledgement
This work was supported by NIH grant HL-088825 and American Heart Association Postdoctoral Fellowship 10POST3870046 to L.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All three authors declare that they do not have any financial and personal relationships with other people and organizations that could inappropriately influence our work on this manuscript.
References
- Balcells M, Martorell J, Olive C, Santacana M, Chitalia V, Cardoso AA, Edelman ER. Smooth muscle cells orchestrate the endothelial cell response to flow and injury. Circulation. 2010;121:2192–2199. doi: 10.1161/CIRCULATIONAHA.109.877282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee KA, Mundel T, Lal R, Davies PF. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am J Physiol. 1995;268:H1765–H1772. doi: 10.1152/ajpheart.1995.268.4.H1765. [DOI] [PubMed] [Google Scholar]
- Brown MA, Wallace CS, Angelos M, Truskey GA. Characterization of Umbilical Cord Blood Derived Late Outgrowth Endothelial Progenitor Cells Exposed to Laminar Shear Stress. Tissue Eng Part A. 2009;15:3575–3587. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Zhang L, Levering VW, Wu JH, Satterwhite LL, Brian L, Freedman NJ, Truskey GA. Human Umbilical Cord Blood-Derived Endothelial Cells Reendothelialize Vein Grafts and Prevent Thrombosis. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield FJ, Reen RK, Shentu TP, Levitan I, Gooch KJ. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech. 2009;42:1114–1119. doi: 10.1016/j.jbiomech.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JJ, Chen LJ, Chang SF, Lee PL, Lee CI, Tsai MC, Lee DY, Hsieh HP, Usami S, Chien S. Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: role of NF-kappaB. Arterioscler Thromb Vasc Biol. 2005;25:963–969. doi: 10.1161/01.ATV.0000159703.43374.19. [DOI] [PubMed] [Google Scholar]
- Costa KD, Yin FC. Analysis of indentation: implications for measuring mechanical properties with atomic force microscopy. J Biomech Eng. 1999;121:462–471. doi: 10.1115/1.2835074. [DOI] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetalvero KM, Shyu M, Nomikos AP, Chiu YF, Wagner RJ, Powell RJ, Hwa J, Martin KA. The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol. 2006;290:H1337–H1346. doi: 10.1152/ajpheart.00936.2005. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame MD, Sarelius IH. Flow-induced cytoskeletal changes in endothelial cells growing on curved surfaces. Microcirculation. 2000;7:419–427. [PubMed] [Google Scholar]
- Franke RP, Grafe M, Schnittler H, Seiffge D, Mittermayer C, Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984;307:648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–960. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Hutter JL, Bechhoefer J. Calibration of Atomic-Force Microscope Tips. Rev Sci Instrum. 1993;64:1868–1873. [Google Scholar]
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- Kang I, Panneerselvam D, Panoskaltsis VP, Eppell SJ, Marchant RE, Doerschuk CM. Changes in the Hyperelastic Properties of Endothelial Cells Induced by Tumor Necrosis Factor-α. Biophys J. 2008;94:3273–3285. doi: 10.1529/biophysj.106.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliche K, Jeggle P, Pavenstadt H, Oberleithner H. Role of cellular mechanics in the function and life span of vascular endothelium. Pflugers Arch. 2011 doi: 10.1007/s00424-011-0929-2. [DOI] [PubMed] [Google Scholar]
- Kusche-Vihrog K, Urbanova K, Blanque A, Wilhelmi M, Schillers H, Kliche K, Pavenstadt H, Brand E, Oberleithner H. C-reactive protein makes human endothelium stiff and tight. Hypertension. 2011;57:231–237. doi: 10.1161/HYPERTENSIONAHA.110.163444. [DOI] [PubMed] [Google Scholar]
- Lavender MD, Pang Z, Wallace CS, Niklason LE, Truskey GA. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials. 2005;26:4642–4653. doi: 10.1016/j.biomaterials.2004.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Little PJ, Ivey ME, Osman N. Endothelin-1 actions on vascular smooth muscle cell functions as a target for the prevention of atherosclerosis. Curr Vasc Pharmacol. 2008;6:195–203. doi: 10.2174/157016108784911966. [DOI] [PubMed] [Google Scholar]
- Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- Mathur AB, Collinsworth AM, Reichert WM, Kraus WE, Truskey GA. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J Biomech. 2001;34:1545–1553. doi: 10.1016/s0021-9290(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Mathur AB, Reichert WM, Truskey GA. Flow and high affinity binding affect the elastic modulus of the nucleus, cell body and the stress fibers of endothelial cells. Ann Biomed Eng. 2007;35:1120–1130. doi: 10.1007/s10439-007-9288-8. [DOI] [PubMed] [Google Scholar]
- Mathur AB, Truskey GA, Reichert WM. Atomic force and total internal reflection fluorescence microscopy for the study of force transmission in endothelial cells. Biophys J. 2000;78:1725–1735. doi: 10.1016/s0006-3495(00)76724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93:141–147. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- Na S, Trache A, Trzeciakowski J, Sun Z, Meininger GA, Humphrey JD. Time-dependent changes in smooth muscle cell stiffness and focal adhesion area in response to cyclic equibiaxial stretch. Ann Biomed Eng. 2008;36:369–380. doi: 10.1007/s10439-008-9438-7. [DOI] [PubMed] [Google Scholar]
- Nerem RM. Role of mechanics in vascular tissue engineering. Biorheology. 2003;40:281–287. [PubMed] [Google Scholar]
- Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmuller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci U S A. 2009;106:2829–2834. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104:16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesen D, Hoh JH. Micromechanical architecture of the endothelial cell cortex. Biophys J. 2005;88:670–679. doi: 10.1529/biophysj.104.049965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell RJ, Hydowski J, Frank O, Bhargava J, Sumpio BE. Endothelial cell effect on smooth muscle cell collagen synthesis. J Surg Res. 1997;69:113–118. doi: 10.1006/jsre.1997.5045. [DOI] [PubMed] [Google Scholar]
- Satcher R, Dewey CF, Jr, Hartwig JH. Mechanical remodeling of the endothelial surface and actin cytoskeleton induced by fluid flow. Microcirculation. 1997;4:439–453. doi: 10.3109/10739689709146808. [DOI] [PubMed] [Google Scholar]
- Sato M, Nagayama K, Kataoka N, Sasaki M, Hane K. Local mechanical properties measured by atomic force microscopy for cultured bovine endothelial cells exposed to shear stress. J Biomech. 2000;33:127–135. doi: 10.1016/s0021-9290(99)00178-5. [DOI] [PubMed] [Google Scholar]
- Sato M, Suzuki K, Ueki Y, Ohashi T. Microelastic mapping of living endothelial cells exposed to shear stress in relation to three-dimensional distribution of actin filaments. Acta Biomater. 2007;3:311–319. doi: 10.1016/j.actbio.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Breymann C, Weber A, Guenter CI, Neuenschwander S, Zund G, Turina M, Hoerstrup SP. Umbilical cord blood derived endothelial progenitor cells for tissue engineering of vascular grafts. Ann Thorac Surg. 2004;78:2094–2098. doi: 10.1016/j.athoracsur.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee SY, Fu J, Chen CS, Janmey PA. Cell shape and substrate rigidity both regulate cell stiffness. Biophys J. 2011;100:L25–L27. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CS. Biomedical Engineering. Durham: Duke University; 2008. Endothelial cell adhesion and function when co-cultured with smooth muscle cells. [DOI] [PubMed] [Google Scholar]
- Wallace CS, Champion JC, Truskey GA. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann Biomed Eng. 2007a;35:375–386. doi: 10.1007/s10439-006-9230-5. [DOI] [PubMed] [Google Scholar]
- Wallace CS, Strike SA, Truskey GA. Smooth muscle cell rigidity and extracellular matrix organization influence endothelial cell spreading and adhesion formation in coculture. Am J Physiol Heart Circ Physiol. 2007b;293:H1978–H1986. doi: 10.1152/ajpheart.00618.2007. [DOI] [PubMed] [Google Scholar]
- Wallace CS, Truskey GA. Direct-contact co-culture between smooth muscle and endothelial cells inhibits TNF-alpha-mediated endothelial cell activation. Am J Physiol Heart Circ Physiol. 2010;299:H338–H346. doi: 10.1152/ajpheart.01029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.