Abstract
Introduction
There has been limited attention to pathological features of basilar artery atherosclerosis. It has been assumed that pathology of basilar artery atherosclerosis mimics that of other vascular beds.
Methods
To define the nature of the basilar artery atherosclerotic lesions we analyzed postmortem intracranial artery samples from eight subjects with history of stroke.
Results
Atherosclerotic lesions were present in 7/8 arteries examined, with a mean estimated stenosis of 34%. Lumen thrombus with a disrupted fibrous cap was seen in 1 lesion; the remaining 6 lesions revealed a thick fibrous cap. Neovascularity and calcification were seen in 1 lesion and mild to moderate inflammation was seen in 3 lesions. Necrotic core was present in 4/7 lesions, and was associated with plaque rupture in the only disrupted lesion.
Conclusions
Basilar artery atherosclerotic lesions were relatively benign in this series of patients presenting with stroke. While confirmation is needed with larger sample size, the relative paucity of neovascularity suggests a possibly distinctive histopathological profile.
Keywords: Intracranial, Atherosclerosis, Stroke, Basilar, Plaque, Artery
Introduction
The presence of intracranial atherosclerosis and its complications play an important role in ischemic stroke [1-4]. In a study of consecutive autopsies of stroke patients with brain infarction, intracranial plaques and stenoses were observed in 62% and 43% of patients respectively [5]. A higher prevalence of atherosclerotic pathology of the vertebral-basilar system, especially the proximal/lower basilar artery has been reported [6-8]. However, the exact nature of the culprit lesion in basilar atherosclerosis has attracted little attention. It is largely assumed that characteristics of basilar atherosclerotic lesions mimic those seen in atherosclerosis of the extra-cranial vasculature. However, there is little evidence to support this contention. The purpose of this study is to begin to define the nature of atherosclerotic lesions of the basilar system. Better understanding of elements of basilar atherosclerosis may have important consequences for identifying optimal therapy.
Methods
Intracranial arteries were removed at autopsy from subjects randomly chosen with clinical symptoms of acute stroke (confirmed by CT), in a study approved by the Ethics Committee of Debrecen University, Hungary. Within 24 hours from time of death, intracranial vessels were removed, frozen, and stored at -80ºC. Vessels were shipped frozen for pathological analysis to the University of California, Irvine (UCI), where they were cut into three pieces. Arterial segments were further processed by dehydration in a series of graded ethanol solutions prior to paraffin embedding. The blocks were cut in serial 5um thick sections and mounted on slides. All gross lesions were studied, with approximately nine stained sections per lesion.
For oil red staining, 7 micron thick sections were cut from specimens embedded in OCT. Sections were stained in 0.3% Oil Red O solution (Nilechemicals) in 60% propylene glycol solution for 10 minutes. The sections were then rinsed in 60% propylene glycol solution for 30 seconds. Cellular nuclei were counterstained in Gill's hematoxylin solution for 3 minutes. The sections were then washed in distilled water and mounted in aqueous medium.
For additional histopathological study, specimens were thawed, formalin-fixed, and embedded in paraffin, followed by cutting serial 5-micron thick sections and mounting on vectabond (Vector SP-1800)-pretreated slides. Deparaffinzation was performed by heating sections for 25 minutes at 56°C. The tissue was then dehydrated twice in xylene bath and a graded series of ethanol. Tissue sections were stained with hematoxylin & eosin, and Masson's trichrome stain.
For immunohistochemistry (IHC), deparaffinized sections were treated with 3% H2O2 in PBS to block endogenous peroxidase for 5 minutes at room temperature. Nonspecific background staining was blocked by incubation in 3% BSA for 1 hour with 0.3% Triton X-100 (TX) at room temperature. Smooth muscle cells were stained with anti-smooth muscle actin antibody (0.25ug/ml, R&D, MAB1420). Sections were incubated with the primary antibodies overnight at 4°C, rinsed 3 times with PBS with 0.1% TX and incubated with biotinylated secondary antibody followed by ABC kit reagent (Vector, Burlingame, CA) for 1 hour each at room temperature. Finally, after washing three times with PBS, the sections were incubated for approximately 2-5 min with diamino-benzidine (DAB) (Vector). Sections were further processed by dehydration in a series of graded ethanol, cleared with xylene, and then coverslipped with DPX (BHD, Biomedical Specialties, CA). H&E, Masson and immunostaining were observed under a Zeiss Axiovert-200 inverted microscope (Carl Zeiss,Thornwood, NY, USA) and images acquired with a Zeiss Axiocam high-resolution digital color camera (1300 × 1030 pixel) using Axiovision 3.1 software (Carl Zeiss). Histological specimens were analyzed on the basis of the classification scheme of the American Heart Association, as previously described [9-11].
Results
Postmortem intracranial artery samples were collected from 8 patients, with a distribution of 3 females and 5 males aged 71-93 years old (median 82.5, mean 82). Four patients had right middle cerebral artery (RMCA) stroke distribution. Five patients had left middle cerebral artery stroke distribution (LMCA). One of the patients had infarction of left cerebellum.
Atherosclerotic lesions were present in 7/8 arteries examined. Stenosis ranged from 10-90% with mean estimated stenosis of 34%. Only one lesion had a thin fibrous cap associated with plaque rupture. All remaining lesions had thick fibrous caps. Neovascularity and calcification were seen in 1/7 arteries examined. Inflammation was found in 3/7 lesions, and when present was only mild or moderate. Necrotic core was present in 4/7 lesions, but was associated with plaque rupture and thin fibrous cap in only one lesion. Overall, lumen thrombus was present in 1/7 lesions, with a corresponding plaque rupture. Erosion was not observed in any sample. Figures 1 and 2 illustrate histological appearance of the characteristics of atherosclerotic lesions from two patients. Plaque characteristics of all 8 patients are tabulated (Table 1)
Figure 1.
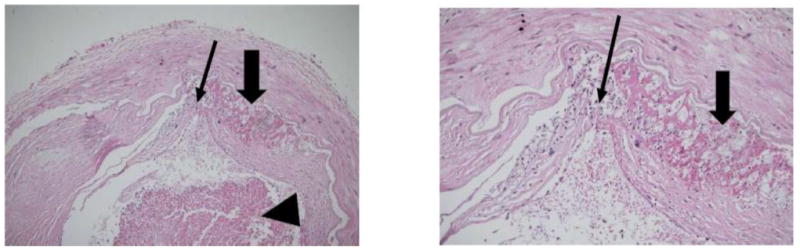
Basilar artery plaque with hemorrhage (thick arrow), ruptured fibrous cap (thin arrow) and luminal thrombus (arrowhead) (patient 5) (Original magnification: Left×100; Right×400)
Figure 2.
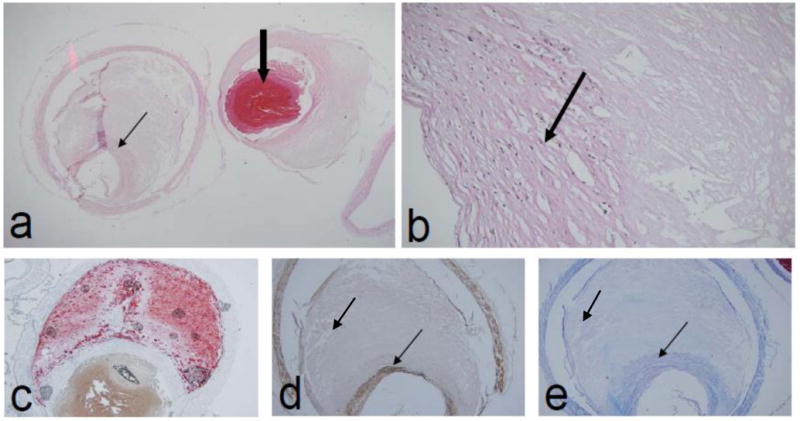
Basilar artery plaque with thick fibrous cap (a,b,d,e) rich in smooth muscle (d) and fibrous tissue (e) (thin arrows) (patient 6). The plaque has a lipid rich core demonstrated by oil red O stain (c) and 90% lumen stenosis. Thrombus (thick arrow) is post-mortem (patient 6) (Original magnification: a, c, d, e ×100; b×400)
Table 1. Characteristics of Atherosclerosis of Basilar Arteries.
| Patient | Percent Stenosis | Lumen Thrombus† | Necrotic Core† | Fibrous Cap | Inflammation‡ | Neovascularity | Calcification | Ruptue | Erosion | Ulcer | Age | Gender | Stroke Distribution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | N | Y | Thick | + | Y | Y | N | N | N | 71 | Female | LMCA |
| 2 | 40 | N | Y | Thick | ++ | N | N | N | N | N | 78 | Female | RMCA |
| 3 | 0 | N | N | - | ° | N | N | N | N | N | 91 | Male | LMCA |
| 4 | 30 | N | N | Thick | ° | N | N | N | N | N | 72 | Male | LMCA/Left Cerebellum |
| 5 | 30 | Y | Y | Thin | ° | N | N | Y | N | N | 88 | Female | RMCA |
| 6 | 90 | Y | Y | Thick | ++ | N | N | N | N | N | 93 | Male | LMCA |
| 7 | 10 | N | N | Thick | ° | N | N | N | N | N | 87 | Male | RMCA |
| 8 | 10 | N | N | Thick | ° | N | N | N | N | N | 77 | Male | LMCA |
Y: presence noted N: presence not noted
++: present in greater than moderate O: none noted (inflammation)
LMCA: left middle cerebral artery RMCA: right middle cerebral artery
Discussion
In this series, basilar artery atherosclerotic lesions had rare neovascularity, infrequent inflammation, and rare ulceration and rupture. This suggests a relatively benign histopathological profile for these lesions. The only plaque associated with lumen thrombus had a thin, ruptured fibrous cap (Figure 1). This is consistent with plaque rupture seen in coronary artery disease, in which plaque rupture is associated with an inflamed fibrous cap overlying a large plaque and necrotic core volume [12]. Undisrupted coronary lesions with similar pathology are considered vulnerable to rupture; the characteristics of the basilar artery plaque in patient 6 was considered vulnerable, given the presence of a large necrotic core and a thin fibrous cap (Figure 2). It is uncertain whether the thick fibrous-capped plaque of patient 4, who had acute cerebellar infarct, was the culprit lesion for the acute stroke.
Vasa vasorum abundance is commonly associated with plaque neovascularization, erythrocyte leak, and intraplaque hemorrhage [13-19]. Red blood cell membrane is a rich source of free cholesterol and contributes to large necrotic cores, and further perpetuates plaque inflammation [13,18,20,21]. Intracranial arteries have been observed to have limited or absent vasa vasorum [17], suggesting the possibility for a fundamentally distinct pathogenesis for intracranial atherosclerotic lesions.
We encountered neovascularity only rarely in this series. The arteries that contained the most inflammation (Patients 2 and 6) did not have associated neovascularity. Moreover, 3/4 arteries with a necrotic core did not show neovascularization. This suggests that the pathogenesis of a necrotic core may be different in the atherosclerosis of basilar artery. There was also lack of neovascularization in the basilar artery with the most extensive stenosis (90%) and inflammation (patient 6). These findings provide some support that neovascularity may have a relatively minor role in basilar artery atherosclerotic lesions.
Our study is limited by the small sample size and presence of relatively mild lesions in these largely asymptomatic basilar artery plaques. While the basilar artery has received limited attention, previous pathology studies of intracranial atherosclerosis have described complex lesions [22-24]. Note, however, that in a larger series examining middle cerebral artery stenosis, neovascularity was encountered in only 16% of plaques examined [22]. This is consistent with what we found in our basilar artery lesions, ie, paucity of neovasculature. Finally, our study focused on grossly visible lesions, and we cannot rule-out possible presence of pathologic changes occurring at non-macroscopic level.
In summary, basilar atherosclerosis lesions were relatively benign in this series. The lesions had rare neovascularity, infrequent inflammation, and rare ulceration and rupture. Confirmation of these results is needed in a larger pathological series. Relative paucity of neovascularity in these lesions suggests a potentially distinctive histopathological profile for basilar artery atherosclerosis.
Acknowledgments
Funding: Supported in part by NIH RO1 NS20989
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moossy J. Cerebral infarcts and the lesions of intracranial and extracranial atherosclerosis. Arch Neurol. 1966;14:124–8. doi: 10.1001/archneur.1966.00470080008002. [DOI] [PubMed] [Google Scholar]
- 2.Lhermitte F, Gautier JC, Derouesne C. Nature of occlusions of the middle cerebral artery. Neurology. 1970;20:82–8. doi: 10.1212/wnl.20.1.82. [DOI] [PubMed] [Google Scholar]
- 3.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–92. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 4.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race–ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 5.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–7. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 6.Castaigne P, Lhermitte F, Gautier JC, Escourolle R, Derouesne C, Der Agopian P, Popa C. Arterial occlusions in the vertebral-basilar system: A study of 44 patients with post-mortem-data. Brain. 1973;96:133–54. doi: 10.1093/brain/96.1.133. [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P, Hauw JJ, Gautier JC. Arterial pathology in cerebellar infarction. Stroke. 1990;21:1299–305. doi: 10.1161/01.str.21.9.1299. [DOI] [PubMed] [Google Scholar]
- 8.Amarenco P, Hauw JJ. Cerebellar infarction in the territory of the anterior and inferior cerebellar artery: A clinicopathological study of 20 cases. Brain. 1990;113:139–55. doi: 10.1093/brain/113.1.139. [DOI] [PubMed] [Google Scholar]
- 9.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1994;14:840–56. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- 10.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–31. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 11.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 12.Burke A, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–82. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 13.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries: a possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–7. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol. 1993;143:164–72. [PMC free article] [PubMed] [Google Scholar]
- 15.Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 1995;26:450–6. doi: 10.1016/0046-8177(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 16.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 17.Takaba M, Endo S, Kurimoto M, Kuwayama N, Nishijima M, Takaku A. Vasa vasorum of the intracranial arteries. Acta Neurochirurgica (Wien) 1998;140:411–6. doi: 10.1007/s007010050118. [DOI] [PubMed] [Google Scholar]
- 18.Moreno P, Purushothaman KR, Sirol M, Levy A, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–52. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 19.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–58. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–54. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 21.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 22.Chen XY, Wong KS, Lam WW, Zhao HL, Ng HK. Middle cerebral artery atherosclerosis: histopathologic comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis. 2008;25:74–80. doi: 10.1159/000111525. [DOI] [PubMed] [Google Scholar]
- 23.Tatsumi S, Yamamoto T. An autopsied case of an apparent pontine branch atheromatous disease. Eur Neurol. 2010;63:184–5. doi: 10.1159/000290248. [DOI] [PubMed] [Google Scholar]
- 24.Yin NS, Benavides S, Starkman S, Liebeskind DS, Saver JA, Salamon N, Jahan R, Duckwiler GR, Tateshima S, Vinuela F, Vespa PM, Chute DJ, Vinters HV. Autopsy findings after intracranial thrombectomy for acute ischemic stroke: A clinicopathologic study of 5 patients. Stroke. 2010;41:938–47. doi: 10.1161/STROKEAHA.109.576793. [DOI] [PMC free article] [PubMed] [Google Scholar]


