Abstract
Introduction
Epidemiological studies have shown that moderate alcohol drinkers have a lower death rate for all causes. Alcohol drinking has also been associated with reduced risk of non-Hodgkin lymphoma (NHL). Here, we examined the role of alcohol consumption on NHL survival by type of alcohol consumed and NHL subtype.
Methods
A cohort of 575 female NHL incident cases diagnosed during 1996–2000 in Connecticut was followed-up for a median of 7.75 years. Demographic, clinical, and lifestyle information was collected at diagnosis. Survival analyses were conducted with Kaplan-Meier methods, and hazard ratios (HR) were estimated from Cox Proportional Hazards models.
Results
Compared to never drinkers, wine drinkers experienced better overall survival (75% vs. 69% five-year survival rates, p-value for log-rank test=0.030) and better disease free survival (70% vs. 67% five-year disease-free survival rates, p-value for log-rank test=0.049). Analysis by NHL subtype shows that the favorable effect of wine consumption was mainly seen for patients diagnosed with diffuse large B-cell lymphoma (DLBCL) (wine drinkers for more than 25 years vs. never drinkers: HR=0.36, 95% CI 0.14–0.94 for overall survival; HR=0.38, 95% CI 0.16–0.94 for disease-free survival), and the adverse effect of liquor consumption was also observed among DLBCL patients (liquor drinkers vs. never drinkers: HR=2.49, 95% CI 1.26–4.93 for disease-free survival).
Conclusions
Our results suggest a moderate relationship between pre-diagnostic alcohol consumption and NHL survival, particularly for DLBCL. The results need to be replicated in larger studies.
Implications for cancer survivors
Pre-diagnostic behaviors might impact the prognosis and survival of NHL patients.
Keywords: Alcohol, Wine, Liquor, Non-Hodgkin lymphoma, Prognosis, Survival
Introduction
Epidemiological studies have consistently shown that moderate alcohol drinkers have a lower death rate for all causes, particularly for cardiovascular disease [1–3]. The role of alcohol consumption in cancer is more complicated: it is an established risk factor for oral cavity cancer, esophagus cancer and liver cancer [4–7], while a recent pooled analysis of nine case-control studies [8] and the major prospective studies [9–12] identified an association of alcohol consumption and reduced risk of non-Hodgkin lymphoma (NHL). The underlying mechanism of carcinogenesis is complex and not clear [13]. On one hand, alcohol produces carcinogenic metabolites and inactivates tumor suppressor genes [14]; on the other hand, there is also evidence indicating that light to moderate alcohol intake improves immunocompetence [13], and wine contains antioxidants and might reduce inflammation and improve endothelial function [1].
Data is sparse about whether alcohol drinking influences NHL prognosis and survival. To our knowledge, only two studies in Italy investigated the issue, and both reported a lower survival rate among NHL patients who were drinkers [15, 16]. Neither of them examined the relationship by beverage type, however. Here, we investigated the role of alcohol consumption on NHL survival by type of alcohol consumed and NHL subtype.
Materials and methods
Study population
The study population has been described elsewhere [17, 18]. In brief, a total of 1,122 potential female NHL cases aged between 21 and 84 years were identified through the Yale Comprehensive Cancer Center’s Rapid Case Ascertainment Shared Resource (RCA), a component of the Connecticut Tumor Registry (CTR), between 1996 and 2000. Among those cases, 167 died before they could be interviewed and 123 were excluded because of doctor refusal, previous diagnosis of cancer, or inability to speak English. Out of 832 eligible cases, 601 gave written consent and completed an in-person interview. Pathology slides or tissue blocks were obtained from the hospitals where the cases had been diagnosed. The specimens were reviewed by two independent study pathologists. All NHL cases were classified according to the World Health Organization (WHO) classification system [19, 20].
Vital status for these NHL cases was abstracted at the CTR in 2008. Other follow-up information was also abstracted, including date of death, most recent follow-up date, type and date of treatments, dates of relapse and/or secondary cancer, B-symptoms and tumor stage. Of the 601 cases, 13 were not able to be identified in the CTR system and 13 were found to have a cancer history prior to diagnosis of NHL, yielding 575 NHL patients in the final analysis. Of these, 182 had diffuse large B-cell lymphoma (DLBCL); 133 had follicular lymphoma (FL); 63 had chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); 39 had marginal zone B-cell lymphoma (MZBL); and 42 had T/NK-cell lymphoma (T-cell).
The study was approved by the Human Investigation Committee at Yale University and the Connecticut Department of Public Health.
Exposure assessment
Through in-person interviews, a standardized, structured questionnaire was used to obtain demographic information, history of alcohol consumption and other known or suspected risk factors for NHL [18]. Women were first asked whether they had ever consumed at least 12 drinks a year of each type of alcohol. If they had, they were further asked to provide information on the age they first drank, the duration and intensity of consumption, and whether they stopped drinking. One drink was considered to be one 12-ounce can or bottle of beer, one 4-ounce glass of wine or one shot of liquor. Additional information on age, education, race, history of cigarette smoking, family history of cancer and other factors was also obtained during the interview.
Each type of alcohol, never/ever drinking, age first drank, intensity (g of ethanol per month), duration (years) of consumption, and the total lifetime consumption (kg of ethanol) were considered. Women who reported consumption of less than 12 drinks a year over their lifetime were classified as never drinkers. The average monthly consumption was calculated by multiplying the average number of days per month a subject reported consumption of the type of alcohol by the average number of drinks consumed on those days. This value was then multiplied by the ethanol content of alcohol types (13.2 g of ethanol per can or bottle of beer, 10.8 g of ethanol per 4-ounce glass of wine, and 15.1 g of ethanol per shot of liquor) to determine the intensity of wine consumption [10]. Finally, lifetime consumption (kg) was calculated by multiplying the intensity and duration. Continuous variables, including age at initiation, intensity, duration of consumption and total lifetime consumption were dichotomized a priori based on the median of drinkers.
Self-reported consumption of beer, wine and liquor were combined to estimate the overall impact of alcohol consumption. Never drinkers were used as the reference group in analysis. The age at initiation of drinking was defined as the youngest reported age a subject began drinking beer, wine or liquor. The duration of drinking was defined as the period of time during which subjects consumed any type of alcohol. The intensity of alcohol consumption (g of ethanol per month) and the lifetime estimate of ethanol consumption (kg of ethanol) were calculated by summing the contribution of each type of alcohol. As for each alcohol type, continuous variables were dichotomized a priori based on the median of the study subjects.
Statistical analysis
Survival analysis was conducted for both overall survival (OS) and disease-free survival (DFS). In OS analysis, deaths were events and alive was censoring. In DFS analysis, deaths, relapses and occurrences of secondary cancer were events and otherwise were censorings.
Univariate analysis was performed by comparing the Kaplan-Meier curves. Log-rank statistics were computed to evaluate the difference in survival. Cox proportional hazards (PH) model was used in multivariate analysis to compute adjusted hazard ratios (HR), and 95% confidence intervals (CI) were estimated. Age at diagnosis (<=45, 46–55, 56–65, 66–75 and >=76), education (high school or less, some college, and college graduate or more), stage (I, II, III, IV, and unknown), B-symptom presence (yes, no, unknown), initial treatment (none, radiation only, chemotherapy-based regimen, and other) and smoking (never, ever) were treated as confounding variables and adjusted. For analysis of never/ever drinking, age at initiation, intensity, duration and lifetime consumption by type of alcohol (beer, wine or liquor), the same consumption variables of the other two types were also adjusted. Adjustment for race did not result in material changes for the observed associations and thus race was not included in the final model. Adjusted OS and DFS rates from Cox models were calculated using the corrected group prognosis method [21, 22] and adjusted survival curves were plotted. Statistical analysis was performed using SAS, version 9.1 (SAS Institute, Cary, NC).
Results
Demographic characteristics for 575 NHL cases are presented in Table 1. A majority of these patients (60%) had stage I or II diseases and 6% had B-symptoms. The most common initial therapy was a chemotherapy-based regimen (52%), followed by observation (34%) and radiation only (12%). During the follow-up, 253 women died, 13 women had only recurrence of NHL, 69 women had only secondary cancer, and 5 women had both recurrence of NHL and secondary cancer. The mean was 6.82 years (SD=3.25, range: 0.33–11.79) for OS and 6.53 years (SD=3.31, range: 0.04–11.79) for DFS.
Table 1.
Selected demographic characteristics of NHL cases, Connecticut, 1996–2000
| Characteristic | Number | Percentage |
|---|---|---|
| Age at diagnosis | ||
| <=45 | 70 | 12.17 |
| 46–55 | 111 | 19.30 |
| 56–65 | 121 | 21.04 |
| 66–75 | 165 | 28.70 |
| >=76 | 108 | 18.78 |
| Race | ||
| White | 547 | 95.13 |
| Black | 18 | 3.13 |
| Other | 10 | 1.74 |
| Education | ||
| High School or Less | 246 | 42.78 |
| Some College | 190 | 33.04 |
| College graduate or more | 139 | 24.17 |
| Family history | ||
| None | 125 | 21.74 |
| Any other cancer | 442 | 76.87 |
| NHL | 8 | 1.39 |
In univariate analysis, when comparing OS and DFS between drinkers of different alcohol types and never drinkers, significant difference was only identified between wine drinkers and never drinkers (5-year OS 0.75 vs. 0.69, 10-year OS 0.62 vs. 0.49, log-rank test p-value=0.030; 5-year DFS 0.70 vs. 0.67, 10-year DFS 0.57 vs. 0.45, log-rank test p-value=0.049). Univariate analysis by NHL subtype showed that this favorable effect of wine drinking was mainly seen in DLBCL patients with borderline significant p-values in log-rank tests (0.057 for OS and 0.082 for DFS).
Table 2 presents HRs from multivariate survival analyses for NHL overall. Compared to never drinkers, women who drank less than 4 cans or bottles of beer per month before diagnosis had an significantly decreased risk of death (HR= 0.40, 95% CI: 0.18–0.89) and a significantly decreased risk of relapse, secondary cancer or death (HR=0.37, 95% CI: 0.17–0.79).
Table 2.
Hazard ratios associated with alcohol consumption among NHL patients
| Deaths/Cases | HR for OSa | HR for DFSb | |
|---|---|---|---|
| Alcohol | |||
| Never drinker | 110/224 | 1 | 1 |
| Ever drinker | 143/351 | 0.90(0.70–1.17) | 0.92(0.72–1.18) |
| Initiation age <=21 | 74/207 | 0.82(0.61–1.12) | 0.82(0.61–1.10) |
| Initiation age > 21 | 69/144 | 1.01(0.74–1.39) | 1.07(0.79–1.44) |
| Intensity <=132 g/month | 74/176 | 0.85(0.63–1.15) | 0.87(0.65–1.16) |
| Intensity > 132 g/month | 69/175 | 0.97(0.71–1.33) | 0.99(0.74–1.34) |
| Duration <=30 years | 67/176 | 1.12(0.81–1.55) | 1.09(0.80–1.48) |
| Duration >30 years | 76/175 | 0.78(0.58–1.06) | 0.82(0.62–1.10) |
| Lifetime consumption <=34.49 kg | 70/176 | 0.87(0.64–1.18) | 0.90(0.68–1.21) |
| Lifetime consumption >34.49 kg | 73/175 | 0.95(0.70–1.29) | 0.94(0.70–1.27) |
| Beer | |||
| Beer drinker | 59/145 | 0.83(0.45–1.55) | 0.76(0.41–1.40) |
| Initiation age <=20 | 30/86 | 0.74(0.37–1.49) | 0.70(0.36–1.37) |
| Initiation age >20 | 29/59 | 0.79(0.38–1.66) | 0.67(0.32–1.37) |
| Intensity <=52.8 g (4 cans) /month | 23/76 | 0.40(0.18–0.89) | 0.37(0.17–0.79) |
| Intensity >52.8 g (4 cans) /month | 36/69 | 1.20(0.62–2.30) | 1.09(0.57–2.07) |
| Duration <=22 years | 31/75 | 0.98(0.50–1.92) | 0.85(0.44–1.65) |
| Duration > 22 years | 28/70 | 0.65(0.31–1.34) | 0.64(0.32–1.28) |
| Lifetime consumption <=11.40 kg | 25/74 | 0.56(0.27–1.17) | 0.47(0.23–0.98) |
| Lifetime consumption > 11.40 kg | 34/71 | 1.18(0.60–2.31) | 1.08(0.56–2.07) |
| Wine | |||
| Wine drinker | 96/260 | 0.88(0.59–1.30) | 0.88(0.61–1.28) |
| Initiation age <=25 | 48/149 | 0.79(0.50–1.24) | 0.76(0.50–1.17) |
| Initiation age >25 | 48/111 | 0.99(0.63–1.53) | 1.03(0.68–1.56) |
| Intensity <=64.8 g (6 glasses) /month | 47/130 | 0.80(0.51–1.24) | 0.84(0.55–1.27) |
| Intensity >64.8 g (6 glasses) /month | 49/130 | 0.96(0.61–1.52) | 0.91(0.59–1.42) |
| Duration <=25 years | 50/132 | 1.18(0.75–1.87) | 1.11(0.72–1.72) |
| Duration > 25 years | 46/128 | 0.72(0.47–1.12) | 0.77(0.51–1.16) |
| Lifetime consumption <=17.63 kg | 42/129 | 0.86(0.55–1.35) | 0.89(0.58–1.36) |
| Lifetime consumption > 17.63 kg | 54/131 | 0.90(0.58–1.40) | 0.87(0.57–1.33) |
| Liquor | |||
| Liquor drinker | 94/232 | 1.01(0.67–1.52) | 1.12(0.76–1.66) |
| Initiation age <=21 | 42/128 | 0.98(0.59–1.63) | 0.97(0.60–1.56) |
| Initiation age >21 | 52/104 | 1.06(0.68–1.64) | 1.26(0.83–1.92) |
| Intensity <=60.4 g (4 shots) /month | 50/130 | 0.99(0.62–1.59) | 0.99(0.63–1.57) |
| Intensity >60.4 g (4 shots) /month | 44/102 | 0.90(0.56–1.45) | 1.08(0.69–1.69) |
| Duration <=26 years | 51/120 | 1.06(0.67–1.68) | 1.22(0.79–1.87) |
| Duration > 26 years | 43/112 | 0.88(0.53–1.45) | 0.94(0.58–1.51) |
| Lifetime consumption <=16.76 kg | 42/116 | 1.06(0.66–1.70) | 1.02(0.65–1.60) |
| Lifetime consumption > 16.76 kg | 52/116 | 0.94(0.59–1.49) | 1.15(0.74–1.79) |
Models adjusted for age, education, smoking, disease stage, B-symptom presence, initial treatment, and consumption of other alcohol types for each type of alcohol.
Hazard ratios for risks of death.
Hazard ratios for risks of relapse, secondary cancer or death.
Table 3 shows HRs from multivariate survival analyses of the two most common NHL subtypes: DLBCL and FL. Compared to never drinkers, wine drinkers had decreased risks while liquor drinkers had increased risks of negative outcomes for DLBCL patients. In addition, an earlier initiation age, a higher intensity, a longer duration, and more lifetime consumption of wine drinking are associated with more reduced risks; an earlier initiation age, higher intensity, and more lifetime consumption of liquor drinking are associated with more increased risks. Particularly, DLBCL patients who drank wine more than 25 years had a significantly reduced risk of death (HR=0.36, 95% CI: 0.14–0.94) and a significantly reduced risk of relapse, secondary cancer, or death (HR=0.38, 95% CI: 0.16–0.94); DLBCL patients who started drinking liquor before 21 years old had a significantly increased risk of death (HR=2.81, 95% CI: 1.05–7.49) and a significantly increased risk of relapse, secondary cancer, or death (HR=3.17, 95% CI: 1.29–7.80). No significant results were found for FL, although the majority of the HRs associated with beer drinking and wine drinking were greater than 1, suggesting an adverse effect of beer drinking and wine drinking for FL survival.
Table 3.
Hazard ratios associated with alcohol consumption among DLBCL and FL patients
| DLBCL |
FL |
|||||
|---|---|---|---|---|---|---|
| Deaths/ Cases |
HR for OSa | HR for DFSb | Deaths/ Cases |
HR for OSa | HR for DFSb | |
| Alcohol | ||||||
| Never drinker | 37/70 | 1 | 1 | 21/55 | 1 | 1 |
| Ever drinker | 47/112 | 0.92(0.57–1.47) | 0.96(0.61–1.51) | 28/78 | 0.89(0.49–1.61) | 1.04(0.60–1.82) |
| Initiation age <=21 | 26/68 | 0.84(0.48–1.47) | 0.84(0.50–1.43) | 11/43 | 0.64(0.30–1.37) | 0.80(0.40–1.60) |
| Initiation age > 21 | 21/44 | 1.02(0.57–1.83) | 1.13(0.65–1.98) | 17/35 | 1.20(0.60–2.41) | 1.37(0.71–2.64) |
| Intensity <=132 g/month | 23/48 | 0.88(0.49–1.57) | 0.82(0.46–1.43) | 18/43 | 1.01(0.52–1.97) | 1.23(0.66–2.29) |
| Intensity > 132 g/month | 24/64 | 0.96(0.55–1.69) | 1.10(0.65–1.87) | 10/35 | 0.72(0.32–1.61) | 0.79(0.37–1.69) |
| Duration <=30 years | 26/63 | 1.51(0.84–2.71) | 1.54(0.89–2.67) | 15/42 | 1.07(0.51–2.24) | 1.25(0.62–2.50) |
| Duration>30 years | 21/49 | 0.63(0.35–1.13) | 0.65(0.37–1.14) | 13/36 | 0.75(0.36–1.56) | 0.90(0.46–1.76) |
| Lifetime consumption <=34.49 kg | 23/49 | 1.01(0.56–1.83) | 0.95(0.54–1.67) | 18/45 | 0.95(0.49–1.86) | 1.29(0.70–2.38) |
| Lifetime consumption>34.49 kg | 24/63 | 0.86(0.50–1.49) | 0.97(0.57–1.62) | 10/33 | 0.79(0.36–1.75) | 0.72(0.33–1.58) |
| Beer | ||||||
| Beer drinker | 24/56 | 1.24(0.41–3.77) | 0.94(0.31–2.88) | 9/28 | 2.27(0.43–12.03) | 1.87(0.36–9.61) |
| Initiation age <=20 | 13/33 | 1.14(0.33–3.95) | 0.87(0.24–3.09) | 5/14 | 1.15(0.14–9.45) | 1.77(0.22–14.35) |
| Initiation age >20 | 11/23 | 1.53(0.41–5.80) | 1.12(0.30–4.13) | 4/14 | 2.01(0.23–17.58) | 1.13(0.13–9.83) |
| Intensity <=52.8 g (4 cans) /month | 7/25 | 0.51(0.12–2.25) | 0.29(0.06–1.35) | 5/18 | 2.47(0.44–14.03) | 2.18(0.41–11.54) |
| Intensity>52.8 g (4 cans) /month | 17/31 | 2.29(0.60–8.72) | 1.61(0.43–6.03) | 4/10 | 12.31(0.64–238.3) | 5.98(0.44–80.71) |
| Duration <=22 years | 17/35 | 1.55(0.48–5.06) | 1.17(0.36–3.75) | 2/10 | 3.67(0.25–54.30) | 2.33(0.19–29.21) |
| Duration > 22 years | 7/21 | 1.54(0.36–6.55) | 1.22(0.30–4.94) | 7/18 | 2.62(0.50–13.88) | 2.09(0.40–10.84) |
| Lifetime consumption <=11.40 kg | 10/28 | 0.97(0.28–3.33) | 0.65(0.18–2.36) | 4/15 | 2.33(0.42–12.84) | 1.70(0.29–9.86) |
| Lifetime consumption > 11.40 kg | 14/28 | 1.79(0.50–6.40) | 1.36(0.39–4.72) | 5/13 | 3.36(0.31–36.33) | 2.59(0.35–19.28) |
| Wine | ||||||
| Wine drinker | 29/82 | 0.58(0.26–1.27) | 0.58(0.27–1.24) | 21/62 | 1.27(0.56–2.89) | 1.56(0.72–3.38) |
| Initiation age <=25 | 15/49 | 0.46(0.19–1.14) | 0.49(0.21–1.12) | 11/35 | 1.27(0.46–3.52) | 1.23(0.47–3.23) |
| Initiation age >25 | 14/33 | 0.74(0.29–1.88) | 0.77(0.31–1.89) | 10/27 | 1.23(0.45–3.37) | 1.83(0.74–4.54) |
| Intensity <=64.8 g (6 glasses) /month | 14/36 | 0.65(0.26–1.65) | 0.64(0.26–1.54) | 10/30 | 1.12(0.44–2.88) | 1.42(0.59–3.43) |
| Intensity >64.8 g (6 glasses) /month | 15/46 | 0.45(0.18–1.14) | 0.43(0.17–1.05) | 11/32 | 1.40(0.50–3.94) | 1.71(0.67–4.38) |
| Duration <=25 years | 16/44 | 0.85(0.35–2.08) | 0.91(0.39–2.13) | 14/35 | 1.81(0.73–4.49) | 2.34(0.98–5.58) |
| Duration > 25 years | 13/38 | 0.36(0.14–0.94) | 0.38(0.16–0.94) | 7/27 | 0.76(0.25–2.25) | 1.03(0.40–2.67) |
| Lifetime consumption <=17.63 kg | 11/37 | 0.61(0.23–1.60) | 0.60(0.24–1.48) | 9/31 | 1.16(0.43–3.12) | 1.55(0.64–3.79) |
| Lifetime consumption > 17.63 kg | 18/45 | 0.52(0.22–1.21) | 0.52(0.23–1.18) | 12/31 | 1.46(0.55–3.89) | 1.63(0.66–4.04) |
| Liquor | ||||||
| Liquor drinker | 33/73 | 1.76(0.87–3.59) | 2.49(1.26–4.93) | 15/52 | 0.93(0.31–2.82) | 0.81(0.26–2.46) |
| Initiation age <=21 | 16/40 | 2.81(1.05–7.49) | 3.17(1.29–7.80) | 6/28 | 0.71(0.17–3.03) | 0.58(0.13–2.54) |
| Initiation age >21 | 17/33 | 1.45(0.64–3.28) | 2.23(1.03–4.83) | 9/24 | 1.00(0.31–3.24) | 0.90(0.28–2.91) |
| Intensity <=60.4 g (4 shots) /month | 14/34 | 1.18(0.48–2.90) | 1.18(0.48–2.88) | 9/29 | 1.11(0.31–4.01) | 1.05(0.29–3.78) |
| Intensity>60.4 g (4 shots) /month | 19/39 | 2.09(0.90–4.89) | 3.31(1.49–7.37) | 6/23 | 0.85(0.23–3.08) | 0.71(0.20–2.46) |
| Duration <=26 years | 19/43 | 2.59(0.93–7.24) | 4.60(1.88–11.28) | 8/27 | 0.89(0.28–2.78) | 0.82(0.26–2.54) |
| Duration > 26 years | 14/30 | 1.62(0.71–3.69) | 1.99(0.88–4.49) | 7/25 | 1.11(0.23–5.26) | 0.77(0.17–3.46) |
| Lifetime consumption <=16.76 kg | 13/32 | 1.61(0.66–3.94) | 1.66(0.72–3.84) | 7/29 | 0.75(0.23–2.44) | 0.75(0.24–2.36) |
| Lifetime consumption > 16.76 kg | 20/41 | 1.80(0.82–3.97) | 3.27(1.50–7.15) | 8/23 | 1.48(0.34–6.49) | 0.80(0.19–3.29) |
Models adjusted for age, education, smoking, disease stage, B-symptom presence, initial treatment, and consumption of other alcohol types for each type of alcohol.
Hazard ratios for risks of death.
Hazard ratios for risks of relapse, secondary cancer or death.
No significant results were found for CLL/SLL, MZBL or T-cell lymphoma given the small number of cases (data not shown).
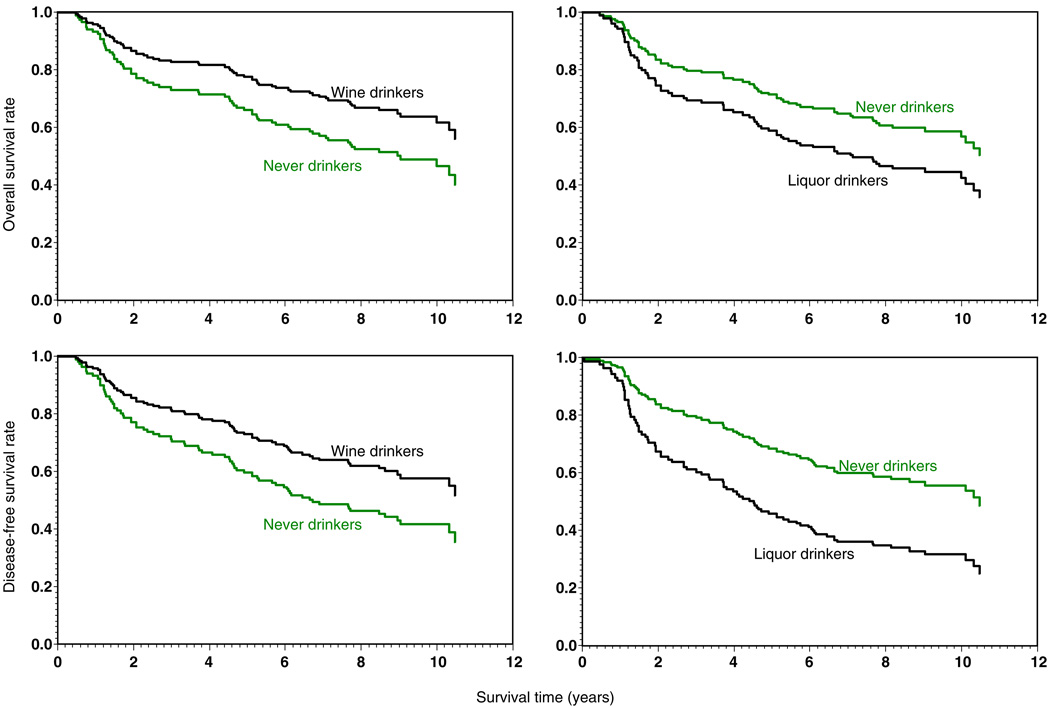
Adjusted OS and DFS curves from Cox PH models for DLBCL patients were plotted in Fig. 1.
FIGURE 1.
Adjusted overall and disease-free survival curves of DLBCL patients by wine-drinking and liquor-drinking status.
Discussion
Our follow-up study of 575 female NHL patients suggests that pre-diagnostic alcohol consumption might affect the prognosis and survival of NHL patients, and the impact varies by NHL subtype and type of alcohol consumed. Wine drinking appeared to favor NHL prognosis and survival, particularly for DLBCL, the most common NHL subtype. Light consumption of beer showed benefit for NHL overall while no effect was observed when different NHL subtypes were analyzed separately. Liquor drinking was associated with an increased risk of negative outcomes in DLBCL patients, such as relapse, occurrence of secondary cancer, and death. The findings warrant replication in other studies.
One of our main findings is that pre-diagnostic wine consumption was associated with a better survival among NHL patients; particularly, a reduced risk of death, relapse and secondary cancer occurrence was observed among DLBCL patients. Extensive laboratory studies have shown that the polyphenolic constituents rich in grapes, which are abundant in wine, such as flavonoids and resveratrol, could block carcinogenesis and inhibit the growth of tumors in animals or cell culture [23]. These polyphenols have been well demonstrated to have anti-oxidative [24, 25] and anti-inflammatory [25, 26] effects; emerging evidence showed that they also exhibited additional biological properties and could interfere with multistage tumor onset and tumor growth, such as preventing DNA alteration by inhibition of phase I enzymes, induction of phase II enzymes, stimulation of DNA repair [27], inhibiting cell proliferation by cell cycle arrest [28–31], modulating growth-related signal transduction pathways through altering expression of protein kinases [32–35], and activating apoptosis [28, 29, 36–38]. Besides the anti-cancer effects, the polyphenols [39–42] or moderate wine drinking [11, 43–45] has been shown inversely associated with the risk of cardiovascular diseases (CVD), the No. 1 killer in the U.S. and to which cancer survivors are especially prone [46], which may also contribute to the better OS and DFS we observed among wine drinkers. The medical history of CVD was not collected at diagnosis or during the follow-up in our study. However, efforts were made by looking at cause of death. There were 11 deaths from CVD out of 224 non-wine drinkers (5%) compared with 10 deaths from CVD out of 260 wine-drinkers (4%).
Another possible explanation of our observation of wine’s favorable effect and liquor’s adverse effect on NHL patients’ prognosis and survival is that moderate wine drinking is associated with a higher socio-economic status and lifestyle leading to a healthier diet, while liquor drinking may indicate the opposite. In our population, wine drinkers had a higher average consumption of vegetables and fruit (3.86 vs. 3.74 medium servings per day) but a higher consumption of saturated fat (27.67 vs. 26.95 gm per day) than never drinkers, and liquor drinkers had a lower average consumption of vegetables and fruit (3.64 vs. 3.74 medium servings per day) and a higher consumption of saturated fat (28.28 vs. 26.95 gm per day) than never drinkers, but the differences were not statistically significant. Besides adjusting for clinical factors and education as a measure of socio-economic status, attempts were also made to control potential confounding by other nutritional factors such as total energy intake, intake of protein, fat, carbohydrates, vegetables and fruits, and body mass index, all of which did not result in material changes of the observed associations and thus were not included in the final models. However, we could not eliminate the possibility that the residual confounding may contribute to the observed associations.
Two studies conducted in Italy examined the relationship between alcohol consumption and survival in NHL patients [15, 16], and both found that drinkers had a poor survival and a higher risk of death than non-drinkers. By taking types of alcohol consumed into consideration, our study added information to the previous studies: our study shows that NHL survival varies by alcohol type and that the favorable effect was mainly seen in wine-drinkers and detrimental effects were mainly seen in liquor-drinkers. Unlike those two studies, we did not observe poor survival or higher risk of death among drinkers of alcohol overall; this discrepancy could be due to the different study populations. Compared to the Italian studies’ populations, our study population has a significant lower proportion of drinkers (61% vs. 79%) [15] and lower levels of alcohol consumption (median intensity 0.5 vs. 4 drinks per day; median duration 30 vs. 40 years; median lifetime consumption 34.5 vs. 295 kg ethanol) [15, 16]. A U- or J-shape relationship has been well established between alcohol consumption and other health outcomes, and could exist for NHL survival as well. A possible explanation for the discrepancy is that the consumption level of our study population might lie before while the Italian studies were far beyond the bottom of the U- or J-shape curve. The discrepancy could also be due to gender differences: our study included only females, while the other studies included both genders—638 men and 495 women in one study [15] and 154 men and 114 women in the other [16]—neither of those studies stratified their analyses by gender.
One strength of our study is that the alcohol consumption was analyzed thoroughly. We analyzed not only whether drinking occurred or not, but also the initiation age, intensity, duration, and life time consumption, and types of drinking. Given that the biological mechanism of alcohol drinking on cancer survival is not clear, any of these indicators could be relevant to the outcome of NHL survival. This thorough analysis allowed us to evaluate each of the effects individually and separate the effects of different types of drinking. We also conducted factor analysis and found four resulting factors. Among them, roughly speaking, three correspond to beer, wine, and liquor drinking, respectively; and the other factor corresponds to intensity of ethanol intake. Using the four factors (as opposed to the original measurements) in the Cox models, we reached a similar conclusion: wine drinking was significantly associated with DLBCL survival.
The second strength of our study is the utilization of CTR to obtain follow-up information. According to the recently submitted SEER database (Nov 2007) [47], among those microscopically confirmed female NHL patients diagnosed in 1996–2000 in CT and aged 21–84, 99.2% were actively followed by CTR through 12/31/2004. Through CTR, we obtained the information on tumor stage, B-symptoms and initial treatment; by adjusting them as confounders in our analysis, we were able to examine the independent effect of alcohol consumption on NHL prognosis and survival. Another strength of our study is the relatively large sample size, which provides power to detect differences among NHL subtypes, especially for the most common subtype DLBCL.
A limitation of our study is that patients were interviewed only at entrance in the study, and some subjects may have changed their drinking habits during follow-up. Therefore, our observation reveals only the association between pre-diagnostic alcohol consumption and NHL survival and cautions should be taken when explaining the relationship between post-diagnostic alcohol consumption and NHL survival. Another limitation lies in the possible incomplete information on relapse and secondary cancer occurrence abstracted from CTR, especially among patients who were no longer CT residents, which could cause our measure of DFS longer than the true. However, this information bias is unlikely to be associated with drinking habits, thus our observed associations on DFS may be biased towards the null due to this non-differential misclassification.
In conclusion, our study shows that pre-diagnostic wine drinking may favor NHL prognosis and survival, especially among DLBCL patients, while liquor might negatively affect DLBCL prognosis and survival. The analyses for different histological subtypes suggest that the effect of alcohol consumption on prognosis and survival might be different among NHL subtypes. Our findings should be confirmed by other studies.
Acknowledgments
This study is supported by grant CA62006 from the National Cancer Institute (NCI), by Hull Argall & Anna Grant 22067A from the Yale Cancer Center, and by Fogarty training grants 1D43TW008323-01 and 1D43TW007864-01 from the National Institute of Health (NIH). This publication was made possible by CTSA Grant number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the NIH and NHL roadmap for medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR. This research was approved by the DPH HIC. Certain data used in this study were obtained from the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data.
Contributor Information
Xuesong Han, School of Public Health, Yale University, New Haven, CT 06520, USA.
Tongzhang Zheng, School of Public Health, Yale University, New Haven, CT 06520, USA.
Francine M. Foss, School of Medicine, Yale University, New Haven, CT 06520, USA
Shuangge Ma, School of Public Health, Yale University, New Haven, CT 06520, USA.
Theodore R. Holford, School of Public Health, Yale University, New Haven, CT 06520, USA
Peter Boyle, International Prevention Research Institute, Lyon 69006, France.
Brian Leaderer, School of Public Health, Yale University, New Haven, CT 06520, USA.
Ping Zhao, Cancer Institute/Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China.
Min Dai, Cancer Institute/Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China.
Yawei Zhang, School of Public Health, Yale University, New Haven, CT 06520, USA; Yale University School of Public Health, 60 College Street, LEPH 440, P.O. Box 208034, New Haven, CT 06520-8034, USA, yawei.zhang@yale.edu.
References
- 1.Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. 2007;116:1306–1317. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- 2.Ginter E, Simko V. Ethanol and cardiovascular diseases: epidemiological, biochemical and clinical aspects. Bratisl Lek Listy. 2008;109:590–594. [PubMed] [Google Scholar]
- 3.Djousse L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Curr Atheroscler Rep. 2008;10:117–120. doi: 10.1007/s11883-008-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullough MJ, Farah CS. The role of alcohol in oral carcinogenesis with particular reference to alcohol-containing mouthwashes. Aust Dent J. 2008;53:302–305. doi: 10.1111/j.1834-7819.2008.00070.x. [DOI] [PubMed] [Google Scholar]
- 5.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA. Hepatocellular carcinoma. Curr Opin Gastroenterol. 2006;22:248–253. doi: 10.1097/01.mog.0000218961.86182.8c. [DOI] [PubMed] [Google Scholar]
- 7.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens–Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 8.Morton LM, Zheng T, Holford TR, Holly EA, Chiu BC, Costantini AS, et al. Alcohol consumption and risk of non-Hodgkin lymphoma: a pooled analysis. Lancet Oncol. 2005;6:469–476. doi: 10.1016/S1470-2045(05)70214-X. [DOI] [PubMed] [Google Scholar]
- 9.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 10.Chiu BC, Cerhan JR, Gapstur SM, Sellers TA, Zheng W, Lutz CT, et al. Alcohol consumption and non-Hodgkin lymphoma in a cohort of older women. Br J Cancer. 1999;80:1476–1482. doi: 10.1038/sj.bjc.6690547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klatsky AL, Li Y, Baer D, Armstrong MA, Udaltsova N, Friedman GD. Alcohol consumption and risk of hematologic malignancies. Ann Epidemiol. 2009 doi: 10.1016/j.annepidem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Lim U, Morton LM, Subar AF, Baris D, Stolzenberg-Solomon R, Leitzmann M, et al. Alcohol, smoking, and body size in relation to incident Hodgkin’s and non-Hodgkin’s lymphoma risk. Am J Epidemiol. 2007;166:697–708. doi: 10.1093/aje/kwm122. [DOI] [PubMed] [Google Scholar]
- 13.Diaz LE, Montero A, Gonzalez-Gross M, Vallejo AI, Romeo J, Marcos A. Influence of alcohol consumption on immunological status: a review. Eur J Clin Nutr. 2002;56 Suppl 3:S50–S53. doi: 10.1038/sj.ejcn.1601486. [DOI] [PubMed] [Google Scholar]
- 14.Poschl G, Seitz HK. Alcohol and cancer. Alcohol and alcoholism. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 15.Battaglioli T, Gorini G, Costantini AS, Crosignani P, Miligi L, Nanni O, et al. Cigarette smoking and alcohol consumption as determinants of survival in non-Hodgkin’s lymphoma: a population-based study. Ann Oncol. 2006;17:1283–1289. doi: 10.1093/annonc/mdl096. [DOI] [PubMed] [Google Scholar]
- 16.Talamini R, Polesel J, Spina M, Chimienti E, Serraino D, Zucchetto A, et al. The impact of tobacco smoking and alcohol drinking on survival of patients with non-Hodgkin lymphoma. Int J Cancer. 2008;122:1624–1629. doi: 10.1002/ijc.23205. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Holford TR, Leaderer B, Boyle P, Zahm SH, Flynn S, et al. Hair-coloring product use and risk of non-Hodgkin’s lymphoma: a population-based case-control study in Connecticut. Am J Epidemiol. 2004;159:148–154. doi: 10.1093/aje/kwh033. [DOI] [PubMed] [Google Scholar]
- 18.Morton LM, Holford TR, Leaderer B, Zhang Y, Zahm SH, Boyle P, et al. Alcohol use and risk of non-Hodgkin’s lymphoma among Connecticut women (United States) Cancer Causes Control. 2003;14:687–694. doi: 10.1023/a:1025626208861. [DOI] [PubMed] [Google Scholar]
- 19.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 20.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 21.Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. Jama. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 22.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143:1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 23.He S, Sun C, Pan Y. Red wine polyphenols for cancer prevention. Int J Mol Sci. 2008;9:842–853. doi: 10.3390/ijms9050842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Panchon MS, Villano D, Troncoso AM, Garcia-Parrilla MC. Antioxidant activity of phenolic compounds: from in vitro results to in vivo evidence. Crit Rev Food Sci Nutr. 2008;48:649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- 25.Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445–454. doi: 10.1111/j.1753-4887.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 26.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 27.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 28.Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellon EA. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J Androl. 2007;28:282–293. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- 29.Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis: inhibition of cell proliferation and induction of apoptosis. Chem Biol Interact. 2009;179:131–144. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Kuo PL, Hsu YL. The grape and wine constituent piceatannol inhibits proliferation of human bladder cancer cells via blocking cell cycle progression and inducing Fas/membrane bound Fas ligand-mediated apoptotic pathway. Mol Nutr Food Res. 2008;52:408–418. doi: 10.1002/mnfr.200700252. [DOI] [PubMed] [Google Scholar]
- 31.Leifert WR, Abeywardena MY. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr Res. 2008;28:842–850. doi: 10.1016/j.nutres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Lee KW, Kang NJ, Heo YS, Rogozin EA, Pugliese A, Hwang MK, et al. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008;68:946–955. doi: 10.1158/0008-5472.CAN-07-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KW, Kang NJ, Rogozin EA, Kim HG, Cho YY, Bode AM, et al. Myricetin is a novel natural inhibitor of neoplastic cell transformation and MEK1. Carcinogenesis. 2007;28:1918–1927. doi: 10.1093/carcin/bgm110. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu T, Nakazato T, Xian MJ, Sagawa M, Ikeda Y, Kizaki M. Resveratrol induces apoptosis of human malignant B cells by activation of caspase-3 and p38 MAP kinase pathways. Biochem Pharmacol. 2006;71:742–750. doi: 10.1016/j.bcp.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 35.King RE, Kent KD, Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem Biol Interact. 2005;151:143–149. doi: 10.1016/j.cbi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 36.D'Archivio M, Santangelo C, Scazzocchio B, Vari R, Filesi C, Masella R, et al. Modulatory effects of polyphenols on apoptosis induction: relevance for cancer prevention. Int J Mol Sci. 2008;9:213–228. doi: 10.3390/ijms9030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juan ME, Wenzel U, Daniel H, Planas JM. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem. 2008;56:4813–4818. doi: 10.1021/jf800175a. [DOI] [PubMed] [Google Scholar]
- 38.Larrosa M, Tomas-Barberan FA, Espin JC. The grape and wine polyphenol piceatannol is a potent inducer of apoptosis in human SK-Mel-28 melanoma cells. Eur J Nutr. 2004;43:275–284. doi: 10.1007/s00394-004-0471-5. [DOI] [PubMed] [Google Scholar]
- 39.Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 40.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirvonen T, Pietinen P, Virtanen M, Ovaskainen ML, Hakkinen S, Albanes D, et al. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology. 2001;12:62–67. doi: 10.1097/00001648-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999;149:943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- 43.Rotondo S, Di Castelnuovo A, de Gaetano G. The relationship between wine consumption and cardiovascular risk: from epidemiological evidence to biological plausibility. Ital Heart J. 2001;2:1–8. [PubMed] [Google Scholar]
- 44.Streppel MT, Ocke MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term wine consumption is related to cardiovascular mortality and life expectancy independently of moderate alcohol intake: the Zutphen Study. J Epidemiol Community Health. 2009 doi: 10.1136/jech.2008.082198. [DOI] [PubMed] [Google Scholar]
- 45.Athyros VG, Liberopoulos EN, Mikhailidis DP, Papageorgiou AA, Ganotakis ES, Tziomalos K, et al. Association of drinking pattern and alcohol beverage type with the prevalence of metabolic syndrome, diabetes, coronary heart disease, stroke, and peripheral arterial disease in a Mediterranean cohort. Angiology. 2007;58:689–697. doi: 10.1177/0003319707306146. [DOI] [PubMed] [Google Scholar]
- 46.Demark-Wahnefried W, Rock CL, Patrick K, Byers T. Lifestyle interventions to reduce cancer risk and improve outcomes. Am Fam Physician. 2008;77:1573–1578. [PubMed] [Google Scholar]
- 47.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2007 Sub (1973–2005 varying)—Linked To County Attributes—Total U.S., 1969–2005 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; released April 2008, based on the November 2007 submission.