Abstract
Objective
Aging is a major risk factor for increased ischemic tissue injury. Whether collateral rarefaction and impaired remodeling contribute to this is unknown. We quantified the number and diameter of native collaterals, and their remodeling in 3-, 16-, 24-, and 31-months-old mice.
Methods and Results
Aging caused an “age-dose-dependent” greater drop in perfusion immediately after femoral artery ligation, followed by a diminished recovery of flow and increase in tissue injury. These effects were associated with a decline in collateral number, diameter and remodeling. Angiogenesis was also impaired. Mechanistically, these changes were not accompanied by reduced recruitment of T-cells or macrophages to remodeling collaterals. However, eNOS signaling was dysfunctional, as indicated by increased protein nitrosylation and less phosphorylated eNOS and VASP in collateral wall cells. The cerebral circulation exhibited a similar age-dose-dependent loss of collateral number and diameter and increased tortuosity, resulting in an increase in collateral resistance and infarct volume (e.g., 6- and 3-fold, respectively, in 24-months-old mice) after artery occlusion. This was not associated with rarefaction of similarly-sized arterioles. Collateral remodeling was also reduced.
Conclusions
Our findings demonstrate that aging causes rarefaction and insufficiency of the collateral circulation in multiple tissues, resulting in more severe ischemic tissue injury.
Keywords: collateral vessels, aging, ischemia, arteriogenesis, angiogenesis
Introduction
The occurrence of coronary and peripheral artery diseases increases with age, even in a population without other major risk factors.1,2 The prevalence of peripheral arterial disease is 5-10 percent, whereas 15-20 percent of individuals over 70 years of age are affected.3,4 The risk for stroke and transient ischemic attack also increases with age.5 Besides prevalence, mortality due to ischemic cardiovascular disease is higher in elderly individuals.2,6 Thus, the severity of tissue injury following acute and chronic ischemia are increased by aging.
Mechanisms of age-associated decline in vascular function are complicated. Changes occur in cell signaling and matrix that predispose toward initiation and progression of cardiovascular diseases. For example, endothelial dysfunction is a prominent feature of the aged vascular wall.7-9 Many of the complications associated with cardiovascular risk factors and aging are initially attributable, at least in part, to attenuated endothelial function.7-9 Subsequent structural changes proceed, eg, intima-medial thickening and changes in extracellular matrix as occurs during atherosclerosis, aging and hypertension.10 Additionally, evidence suggests that collateral remodeling is impaired, in part, by dysfunctional eNOS signaling and oxidative stress.11,12
Recent studies in mice suggest that the extent of the native collateral circulation (ie, pre-existing collateral number and diameter) is a primary determinant of the severity of tissue injury following acute arterial obstruction. Native collateral extent defines not only the initial conductance of the network after obstruction, but also the number of collaterals available for remodeling—a process that takes days-to-weeks.13,14However, no studies have determined whether aging reduces the extent of the collateral circulation and results in greater ischemic injury.
Here we report that aging is accompanied by a progressive loss of collateral number and diameter and increased tortuosity—changes that significantly increase resistance of the collateral circulation. These alterations, which are associated with impaired eNOS signaling, are compounded by impaired collateral remodeling after vascular occlusion, resulting in worse ischemic injury. The findings may explain, in part, the inconclusive results of previous therapeutic trials aimed at enhancing collateral function in older individuals with ischemic cardiovascular disease.
Methods
An expanded Materials and Methods section is available at http://atvb.org.
Male C57BL/6 mice received right femoral artery ligation (FAL) distal to the lateral caudal femoral artery. Plantar foot (index of overall leg perfusion14) of each leg was measured before and immediately, 3 and 7 days after FAL. Right hindlimb appearance and use scores (indexes of muscle ischemia and function) were obtained.11,14 Seven days later, histology was performed on the center-most region of the adductor musculature of the chronically ligated right and acutely ligated left legs to obtain lumen diameter at the ~midpoint of the anterior and posterior gracilis muscle collaterals,11,14and to determine the number of CD3+ and CD11b+ cells11 and phospho-eNOS and phospho-VASP levels in their peri-collateral region. In adjacent sections, the number of α-smooth muscle actin (SMA)-positive vessels (primarily collaterals) crossing the midpoint of the main adductor muscle (semimembranosis) were counted.11,14
Capillary density, fiber size and number were obtained in the gastrocnemius of both legs. Collaterals connecting the middle and anterior cerebral artery (MCA, ACA) trees of both hemispheres were examined for number, lumen diameter (D) at their midpoint, length, tortuosity (vector length/axial length (l)) and collateral circuit resistance (l/n*D4).13 In 31-months-old mice number and diameter of distal-most Type I or Type II arterioles (DMA) (not connecting or connecting to a collateral, respectively) of the MCA tree were also quantified, as was cerebral cortical area and territories of the MCA, ACA and posterior cerebral artery (PCA) trees.13,14 The number of penetrating arterioles branching from pial collaterals was quantified. These measures were made in 31-months-old mice that showed the greatest rarefaction.
In a separate group of 24-months-old mice, infarct volume and collateral remodeling were determined 3 days after permanent right MCA occlusion (MCAO).13 31-months-old mice were not examined because of reduced chance of surviving MCAO. Circulating blood cell differential analysis (CBC) was performed. Western blotting was conducted on mesenteric artery for nitrotyrosine, because the minute size of native collaterals prevents such analysis. Data were subjected to ANOVA followed by Dunn-Bonferroni corrected t-tests for pre-planned comparisons, or Student’s t-tests.
Results
Aging decreases collateral number and diameter and impairs collateral remodeling, angiogenesis and recovery of perfusion in ischemic hindlimb
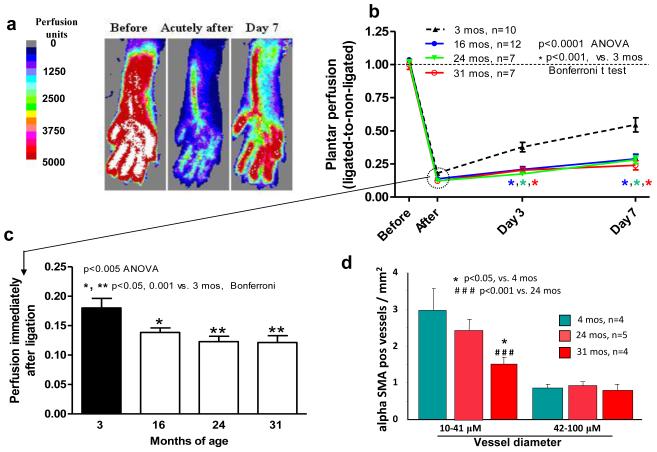
We first examined whether aging causes collateral rarefaction using mouse hindlimb. Approximately 5 minutes after FAL (time required for Doppler scanning), perfusion was lower in 16-, 24- and 31-months-old compared to young (3-months-old) mice (Fig 1a-c). Baseline perfusion before ligation did not differ between right and left legs or with aging (1236±38, 1214±22, 1190±30 and 1119±62 perfusion units for 3-, 16-, 24- and 31-months-old mice; p=0.11). Aging is well-known to favor an increase in arterial pressure, thus indicating that lower perfusion immediately after acute femoral artery ligation in the aged groups cannot be attributed to lower arterial pressure. These data therefore suggest aging causes a loss of number and/or diameter of native collaterals. Aged mice also had reduced recovery of perfusion (Fig 1b), suggesting impaired collateral remodeling (arteriogenesis). Later time-points were not obtained because our main goal was to assess the effect of aging on the native collateral circulation, and because others have demonstrated lower recovery of perfusion after FAL with aging, although mechanisms vis-à-vis rarefaction and collateral remodeling were not studied.15-17
Figure 1. Aging causes greater drop in perfusion immediately after femoral artery ligation (FAL), poorer recovery of perfusion, and fewer α-smooth muscle actin (SMA)-positive vessels in adductor collateral zone.
a, Laser Doppler perfusion imaging of plantar foot (correlates with leg flow). b, Plantar perfusion. c, Comparison of perfusion immediately after FAL. d, Number of αSMA-positive arterioles in collateral zone of semimembranosis muscle (16 months-old-group missing due to processing complications). Data are mean ± SEM and n-sizes are for number of mice, in this and other figures and tables.
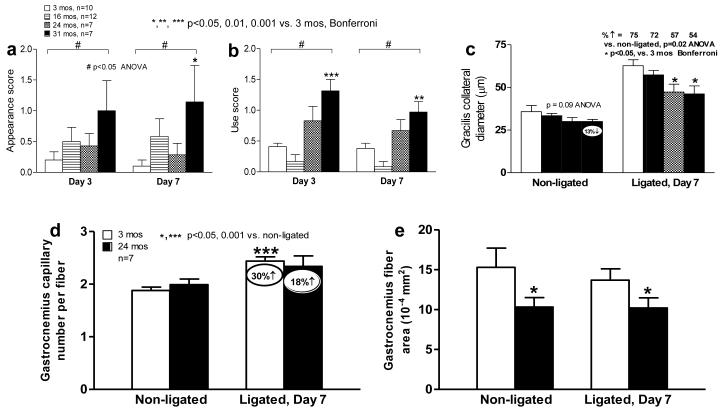
The number of α-SMA-positive vessels at the mid-point (i.e., collateral zone) of the semimembranosis muscle was determined in the non-ligated limb, as an index of native collaterals.11,14 The number of 10-41 micron-diameter vessels declined with aging (Fig 1d). Since venules of these diameters lack smooth muscle and native collaterals in the adductor muscles average ~22 microns diameter,11,14 these data suggest loss of collaterals with aging. This conclusion, which is consistent with the greater drop in perfusion immediately after ligation, is also supported by the higher appearance and use scores for the ligated limb, indicating worse ischemia (Fig 2a,b).
Figure 2. Aging causes collateral circulatory insufficiency in hindlimb.
a-c, Greater hindlimb ischemia, use impairment, tendency for decline in native collateral diameter (before FAL), and reduced collateral remodeling after FAL. d, Aging impairs ischemic angiogenesis. e, Baseline muscle atrophy (average muscle fiber cross-sectional area) in aged mice. No ligation-induced atrophy at day-7 after FAL in this moderate ligation model. Decline in baseline collateral diameter and subsequent outward remodeling contribute to findings in Fig 1; less ischemic angiogenesis may also contribute to reduced recovery of perfusion on day-3 and day-7 after FAL.
These findings were confirmed for the anatomically distinct collateral present in each of the anterior and posterior gracilis muscles. Lumen diameter trended smaller (eg, 13% lower in the non-ligated limb of 31-months-old mice, p=0.09) (Fig 2c). Remodeling of these collaterals in the ligated limb (75% increase 7 days after ligation in young mice) was less with aging (54% in 31-months-old mice, p=0.02) (Fig 2c).
In 24-months-old mice, baseline capillary density in the non-ligated left leg was not different from young mice (Fig 2d). This suggests, together with the above evidence for fewer collaterals of smaller diameter in aged mice, that the greater drop in perfusion immediately after ligation (Fig 1b,c) is not due to reduction of capillary density. Aged mice had impaired angiogenesis (Fig 2c), which could contribute modestly to their impaired recovery of perfusion (Fig 1b). Gastrocnemius fiber size was smaller in aged mice, reflecting known age-associated muscle atrophy (Fig 2e). Aged mice did not evidence greater atrophy after FAL, likely because atrophy was already evident, a model of moderate rather than severe ischemia was used, and C57BL/6 mice have abundant collaterals.11,14 The latter two considerations also explain the modest angiogenesis observed. The data in figure 2 for the 3-months-old mice agree with our previous studies in C57BL/6 mice of the same age.11,14
Collateral remodeling is initiated by increased shear stress after arterial obstruction, followed by recruitment of hematopoietic cells to the peri-collateral region where they release cytokines and growth factors that direct remodeling. However, we did not detect a difference in abundance of peri-collateral T lymphocytes (CD3+)and macrophages (CD11b+) in young and 24-months-old mice (Supplemental fig I; for immunohistochemical controls, see Supplemental figs II,III). Also, the number of circulating lymphocytes and monocytes did not differ (Supplemental tables I,II). These data suggest that impaired collateral remodeling with aging (Fig 2c) is not due to reduced leukocyte recruitment.
Aging decreases the number and diameter of native collaterals and impairs collateral remodeling in the brain, resulting in increased infarct volume
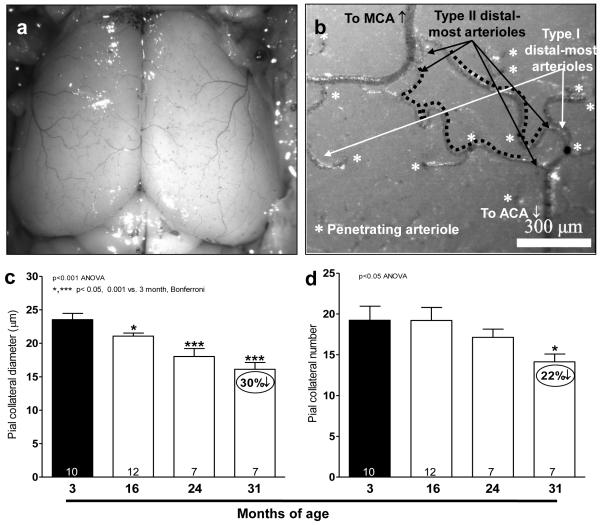
Similar to skeletal muscle, native pial collateral number and diameter also declined (Fig 3c,d), and collateral tortuosity doubled by 16-months-age (Fig 3e-g). Collateral span increased slightly, reflecting tortuosity acquired by the ends of the distal-most arterioles (DMA) connecting to collaterals. The ~40% decline in tortuosity in the 31-months-old mice (relative to the increase in 16-versus 3-months-old mice) is significantly larger than the 5% shrinkage of the cortex (see below) in this advanced age group. Thus, collateral tortuosity increases and then subsides with aging. The decrease in collateral number and diameter, plus increase in length, increased relative resistance of the collateral circulation by as much as 10-fold (Fig 3h).
Figure 3. Aging causes rarefaction of native cerebral collateral diameter and number (a-d).
a,b, Image of dilated, fixed and filled pial cortical circulation; higher magnification image showing two collaterals (black dotted lines) and penetrating arterioles (stars) branching from type II (black arrows) and type I (white arrows) distal-most arterioles that either are or are not cross-connected by collaterals, respectively. MCA, ACA, middle and anterior cerebral artery, respectively. c,d, All collaterals interconnecting MCA and ACA trees in both cerebral hemispheres were quantified. Increased collateral tortuosity and resistance of the native collateral circulation with aging (e-h). e,f, Collateral length (l), axial length of pial collateral. Collateral span (L), scalar length connecting both ends of collateral. h, Relative resistance of the pial collateral circulation, calculated as collateral length / (collateral number x diameter4), is 6- and 10-fold higher in 24- and 31-months-old groups than 3-months-old group. All collaterals between MCA and ACA in both hemispheres were quantified.
This collateral insufficiency, which resulted in a 6-fold increase in collateral resistance (Fig 3h), was associated with 3-fold larger infarct volume3 days after permanent MCAO in 24-months-old mice (Fig 4a-c). This cannot be attributed to increased MCA territory, because territory decreased by 5% (discussed below). Pial collateral remodeling was also examined 3 days after MCAO, when it has reached maximum in C57BL/6 mice.13 Aged mice had smaller baseline collateral diameters (Fig 3d), confirming the trend we detected in skeletal muscle (Fig 2). Collateral remodeling was 44% less in old mice (p<0.05) (Fig 4d insert graph). The above cerebral and hindlimb data suggest that aging is associated in multiple tissues with a loss of native collateral number and diameter, plus impaired collateral remodeling.
Figure 4. Increased cerebral infarct volume and less collateral remodeling in aged mice.
a,b, Cerebral infarct volume (A, white area in each slice) was measured 3 days after permanent MCA occlusion. c, Pial collaterals (black arrows) after dilation, fixation and filling with contrast material. d, Native collateral diameter is smaller in aged mice (non-occluded). Aging impairs collateral remodeling (% increase is relative to 3-months-old, p=0.03); inset, change in diameter. White bar, 3-months-old; black bar, 24-months-old.
Mechanisms of aging-associated collateral circulatory insufficiency
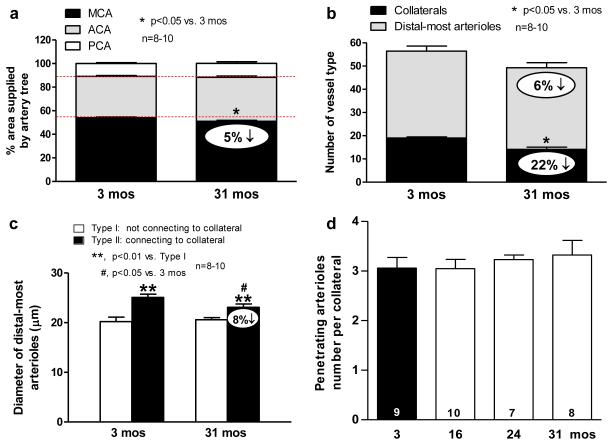
The MCA tree was 5% smaller in 31- versus 3-months-old mice (Fig 5a), and thus showed a similar 6% reduction in the number of DMAs (Fig 3b) within the crown of the MCA tree (Fig 5b). These changes agree with the 5% smaller cortex in this aged group (Supplemental fig IV). They are also consistent with absence of correlation of differences in collateral number and MCA tree size among 15 inbred mouse strains.13
Figure 5. Cerebral morphometry in young and old mice.
a. Percent area of cortex supplied by MCA, ACA and PCA trees. 5% smaller MCA territory with aging; this favors smaller infarct volumes, opposite to Figure 6. b. MCA tree morphometry: similar 6% decrease in distal-most arterioles (DMA, both Type I and II) of MCA tree as 5% decrease in MCA territory. Collateral number decreased by similar absolute amount (though greater percentage) as DMAs, indicating concomitant loss of both. c, Type II DMAs are larger than Type I, consistent with greater flow of Type 2 to supply penetrating arterioles branching from collaterals. Aging decreases Type II diameter. This is consistent with decline in diameter of the collaterals they supply (Figure 3), which may relate to the mechanism of collateral loss (see Text). d, Collaterals not lost have same number of penetrating arterioles branching from them.
While the 6% decrease in DMAs is smaller than the 22% decrease in collateral number (Fig 5b), the absolute decreases in collaterals and DMAs are comparable (Fig 5b). This suggests that aging causes loss of native collaterals by a pruning process that involves or is preceded by a decline in diameter (Figs 2c,3d, 4d)—a process unique to collaterals (see below)—that extends retrograde to include the DMA from which the collateral arises. Consistent with this, the diameters of DMAs that connect to the extant collaterals (“Type II” DMAs, Fig 3b) in aged mice are smaller than in young mice, while the diameters of “Type I” DMAs (which do not connect to collaterals) are not different (Fig 5c). The larger diameter of Type II DMAs in both young and old mice would be expected given the greater flow carried by these arterioles to the collaterals which, in turn, supply penetrating arterioles that branch from the collaterals into the cortex (Fig 3b). The number of penetrating arterioles branching from collaterals did not differ among aged mice (Fig 5d), suggesting that collaterals are not lost with aging because of loss of penetrating arterioles branching from them. Also, as with hindlimb capillary density, DMAs and penetrating arterioles did not decline in aged mice. These findings suggest that the collateral circulation may be especially susceptible to rarefaction with aging.
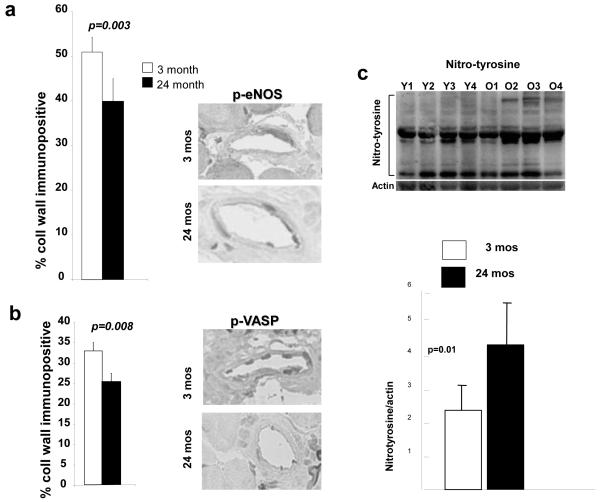
eNOS activation contributes to shear-stress-induced collateral remodeling,11and aging is accompanied by reduced eNOS/NO activity/bioavailability.7-9,18-20 Moreover, native collateral number is reduced in eNOS deficient mice, suggesting that NO is a maintenance factor for the collateral circulation.11 We therefore examined gracilis collaterals for phospho-eNOS (necessary for eNOS activation) and phospho-VASP (which undergoes phosphorylation when NO is increased). Before ligation, baseline phospho-eNOS and phospho-VASP were lower in aged mice (Fig 6a,b). Nitrotyrosine was increased in mesenteric arterioles (Fig 6c). These and previous findings11 suggest a role for impaired eNOS/NO signaling in collateral loss and reduced remodeling with aging.
Figure 6. Phospho-eNOS and phospho-VASP are decreased in aged collaterals.
Immunohistochemistry of gracilis muscle collaterals for phosphorylated eNOS (a) and phosphorylated VASP (b), and for nitrotyrosine in mesenteric arterioles (c) in 3-months-old (Y1-Y4, young) and 24-months-old (O1-O4, old) mice.
Discussion
The extent (density and diameter) of the native collateral circulation in healthy tissues varies widely as a function of differences in genetic background.13,14 Environmental factors may also impact collateral extent. The present study examined the hypothesis that one omnipresent environmental factor—the natural aging process—adversely affects the collateral circulation. Indeed, nitric oxide (NO) deficiency results in loss of collaterals during natural growth to adulthood,11 and endothelial/NO dysfunction accompanies aging.7-9,19,20 We found that aging causes a decline in the number and diameter of collaterals in skeletal muscle, resulting in larger decreases in blood flow and greater tissue injury following occlusion. Similar collateral insufficiency also occurred in brain, resulting in a 6-fold increase in collateral resistance and 3-fold increase in severity of infarct volume after middle cerebral artery occlusion in 24- versus 3-months-old-mice. Rarefaction was specific to the collateral circulation, and was associated with impaired eNOS/NO signaling in the collateral wall—a finding congruent with evidence that eNOS-deficient mice loose collaterals in these same tissues by 3-months-age.11 While multiple mechanisms are likely involved, these data suggest that aging-induced collateral insufficiency in humans could contribute significantly to the increase in severity of ischemic tissue injury in the later decades of life.
Our previous studies have shown that strain-specific differences in the extent of the native collateral circulation in the cerebral cortex mirror similar changes in other tissues.14,21 However, unlike in other tissues, the arterial trees and interconnecting collaterals are restricted to the pial cerebral circulation, permitting robust morphometry.11 These considerations allowed us to examine whether the magnitude of decline in collateral density and diameter with aging simply reflected arteriolar rarefaction in the general arterio-venous circulation. However, these declines were not observed for similarly-sized distal-most arterioles (DMAs) and penetrating arterioles. The diameter of DMAs in the MCA tree that end by descending into the cortex was the same in 3-and 24-months-old mice. However, those DMAs that continued as collaterals had smaller diameters in the aged mice, in accordance with the smaller diameter of their cognate collaterals.
Neither did aging cause a generalized loss of DMAs (loss of DMAs and collaterals were similar) or penetrating arterioles branching from the collaterals. Others have reported fewer collaterals in the cerebral circulation of aged rats and cats.22,23 In patients with acute myocardial infarction or stable coronary artery disease, the presence of Rentrop-defined coronary collaterals is inversely related to age.33,34 However, whether this results from a decline in native collateral extent before or from reduced collateral remodeling after onset of coronary atherosclerosis, and thus less detection, cannot be distinguished in such studies.
We speculate that collaterals are progressively pruned with aging, and that this is accompanied by pruning of the DMAs that they connect to, rather that visa versa. Potential reasons why collaterals might be especially susceptible to age-induced pruning are discussed below. Similar to the absence of a generalized rarefaction of pial arterioles, capillarity in skeletal muscle did not decline with aging even though, as expected, skeletal muscle fibers underwent atrophy. Decline in capillary density in aged rats is controversial and may be tissue-specific.24-34
Aging also inhibited remodeling of skeletal muscle and cerebral collaterals. This provides direct evidence that impaired remodeling likely contributed to reduced recovery of hindlimb flow after FAL reported in aged animals,16,18,35 wherein impaired increases in Hif1α, VEGF, angiopoietins, SDF1 and other cytokines in ischemic skeletal muscle were also observed. A probable mechanism for these observations is that shear-mediated increase in eNOS expression is reduced by aging,36 as are Hif1α and VEGF,8,16 each normally contributing significantly to collateral remodeling.11,21 Our current findings that phospho-eNOS (serine 1177) and a downstream marker/target of normal eNOS signaling—phospho-VASP are decreased in the wall of collateral vessels in old mice provide evidence that aging-induced impaired eNOS signaling contributes to the reduced collateral remodeling seen with aging.
Interestingly, exogenous VEGF35 or restoration of Hif1α expression16 ameliorated the decline in recovery of limb blood flow in aged mice, although the VEGF-induced improvement was not confirmed in humans with peripheral artery disease.37 Ligation-induced remodeling of ileal arteries upstream of a collateral circuit within the intestine was reduced in aged rats and in a rat strain with accelerated aging.38
The complexity of the eNOS system is illustrated by that fact that other studies demonstrated phospho-eNOS (serine 1177) in endothelial cells from brachial arteries and antecubital veins of 61- versus 21-year-old individuals was higher and total eNOS unchanged.19 Aside from the heterogeneity of human samples, increased phospho-eNOS does not provide definitive evidence of intact eNOS signaling. Downstream signaling triggered by phosphorylation of eNOS is dysfunctional when eNOS is uncoupled by aging and other conditions associated with cardiovascular risk. Thus, Akt-mediated eNOS phosphorylation, which normally enhances production of NO, increased eNOS-derived superoxide rather than NO when eNOS was uncoupled.39 We also found aged mice exhibited increased protein nitrosylation (a marker of oxidative stress) in mesenteric arteries. This finding, plus the decreased formation of phospho-eNOS and phospho-VASP in the collateral wall of aging mice, are consistent with the concept that aging-associated disturbance in eNOS signaling19 occurs in collateral vessels, as it does in the general circulation, resulting in impaired collateral remodeling in occlusive arterial disease.
Tortuosity, a signature characteristic of collaterals, increases as collaterals remodel during chronic increases in shear stress.13 We found that tortuosity of native pial collaterals more than doubled between the ages of 3 and 16 months of age (the latter equivalent to ~55-year-old human). Tortuosity also increases, though much more modestly, in arteries and arterioles of healthy aged rats and humans.40
In the present study aging caused an “age-dose-dependent” rarefaction of collaterals. This was associated with a progressively more severe ischemic injury of hindlimb tissue, decrease in conductance of the cerebral collateral network and worse ischemic stroke. Many homeostatic systems decline with aging, thus direct proof for cause and effect is difficult to establish. Future work studying eNOS-transgenic or VEGF hypermorphic aged mice could help address this, since eNOS/NO and VEGF signaling, which decline with aging, also act as collateral “maintenance factors” and oppose rarefaction.10,21 One could also conduct logit regression analysis of collateral extent and stroke severity across a wide range of ages in CD1 mice, given the large variation in collateral extent in this genetically varying outbred strain.21 Nevertheless, a central role played by collateral extent, per se, in ischemic tissue injury was strongly suggested in a study of 15 strains of 3-month-old mice, where variation in stroke volume tightly correlated with variation in collateral number and diameter,13 and in a subsequent study of a smaller number of strains subjected to hindlimb ischemia.14
Although identification of the mechanisms responsible for the loss of collateral density and diameter require additional study, we offer two hypotheses. One is based on the unique hemodynamic stress that characterizes the normal collateral environment. The absence of a net pressure drop across collaterals in healthy tissues sets the prevailing environment as one of low and disturbed (oscillatory) shear stress and high circumferential wall stress.41 These conditions could predispose the collateral circulation to “accelerated aging”, compared to the general circulation, resulting in collateral rarefaction. A second possible cause relates to eNOS/NO signaling as a “collateral maintenance factor”.11 Thus, we have found that endothelial cells derived from aged mice exhibit an increased propensity to apoptosis in association with impaired eNOS/NO activity (unpublished results). This predisposition is “rescued” by exposure to an NO donor, findings compatible with the concept that endothelial cell dropout may, perhaps in conjunction with the first speculation above, underlie collateral rarefaction. Consistent with this, genetic eNOS-deficiency which has many of the features of the aged general cardiovascular system causes accelerated loss of collaterals in young mice.11 Future investigation is needed to identify the underlying mechanisms and promote new therapeutic strategies to prevent or reverse collateral rarefaction in the aging vasculature.
Supplementary Material
Acknowledgement
We thank Kirk McNaughton and Kim Etzel (UNC) for histological sectioning.
Sources of Funding
This work was supported by NIH grants HL62584 and HL090655 (JEF) and HL085003 (SEE).
Non-standard Abbreviations and Acronyms
- FAL
femoral artery ligation
- DMA
distal-most arteriole
- MCA
middle cerebral artery
- ACA
anterior cerebral artery
- PCA
posterior cerebral artery
- MCAO
middle cerebral artery occlusion
- VASP
vasodilator-stimulated phosphoprotein
- SMA
smooth muscle α-actin
- CBC
circulating blood cell analysis
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper LT, Cooke JP, Dzau VJ. The vasculopathy of aging. J Gerontol. 1994;49:B191–196. doi: 10.1093/geronj/49.5.b191. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Pedelty L, Gorelick PB. Management of hypertension and cerebrovascular disease in the elderly. Am J Med. 2008;121(8 Suppl):S23–31. doi: 10.1016/j.amjmed.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Eliason JL, Wainess RM, Proctor MC, Dimick JB, Cowan JA, Jr, Upchurch GR, Jr, Stanley JC, Henke PK. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–90. doi: 10.1097/01.sla.0000086663.49670.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohta T, Hosaka M, Ishibashi H, Sugimoto I, Mihara E, Hida K, Takeuchi N, Hachiya J, Kato M, Kazui H, Nagata Y. Limb salvage and survival rates among elderly patients with advanced limb ischemia. Surg Today. 1998;28:156–61. doi: 10.1007/s005950050098. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Reeves MJ, Zhao X, Olson DM, Smith EE, Saver JL, Schwamm LH. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121:879–891. doi: 10.1161/CIRCULATIONAHA.109.892497. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–207. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: New perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–41. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen A, Thorin-Trescases N, Thorin E. Working under pressure: Coronary arteries and the endothelin system. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1188–94. doi: 10.1152/ajpregu.00653.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004;26:689–699. doi: 10.1081/ceh-200031982. [DOI] [PubMed] [Google Scholar]
- 11.Dai X, Faber JE. eNOS deficiency causes collateral vessel rarefaction and impairsactivation of a cell cycle gene network during arteriogenesis. Circ Res. 2010;106:1870–81. doi: 10.1161/CIRCRESAHA.109.212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsunaga T, Warltier DC, Weihrauch DW, Moniz M, Tessmer J, Chilian WM. Ischemia-induced coronary collateral growth is dependent on vascular endothelial growth factor and nitric oxide. Circulation. 2000;102:3098–3103. doi: 10.1161/01.cir.102.25.3098. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–34. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalothorn D, Faber JE. Strain-dependent variation in native collateral function in mouse hindlimb. Physiol Genomics. 2010;42:469–79. doi: 10.1152/physiolgenomics.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res. 2007;101:1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 17.Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 18.Wang CY, Wen MS, Wang HW, Hsieh IC, Li Y, Liu PY, Lin FC, Liao JK. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–73. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297:H1829–36. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–36. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–20. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi S, Kobayashi S, Murata A, Yamashita K, Tsunematsu T. Effect of aging on collateral circulation via pial anastomoses in cats. Gerontology. 1988;34:157–64. doi: 10.1159/000212946. [DOI] [PubMed] [Google Scholar]
- 24.Kurotobi T, Sato H, Kinjo K, Nakatani D, Mizuno H, Shimizu M, Imai K, Hirayama A, Kodama K, Hori M, OACIS Group Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J Am Coll Cardiol. 2004;44:28–34. doi: 10.1016/j.jacc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 25.Nathoe HM, Koerselman J, Buskens E, van Dijk D, Stella PR, Plokker TH, Doevendans PA, Grobbee DE, de Jaegere PP. Determinants and prognostic significance of collaterals in patients undergoing coronary revascularization. Am J Cardiol. 2006;98:31–5. doi: 10.1016/j.amjcard.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 26.Degens H, Morse CI, Hopman MT. Heterogeneity of capillary spacing in the hypertrophied plantaris muscle from young-adult and old rats. Adv Exp Med Biol. 2009;645:61–6. doi: 10.1007/978-0-387-85998-9_10. [DOI] [PubMed] [Google Scholar]
- 27.McCully KK, Posner JD. The application of blood flow measurements to the study of aging muscle. J Gerontol A BiolSci Med Sci. 1995;50:130–6. doi: 10.1093/gerona/50a.special_issue.130. Spec No. [DOI] [PubMed] [Google Scholar]
- 28.Bär T. Morphometric evaluation of capillaries in different laminae of rat cerebral cortex by automatic image analysis: changes during development and aging. Adv Neurol. 1978;20:1–9. [PubMed] [Google Scholar]
- 29.Hunziker O, Abdel’Al S, Schulz U. The aging human cerebral cortex: a stereological characterization of changes in the capillary net. Gerontol. 1979;34:345–50. doi: 10.1093/geronj/34.3.345. [DOI] [PubMed] [Google Scholar]
- 30.Meier-Ruge W, Hunziker O, Schulz U, Tobler HJ, Schweizer A. Stereological changes in the capillary network and nerve cells of the aging human brain. Mech Aging Dev. 1980;14:233–43. doi: 10.1016/0047-6374(80)90123-2. [DOI] [PubMed] [Google Scholar]
- 31.Hughes CC, Lantos PL. A morphometric study of blood vessel, neuron and glial cell distribution in young and old rat brain. J Neurol Sci. 1987;79:101–10. doi: 10.1016/0022-510x(87)90264-4. [DOI] [PubMed] [Google Scholar]
- 32.Jucker M, Bättig K, Meier-Ruge W. Effects of aging and vincamine derivatives on pericapillary microenvironment: stereological characterization of the cerebral capillary network. Neurobiol Aging. 1990;11:39–46. doi: 10.1016/0197-4580(90)90060-d. [DOI] [PubMed] [Google Scholar]
- 33.Hutchins PM, Duseau JW, Marr MC, Green AW. The role of arteriolar structural changes in hypertension. In: Henrich H, editor. Microvascular Aspects of Spontaneous Hypertension. Hans Huber; Bern: 1982. pp. 41–53. [Google Scholar]
- 34.Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–29. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Lei L, Liang Y, Hinh L, Hickey RP, Huang Y, Liu D, Yeh JL, Rebar E, Case C, Spratt K, Sessa WC, Giordano FJ. An engineered VEGF-activating zinc finger protein transcription factor improves blood flow and limb salvage in advanced-age mice. FASEB J. 2006;20:479–81. doi: 10.1096/fj.04-3670fje. [DOI] [PubMed] [Google Scholar]
- 36.Miyashiro JK, Poppa V, Berk BC. Flow-induced vascular remodeling in the rat carotid artery diminishes with age. Circ Res. 1997;81:311–9. doi: 10.1161/01.res.81.3.311. [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan S, Mohler ER, 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–8. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 38.Sheridan KM, Ferguson MJ, Distasi MR, Witzmann FA, Dalsing MC, Miller SJ, Unthank JL. Impact of genetic background and aging on mesenteric collateral growth capacity in Fischer 344, Brown Norway, and Fischer 344 x Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2007;293:H3498–505. doi: 10.1152/ajpheart.00040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan JC, Loomis ED, Collins M, Imig JD, Inscho EW, Pollock JS. Age-related alterations in NOS and oxidative stress in mesenteric arteries from male and female rats. J Appl Physiol. 2004;97:1268–74. doi: 10.1152/japplphysiol.00242.2004. [DOI] [PubMed] [Google Scholar]
- 40.Cook JJ, Wailgum TD, Vasthare US, Mayrovitz HN, Tuma RF. Age-related alterations in the arterial microvasculature of skeletal muscle. J Gerontol. 1992;47:B83–88. doi: 10.1093/geronj/47.3.b83. [DOI] [PubMed] [Google Scholar]
- 41.Chalothorn D, Faber Formation and maturation or the murine native cerebral collateral circulation. J Molec Cell Cardiol. 2010;49:251–259. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.