Abstract
The purpose of this article is to review the basic and clinical science relating plasma triglycerides and cardiovascular disease. Although many aspects of the basic physiology of triglyceride production, its plasma transport and tissue uptake have been known for several decades, the relationship of plasma triglyceride levels to vascular disease is uncertain. Are triglyceride rich lipoproteins, their influence on HDL and LDL, or the underlying diseases leading to defects in triglyceride metabolism the culprit? Animal models have failed to confirm that anything other than early fatty lesions can be produced by triglyceride-rich lipoproteins. Metabolic products of triglyceride metabolism can be toxic to arterial cells; however, these studies are primarily in vitro. Correlative studies of fasting and postprandial triglycerides and genetic diseases implicate VLDL and their remnants, and chylomicron remnants in atherosclerosis development; but the concomitant alterations in other lipoproteins and other risk factors obscure any conclusions about direct relationships between disease and triglycerides. Genes that regulate triglyceride levels also correlate with vascular disease. Human intervention trials, however, have lacked an appropriately defined population, and have produced outcomes without definitive conclusions. The time is more than ripe for new and creative approaches to understanding the relationship of triglycerides and heart disease.
Keywords: lipoproteins, lipase, atherosclerosis, hyperlipidemia, vascular disease
INTRODUCTION
Why has defining the relationship between circulating triglycerides and vascular disease been so evasive? Experimental biologists, epidemiologists, and clinical trialists have struggled with this issue. Guideline panels have skirted the issue with recommendations to calculate and treat non-HDL cholesterol. As we will discuss, some view triglycerides as merely the markers for other lipoprotein or non-lipoprotein abnormalities, which might be the real disease culprits. After all, the hallmark of atherosclerosis is cholesterol not triglyceride accumulation in the artery. At this time a number of panels are considering guidelines for evaluation and treatment of human hypertriglyceridemia. This review is not meant to duplicate these efforts. Rather its role is to illustrate controversy and thereby suggest areas in need of experimental and clinical investigation.
TRIGLYCERIDE UPTAKE AND TRANSPORT
Plasma triglycerides are primarily produced by the intestines and the liver. Dietary triglycerides enter the circulation within chylomicrons. Triglycerides assembled from de novo synthesized fatty acids and from lipids returning to the liver are secreted in VLDL.
Chylomicron production and catabolism
Most dietary fat is digested by pancreatic enzymes that hydrolyze ester bonds and release free fatty acids (FFAs). In a seemingly wasteful process but one that allows transcellular movement of lipids, the FFAs are absorbed in enterocytes and then reesterified. Chylomicron formation requires assembly of triglycerides, phospholipids and apoproteins including apolipoprotein (apo) B48, apoA-I, apoA-IV, apoE and apoCs, and microsomal triglyceride transport protein (MTTP). Chylomicrons flow from the lymphatics to the circulation where they exchange some of their surface apoproteins; apoA-IV dissociates and the particles are enriched with apoC-II, the activator of lipoprotein lipase (LpL).
Several factors regulate plasma triglyceride levels either by altering lipolysis or secretion. One is low HDL itself. Almost all human HDL deficiencies are associated with hypertriglyceridemia. This was recapitulated in the apoA-I knockout mice. Increased triglyceride in this animal was ascribed to a defect in lipolysis due to a deficiency of HDL apoC-II1. Another possible cause of increased triglyceride, greater production, was found in liver specific ABCAI knockout mice 2.
Regulation of remnant catabolism
Triglyceride within the chylomicrons is converted into FFAs, monoglycerides and glycerol producing a smaller, lipid depleted remnant particle. Most remnant particles are cleared from the circulation via hepatic LDL receptors. The lack of major hypertriglyceridemia in LDL receptor knockout mice shows the importance of backup processes. In this regard knockouts of syndecan 1 proteoglycan3, LDL receptor related protein (LRP1)4 and scavenger receptor-B15 lead to defective uptake of remnant lipoproteins and, in some cases, modest hypertriglyceridemia. In the setting of lipoprotein overproduction, deficiency of the VLDL receptor also leads to hypertriglyceridemia, probably due to a defect in LpL actions 6–8
Hepatic synthesis of VLDL triglyceride
Hepatic production of triglycerides is coupled to that of apoB-100 to form VLDL. ApoB production is relatively stable such that changes in liver triglyceride production with carbohydrate feeding leads to large VLDL with unchanged apoB production9. Fatty acids block apoB degradation and might be one reason for greater VLDL production in poorly controlled diabetes. In addition, insulin has been reported to increase apoB degradation, and in insulin resistant states less apo B degradation could lead to increased production and secretion of VLDL10. Moreover, insulin stimulates SREBP1c leading to increased de novo FFA synthesis11.
Regulation of plasma triglyceride lipolysis
LpL-mediated triglyceride lipolysis creates remnants and begins the conversion of VLDL to LDL. Although most triglyceride within lipoproteins is within the core, it is believed that there is always some TG that is exposed on the lipoprotein surface12. Associated apoproteins may assist with surface triglyceride exposure. Chylomicrons are more rapidly removed from the bloodstream than VLDL. The larger size of the chylomicron means that each particle has more triglyceride, so lipoprotein LpL interaction occurs with a lower LpL to triglyceride ratio. Two other factors might increase chylomicron lipolysis in vivo. The lower density and greater size increase the chance of a particle colliding with the capillary wall13. Both in vitro and in vivo data are consistent with a saturation of LpL at triglyceride concentrations of approximately 0.5 μM, 500 mg/dl 14, 15. VLDL triglyceride levels above this are thought to prevent efficient hydrolysis of chylomicrons.
LpL is primarily synthesized in muscle (cardiac and skeletal) and adipose. Loss of LpL in the mouse heart causes hypertriglyceridemia16 and transgenic expression in the heart completely corrects the hypertriglyceridemia that occurs in LpL knockout mice17. LpL is regulated by physical activity. Loss of skeletal muscle LpL, which is akin to detraining or forced inactivity, causes a shift from fatty acid to glucose oxidation, leads to a redistribution of triglyceride to heart and liver, increases lipid concentrations in these tissues, and leads to insulin resistance18. As opposed to the relatively minor changes in plasma triglycerides that accompany complete loss of receptors thought to mediate uptake of remnants into the liver, factors that reduce LpL actions produce dramatic hypertriglyceridemia. Several new proteins that affect lipolysis and that are involved in human disorders of chylomicron metabolism have been described recently and were reviewed in detail by Olivecrona et al.19. Angiopoietin like proteins 3 and 4 are tissue inhibitors of LpL actions and molecular defects are associated with lower triglyceride levels. ApoA-5 reduces plasma triglyceride but its mode of action is still uncertain. Glycosylphostidylinositol HDL binding protein (GPIHBP) appears to assist with LpL attachment to its physiological site of action on the luminal surface of endothelial20.
Hypertriglyceridemia and LDL and HDL metabolism
Hypertriglyceridemia is associated with reduced HDL and small dense LDL (sdLDL) in humans. Experimental studies have shown how this occurs. Decades ago Havel et al. demonstrated that lipolysis releases apoCs that transfer to HDL and return to chylomicrons after feeding 21. This transfer of apoproteins is accompanied by movement of lipids. In part for that reason, LpL inhibition leads to rapid and dramatic reductions in circulating HDL 22. In the presence of cholesteryl ester transfer protein (CETP), which is not found in rodents, HDL reduction is due to both exchange of cholesteryl ester for triglyceride in triglyceride-rich lipoproteins and more rapid catabolism of the smaller triglyceride-enriched HDL 23, 24.
Why is hypertriglyceridemia associated with sdLDL? CETP will also mediate exchange of LDL cholesteryl esters for triglyceride. Triglyceride-enriched LDL are a substrate for LpL and hepatic lipase and may be the precursor to sdLDL25. Recently a kinetic study in humans suggested that large apoC-III-rich VLDL were preferentially converted to sdLDL26.
EXPERIMENTAL EVIDENCE FOR THE ATHEROGENICITY OF TRIGLYCERIDE-RICH LIPOPROTEINS
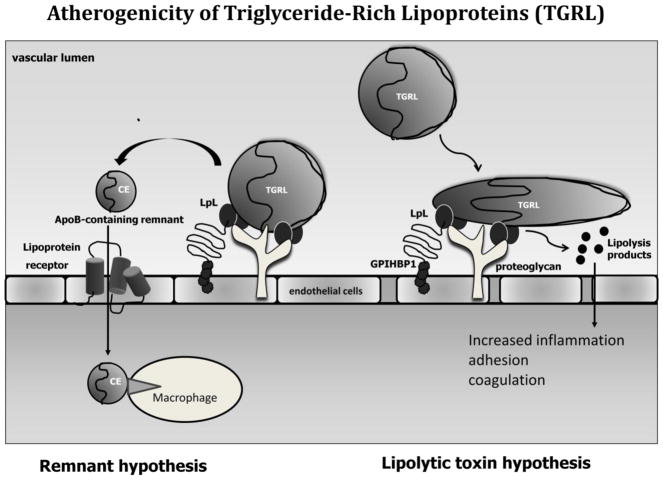
Two pathways have been hypothesized to connect levels of circulating triglycerides with atherosclerosis. Zilversmit proposed that during the postprandial period chylomicrons were converted to remnants that could then penetrate the arterial wall and deposit cholesterol27 (Figure 1 left). This process, he conjectured, correlated with the greater LpL activity in the artery associated with macrophage infiltration28. Evidence that chylomicron remnants are atherogenic is abundant. Remnant lipoproteins have been identified in the arteries of humans 29. And unlike LDL, remnant lipoproteins can convert macrophages into foam cells30. Remnants are the causative factors in many accepted atherosclerosis models including the apoE knockout mouse and cholesterol-fed rabbits and monkeys. In the clinical context, a major issue is quantification of remnants. In humans, chylomicron remnants contain apoB48 but measurements of this apoprotein have not been used in large clinical trials. In kinetic studies, retinyl ester has been used as a tracker that is contained in both chylomicrons and remnants; so triglyceride poor retinyl ester particles or apoB48 particles with a higher density than chylomicrons are operationally considered remnants.
Figure 1. Atherogenicity of triglyceride-rich lipoproteins.
Two hypotheses for pathways by which triglyceride-rich lipoproteins (TGRL) might increase atherosclerosis are illustrated. Left panel shows the remnant infiltration hypothesis. Conversion of TGRLs to remnants produces particles that then enter the arterial wall, carrying both triglyceride and cholesterol. Arterial LpL may be important to increase the local concentration of these particles. Remnants can be internalized by macrophages and convert these cells into foam cells. Right panel illustrates the toxic lipolysis product hypothesis. During lipolysis of TGRLs a number of inflammatory lipids are released that alter endothelial biology. These lipids - including fatty acids lysolecithin and oxidized lipids - increase expression of adhesion molecules and cytokines, and promote coagulation.
Are non-remnant triglyceride-rich lipoproteins atherogenic? Atherosclerosis is a disease whose pathological fingerprint is cholesterol and not triglyceride accumulation within the artery. Thus, one must ask if nascent triglyceride-rich lipoproteins can deliver cholesterol to the vascular wall exclusive of their hydrolysis to remnants. The classic experiments by Duff et al.31 showed that diabetes increased concentrations of large triglyceride-rich lipoproteins and reduced the number of remnants in the circulation of cholesterol-fed rabbits. This change in lipoprotein profile resulted in less atherosclerosis, i.e. diabetes was protective in this model. Several decades later the reason for this observation was uncovered when it was shown that the very large triglyceride-rich lipoproteins, perhaps equivalent to nascent chylomicrons, circulating in the diabetic rabbits were unable to enter the artery32. These studies contrast, but do not contradict, the demonstration by Rutledge et al. that VLDL can deposit in the wall of the perfused artery, especially in the presence of LpL33. Nor do they contradict the data showing that VLDL cholesterol levels correlate with atherosclerosis formation in LDL receptor knockout mice34. But it leaves unanswered whether VLDL size lipoproteins are a source of atherogenic cholesterol.
Studies of humans with genetic defects in LpL activity suggest that nascent lipoproteins are relatively, but not absolutely non-atherogenic. Until recently, a generally accepted view was that patients with severe hypertriglyceridemia were resistant to atherosclerosis35. A similar argument was made to account for the variation in coronary artery disease (CAD) risk in patients with genetic familial hypertriglyceridemias. Patients with familial hypertriglyceridemia (FHTG) appear to develop less CAD than those with familial combined hyperlipidemia (FCHL), perhaps due to their relatively cholesterol-poor VLDL36. However, the demonstration that LpL deficient patients are not entirely immune to vascular disease37 has led to some uncertainty as to CAD risk with genetic hypertriglyceridemia syndromes.
Animal models support the hypothesis that larger triglyceride-rich lipoproteins are not totally harmless but are less atherogenic than smaller lipoproteins. Hypertriglyceridemic mice with deficiencies of LpL38 or GPIHBP39 develop small lesions, but at relatively advanced age. Thus, it appears that large triglyceride-rich lipoproteins can be atherogenic and deposit cholesterol in the artery, but they are much less atherogenic than smaller lipoproteins.
Atherogenicity of lipolysis products
Perhaps the major toxicity of triglyceride-rich lipoproteins is not from intact particles but from their lipolysis along the artery wall. Lipolysis of triglyceride-rich lipoproteins produces FFAs and lysolecithin (Figure 1 right). Recently, more complex lipid analysis has shown that lipolysis of VLDL leads to release of a number of additional potentially toxic oxidized fatty acids40. In vitro studies have implicated these lipids (and in some experiments triglyceride lipolysis) in inflammation 41, macrophage cytotoxicity 42, expression of adhesion molecules 43, 44, and promotion of coagulation45, 46. VLDL lipolysis, but not VLDL alone promotes an inflammatory response in cultured macrophages47. Extreme levels of lipolysis products created in the circulation by infusion of lipid emulsions in the presence of heparin activate toll-like receptors and downstream inflammatory pathways48, whereas removal of FFA by their esterification to triglyceride 49, 50 reduces cellular inflammation. It should be noted that in vitro studies have also implicated lipolysis products as anti-inflammatory molecules due to their activation of PPARs 51, 52.
Gain and loss of lipolysis has been studied in isolated arteries and using mice with genetic modifications of LpL. VLDL and LpL together allowed more LDL to enter the wall of an isolated perfused artery due to disruption in endothelial barrier function53. Loss of macrophage LpL in genetically modulated mice reduced plaques37, 54, 55. Similarly mice with heterozygous LpL deficiency had less atherosclerosis despite hypertriglyceridemia56. Although endothelial cells do not express LpL, an endothelial cell-expressing LpL transgene does create active enzyme57. Mice expressing endothelial cell LpL have defective vascular relaxation, however, this was only found in the presence of TNF. Smooth muscle LpL expression also leads to defective arterial relaxation58. Effects of these two LpL transgenes on atherosclerosis are under study. The many effects of LpL including its ability to alter macrophage inflammatory function and to anchor lipoproteins to matrix proteoglycans will complicate the interpretation of these studies13, which are unlikely to provide more than correlative information relating lipolysis and vascular disease.
RELATIONSHIP OF TRIGLYCERIDES TO CLINCIAL CARDIOVASCULAR DISEASE (CAD)
Hypertriglyceridemia is perhaps the most difficult lipid disorder to evaluate and treat. Why is this? First, genetic disorders are quite common but most genes relevant to this common metabolic disorder have yet to be clearly identified. Second, hypertriglyceridemia associates with a number of acquired disorders, e.g. insulin resistant states. This raises an important issue as to whether the association of hypertriglyceridemia with CAD or other forms of atherosclerosis is a direct effect of the triglyceride-rich lipoproteins themselves or the company they keep. In addition, as reviewed elsewhere59, triglyceride levels can fluctuate widely with diet and exercise.
Problems also exist in defining hypertriglyceridemia. Fasting levels are highly variable and non-fasting levels even more so, population data are skewed, and relationships between fasting or non-fasting triglycerides and CAD often do not relate to the extent of their elevation. When using a triglyceride value of <150 mg/dl to define normal, as is done in existing guidelines, the prevalence of hypertriglyceridemia in the US is 27.5% for subjects with a BMI of < 25 kg/m2, 31% for those with a BMI between 25 and 30 kg/m2, and 37% for those with a BMI > 30 kg/m60. And, with the obesity epidemic still expanding, this prevalence will certainly increase.
Triglycerides and other metabolic derangements
The metabolic syndrome is the best example illustrating the company that elevated plasma triglycerides keep61. The metabolic syndrome is a common metabolic disorder strongly related to central obesity. The pathophysiology seems to be largely attributable to insulin resistance and excessive flux of FFA. An inflammatory state likely contributes to the syndrome and an associated prothrombotic state contributes to CAD risk. The controversy about the definition of the metabolic syndrome was resolved and there is now a single global definition62. Criteria include increased waist circumference (population specific), increased triglyceride (>150,mg/dl), reduced HDL-cholesterol (<40 in man and <50 in women), hypertension (>130 systolic or 85 diastolic), and fasting glucose (>100 mg/dL). Because the metabolic syndrome relates to insulin resistance and captures a broad range of risk factors that relate to CAD, the independent relationship of triglyceride elevation per se to CAD is reduced. Similarly the other lipoprotein abnormalities that accompany metabolic syndrome, low HDL and sdLDL, might also be markers of the real process that increases CAD risk.
Clinical correlative studies of triglycerides and CAD
One of the earliest reports linking triglycerides to CAD was by Margaret Albrink63. In a small cross-sectional study, hypertriglyceridemia appeared to be more important than hypercholesterolemia in its association with CAD in men. Other data such as that from Framingham suggested that high plasma triglyceride levels were an even greater CAD risk factor for women64. This observation has been replicated in other studies65, 66. Within the setting of the metabolic syndrome, type 2 diabetes, and familial combined hyperlipidemia, a recent review has listed 11 published reports of the independence of plasma triglycerides as a risk factor for CAD, but also acknowledges the inconsistency of this relationship67. Thus, are plasma triglycerides a biomarker rather than an independent risk factor of CAD risk?
More recently non-fasting triglycerides have been implicated as equally predictive of CAD risk as fasting hypertriglyceridemia68, 69. In addition, non-fasting triglycerides were associated with stroke risk70. However, in general when levels of fasting triglycerides are elevated so are the postprandial triglyceride levels71, 72. In addition, non-fasting hypertriglyceridemia predicts a higher level of remnant cholesterol, another risk factor for CAD. The relative risk of either a 1.0 mM (90 mg/dl) elevation in fasting triglycerides or levels above 200 mg/dl is ~1.773–75. However, when adjusting for levels of HDL cholesterol, the relative risk became minimally significant in men (1.14) and reduced in women (1.37)74. In general, this relatively higher independent risk in women is reproducible and without obvious explanation. Moreover, a more recent analysis by the Emerging Risk Factors Collaborators in over 300,000 people without CAD demonstrated a risk of 2.6 in the bottom tertile vs. 6.2 in the top tertile76. However, after adjustment for HDL cholesterol and other risk factors, this risk disappeared. Thus, these data suggest that plasma triglycerides are a biomarker rather than an independent CAD risk factor. In contrast, non-fasting triglycerides were associated with stroke risk in both men and women.
GENETIC INSIGHTS
Do genetic studies support a relationship between triglycerides and CAD? Recent genome-wide association studies (GWAS) have provided new insights into the regulation of plasma triglycerides with the identification of novel loci harboring genes that were subsequently identified to have a previously unknown function in triglyceride metabolism. For example, a region on chromosome 8 downstream of TRIB1 (encoding tribbles homolog 1) was found to strongly associate with both plasma triglycerides and CAD risk. Further studies in mice demonstrated reciprocal effects of Trib1 overexpression or knockdown on hepatic triglyceride synthesis and VLDL secretion 77. GWAS have also lent plausibility to the hypothesis that triglycerides are causally related to CAD risk. The main rationale underlying the concept of “Mendelian randomization” is that if a given trait (e.g. elevated triglycerides) is causally related to an outcome (e.g. CAD), the genetic variants associated with the trait should have a similar relation to the outcome. Although the concept is simple, a number of conditions must be met including lack of pleiotrophic effects for a given genetic variant and lack of statistical interactions with other genetic or environmental factors. These criteria do not consistently apply to genetic variants affecting triglyceride metabolism78.
Each step in hepatic and intestinal triglyceride synthesis, VLDL assembly and secretion, and intravascular lipolysis is regulated by multiple gene products. Coding and regulatory variants in a number of genes associate with alterations in HDL- and/or LDL-cholesterol as well as triglycerides. Importantly, triglyceride metabolism is highly sensitive to clinical and environmental conditions including obesity, physical activity, diet, hormonal states, circulating glucose levels and pro-inflammatory cytokines. These interact with genetic factors to regulate triglyceride metabolism. Of note, several triglyceride raising alleles exert greater effects in obese sedentary versus lean physically active individuals. Significant gene/gene interactions are also evident79, 80. For example, the effects of the common APOA5 1132T>C variant on plasma triglycerides is enhanced in carriers of the APOE4 allele.
Furthermore, pleiotrophy is well documented at several genetic loci. For example, a common polymorphism (rs780094) -30G>A in GCKR, the gene encoding the glucokinase regulatory protein, is associated with both elevated fasting triglycerides and lower fasting glucose and reduced risk for diabetes81. No association of rs780094 with CAD has been documented, which may be explained by the opposing effects on CAD risk factors. In contrast, the gene encoding insulin receptor substrate 1, IRS1, is associated with higher plasma triglycerides and with CAD risk. Because the risk alleles also confer a greater propensity for insulin resistance and type 2 diabetes, the effects on CAD risk are likely due to multiple metabolic effects 82.
Associations of genes for triglyceride metabolism and CAD
Despite these confounders, genetic studies are generally consistent with a role for triglyceride-mediated pathways in CAD. The Global Lipids Consortium recently reported on 95 loci associated with plasma lipid traits in over 100,000 individuals of European ancestry83. In a further analysis including 24,607 individuals with CAD and 66,197 controls, association of lipid SNPs with CAD was tested (Table 1). Most loci associated with LDL-cholesterol altered CAD risk in the expected direction. A smaller number of loci primarily associated with triglycerides also increased CAD risk. These included single nucleotide polymorphisms in genes encoding LpL, tribbles 1 (TRIB1), N-acetyltransferase 2 (NAT2) and the ZNF259/APOA5/APOA4/APOC3/APOA1 gene cluster. Certain of these genes/gene clusters are associated with multiple lipid traits. The gene product of TRIB1 regulates hepatic triglyceride synthesis and, although most strongly associated with triglycerides, also associates with HDL- and LDL-cholesterol levels77. The 11q23 locus encompassing ZNF259, APOA5, APOA4, APOC3 and APOA1 is most strongly linked to triglycerides but has multiple effects on other lipoproteins. Studies in genetic isolates also support a role for regulators of the triglyceride lipolytic pathway in atherosclerosis. ApoC-III inhibits triglyceride lipolysis and a null mutation in human APOC3 (R19X) with a carrier frequency of 5% in the Lancaster Amish is associated with lower plasma triglycerides and cardioprotection84. Moreover, increases in apoC-III-containing lipoproteins may confer risk beyond that of other risk factors 85.
Table 1.
Association of Triglyceride SNPs Identified in Genome wide Association Studies with CAD
| Gene | Chr | lead SNP | Alleles | MAF | effect size | CAD Association |
|---|---|---|---|---|---|---|
| (mg/dl) | ||||||
| ANGPTL3 | 1 | rs2131925 | T/G | 0.32 | −4.94 | |
| APOB | 2 | rs1042034 | T/C | 0.22 | −5.99 | |
| GCKR | 2 | rs1260326 | C/T | 0.41 | +8.76 | |
| COBLL1 | 2 | rs10195252 | T/C | 0.40 | −2.01 | |
| MSL2L1 | 3 | rs645040 | T/G | 0.22 | −2.22 | |
| KLHL8 | 4 | rs442177 | T/G | 0.41 | −2.25 | |
| MAP3K1 | 5 | rs9686661 | C/T | 0.20 | +2.57 | |
| HLA | 6 | rs2247056 | C/T | 0.25 | −2.99 | |
| TYW1B | 7 | rs13238203 | C/T | 0.04 | −7.91 | |
| MLXIPL | 7 | rs17145738 | C/T | 0.12 | −9.32 | |
| PINX1 | 8 | rs11776767 | G/C | 0.37 | +2.01 | |
| NAT2 | 8 | rs1495741 | A/G | 0.22 | +2.85 | + |
| LPL | 8 | rs12678919 | A/G | 0.12 | −13.64 | + |
| TRIB1 | 8 | rs2954029 | A/T | 0.47 | −5.64 | + |
| JMJD1C | 10 | rs10761731 | A/T | 0.43 | −2.38 | |
| CYP26A1 | 10 | rs2068888 | G/A | 0.46 | −2.28 | |
| FADS1-2-3 | 11 | rs174546 | C/T | 0.34 | +3.82 | |
| APOA5 | 11 | rs964184 | C/G | 0.13 | +16.95 | + |
| LRP1 | 12 | rs11613352 | C/T | 0.23 | −2.70 | |
| CAPN3 | 15 | rs2412710 | G/A | 0.02 | +7.00 | |
| FRMD5 | 15 | rs2929282 | A/T | 0.05 | +5.13 | |
| CTF1 | 16 | rs11649653 | C/G | 0.40 | −2.13 | |
| CILP2 | 19 | rs10401969 | T/C | 0.07 | −4.74 | + |
| APOE | 19 | rs439401 | C/T | 0.36 | −5.50 | + |
| PLA2G6 | 22 | rs5756931 | T/C | 0.40 | −1.54 | |
MAF (minor allele frequency) (reference 80 Teslovich et al.)
The Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration recently reported on the association of the -1131T>C (rs662799) promoter polymorphism of the apoA5 (APOA5) gene with triglycerides and CAD in 20,842 CAD patients and 35,206 controls86. The -1131T>C polymorphism is in almost complete linkage disequilibrium with two other APOA5 polymorphisms, -3A>G (rs651821) and +1891T>C (rs2266788) and, by decreasing APOA5 gene expression, the risk haplotype would be expected to lead to impaired lipolysis of TG-rich lipoproteins 58, 87. In this large meta analysis, the minor allele of rs662799 (minor allele frequency, 0.08) was associated with an allele specific increase in triglycerides of 16% (CI 12.9–18.7) and an odds ratio for CAD of 1.18 (CI 1.11–1.26; p=2.6X10−7), consistent with the reported hazard ratio of 1.10 (CI 1.08–1.12) for a 16% increase in triglycerides in prospective population studies. Although this locus primarily associates with plasma triglycerides, a smaller but significant effect on HDL-cholesterol (−3.5%) may have also contributed to the observed CAD risk.
Overall genetic studies support the hypothesis that impairment of triglyceride lipolytic pathways is associated with increased CAD risk although the extent to which risk is mediated by increased triglyceride-rich lipoproteins, altered LDL size and composition, or decreased HDL-cholesterol is not entirely clear. Other loci identified to associate with triglycerides in multiple genome-wide association studies are less clearly associated with increased CAD risk58, 88 (Table 1). These include MLXIPL (CHREBP) encoding the carbohydrate response element binding protein and FADS2,3 encoding the fatty acid desaturases, which play important roles in hepatic triglyceride synthesis. This may lead to the conclusion that regulation of hepatic triglyceride synthesis is less relevant for atherosclerosis than regulation of lipolysis. However, the TRIB1 gene product is another regulator of hepatic triglyceride synthesis and, unlike MLXIPL and FADS2,3, is clearly related to CAD risk. Further functional analysis of these triglyceride associated loci may provide further mechanistic insight into triglyceride metabolism and atherosclerosis susceptibility.
As reported by Hegele et al.89, multiple SNPs associated with triglycerides in genome wide association and candidate gene studies are significantly associated with hypertriglyceridemia phenotypes including FCHL and FHTG, indicating that FCHL and FHTG are polygenic traits with considerable overlap. Rare variants90 and common SNPs91 at multiple triglyceride-associated loci contribute cumulatively to the severity of hypertriglyceridemia. Notably, both FCHL and FHTG are strongly associated with CAD risk particularly when other features of the metabolic syndrome are present 92.
CLINICAL TRIALS OF TRIGLYCERIDE REDUCING THERAPIES
Do human clinical trials support triglyceride reduction as a means to reduce CAD risk? Unfortunately such trials have suffered from experimental design and are few in number. The overall findings are hypothesis-generating at best. Because of the complex interactions of plasma lipoproteins and the lack of drugs to specifically lower triglyceride levels, only limited pathophysiologic insights have been unveiled by clinical trials.
The most selective of the triglyceride-reducing drugs are the fibrates. It s unfortunate that the trials to examine if fibrates reduce CAD events have been inadequate to address the hypothesis. At best, we are left with post hoc analysis to establish the optimal trial to follow, i.e. in hypertriglyceridemic patients in whom LDL lowering therapy with a statin has already been instituted. Four major fibrate trials to test the hypothesis that the reduction in fasting (and presumably postprandial) plasma triglycerides reduces the incidence of acute myocardial infarction (MI) or death from cardiovascular causes have been completed in patients with CAD or high risk (Table 2). This subgroup analysis suggests that hypertriglyceridemic patients with low HDL do benefit from fibrates.
TABLE 2.
Fibric acid intervention trials
| Study | Drug | n | TG (Criteria) | TG ↓ | Primary Endpoint |
|---|---|---|---|---|---|
| VA-HIT (DM) | Gemfibrozil | 2531 (550) | 160 mg/dl (<300) | 31% | 24% |
| BIP (DM) | Bezafibrate | 3090 (330) | 145 mg/dl (<300) | 21% | 7% |
| FIELD (DM) | Fenofibrate | 9795 | 156 mg/dl (90–450) | 29% | 11% |
| ACCORD-LIPID (DM) | Fenofibrate | 2532 | 162 mg/dl (<400) | 20% | 8% |
Should fibrates remain the drug class of choice to lower triglycerides in patients with hypertriglyceridemia? Based on the data we have, the answer appears to be yes. As stated in a very recent meta-analysis of the effects of fibrates on CAD outcomes93, fibrates can reduce the risk of major cardiovascular events and might have a role in individuals at high risk of cardiovascular events and in those with combined dyslipidemia. This however remains to be proven.
Higher doses of omega-3 ethyl esters (EEC) (2.0–4.5 g of EPA + DHA) lower plasma triglycerides with very little effect on plasma levels of LDL or HDL cholesterol; high doses of omega-3 EEC lower triglycerides by ~30–35% by a number of mechanisms94. The studies of effects of omega-3 fatty acids on cardiovascular disease are conflicting but their actions might be via processes exclusive of triglyceride reduction. In the GISSI-Prevenzione study, 11,324 CHD patients with a recent MI appeared to benefit from omega-3 EEC 1000 mg/day although the amount of triglyceride lowering was small and was not related to the CVD benefit95. However, a recently published trial performed in northern Europe failed to show a beneficial effect of a similar treatment96. Two other omega-3 EEC studies are worth noting; the Diet and Reinfarction Trial (DART)97, and The Japan EPA Lipid Intervention Study (JELIS)98. In DART, the omega-3 EEC group had an initial reduction in re-infarction by 32% and a 29% reduction in all cause mortality, but the 21,147 person year follow-up failed to demonstrate a long-term survival benefit of higher levels of fish consumption; the fish oil consumption group had a significant increase in sudden cardiac death (P=0.018). JELIS was a large trial in 18,645 patients with hyperlipidemia of whom more than 3500 had a history of a CAD event. Subjects were randomly assigned to either a statin alone or a combination of statin and 1.8 g of EPA daily. After 5 years, the combination treatment was associated with a significant 19% reduction of the primary composite endpoint comprising death, revascularization, MI and unstable angina compared to the statin alone group. The greatest relative risk reduction of 53% was experienced in patients in the primary prevention arm who had hypertriglyceridemia (mean, 272 mg/dl) and decreased HDL-cholesterol levels despite only a 5% difference in the amount of triglyceride lowering in the statin-EPA vs. the statin alone group. It is important to point out that subjects in both GISSI-Prevenzione and JELIS had levels of LDL cholesterol far above current goals. Thus, although omega-3 fatty acids appear to reduce CAD events in some high risk populations, as for other triglyceride lowering therapies the benefit appears unrelated to the triglyceride lowering effect.
Niacin (nicotinic acid) is a lipid altering vitamin used in high doses to favorably modify triglycerides; the molecular/cellular effects of niacin remain uncertain99. Presently the completed niacin trials have been insufficient in size and design to determine whether or not niacin has independent benefits on CAD, and whether or not the reduction in risk for CAD is secondary to triglyceride lowering. Large randomized clinical trials are in progress. Smaller studies of CAD in consort with other lipid reducing agents 100 or of carotid plaques in statin-treated subjects101 show beneficial effects of niacin. However, because niacin also reduces LDL and raises HDL - and may lower lipoprotein (a) - benefit in clinical studies cannot be ascribed solely to reduction in triglyceride.
Moderate to high doses of the more potent statins lower triglycerides. Statins have been reported to decrease hepatic secretion of apoB containing lipoproteins102. They might also increase VLDL and chylomicron clearance secondary to upregulation of the LDL receptor. Observational evidence from the GREACE Study suggests that that the decrease in triglycerides in statin (mainly atorvastatin)-treated CAD patients correlated with CAD event reduction; this effect was pronounced in patients with the metabolic syndrome103. In contrast, data from the Treating to New Targets study showed that benefit due to intensive lipid-lowering therapy with atorvastatin was not related to the modest 10% fall in fasting plasma triglycerides104.
CONCLUSIONS AND AREAS IN NEED OF SCIENTIFIC STUDY
Although hypertriglyceridemia is a common biochemical abnormality in humans, except for pancreatitis risk, its relationship to human disease is far from certain. Is hypertriglyceridemia merely a marker of other metabolic abnormalities, or does it initiate or accelerate progression of vascular disease? Does this only occur in specific genetic or environmental settings? Despite the plethora of data showing atherogenic-like effects of lipids or lipolysis products on cultured cells, animal models to allow the study of the effects of hypertriglyceridemia on arteries in vivo are needed. Perhaps it s not the triglycerides themselves, but VLDL particle number or changes in HDL or LDL that are most important for vascular biology. Studies further exploring the link of genetic variants to hypertriglyceridemia and CAD are in progress. Importantly, the development of specific drugs to lower triglycerides would allow one to directly test in humans whether triglyceride reduction reduces CAD events. The correct trial in patients with hypertriglyceridemia needs to be performed. At this point, such drugs and clinical trials are but a dream. Thus, the hypothesis that triglycerides are an independent risk for human vascular disease will be around to challenge the next generation of basic and clinical scientists.
Footnotes
Disclosure - The authors have no relationships that could be perceived as real or apparent conflict(s) of interest.
References
- 1.Voyiaziakis E, Goldberg IJ, Plump AS, Rubin EM, Breslow JL, Huang LS. ApoA-I deficiency causes both hypertriglyceridemia and increased atherosclerosis in human apoB transgenic mice. J Lipid Res. 1998;39:313–321. [PubMed] [Google Scholar]
- 2.Chung S, Timmins JM, Duong M, Degirolamo C, Rong S, Sawyer JK, Singaraja RR, Hayden MR, Maeda N, Rudel LL, Shelness GS, Parks JS. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J Biol Chem. 285:12197–12209. doi: 10.1074/jbc.M109.096933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willnow TE, Sheng Z, Ishibashi S, Herz J. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 1994;264:1471–1474. doi: 10.1126/science.7515194. [DOI] [PubMed] [Google Scholar]
- 5.Out R, Kruijt JK, Rensen PC, Hildebrand RB, de Vos P, Van Eck M, Van Berkel TJ. Scavenger receptor BI plays a role in facilitating chylomicron metabolism. J Biol Chem. 2004;279:18401–18406. doi: 10.1074/jbc.M401170200. [DOI] [PubMed] [Google Scholar]
- 6.Yagyu H, Lutz EP, Kako Y, Marks S, Hu Y, Choi SY, Bensadoun A, Goldberg IJ. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem. 2002;277:10037–10043. doi: 10.1074/jbc.M109966200. [DOI] [PubMed] [Google Scholar]
- 7.Tacken PJ, Teusink B, Jong MC, Harats D, Havekes LM, van Dijk KW, Hofker MH. LDL receptor deficiency unmasks altered VLDL triglyceride metabolism in VLDL receptor transgenic and knockout mice. J Lipid Res. 2000;41:2055–2062. [PubMed] [Google Scholar]
- 8.Goudriaan JR, Espirito Santo SM, Voshol PJ, Teusink B, van Dijk KW, van Vlijmen BJ, Romijn JA, Havekes LM, Rensen PC. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res. 2004;45:1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Melish J, Le NA, Ginsberg H, Steinberg D, Brown WV. Dissociation of apoprotein B and triglyceride production in very-low-density lipoproteins. Am J Physiol. 1980;239:E354–362. doi: 10.1152/ajpendo.1980.239.5.E354. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res. 2009;50 (Suppl):S162–166. doi: 10.1194/jlr.R800090-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller KW, Small DM. Surface-to-core and interparticle equilibrium distributions of triglyceride-rich lipoprotein lipids. J Biol Chem. 1983;258:13772–13784. [PubMed] [Google Scholar]
- 13.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 14.Brunzell JD, Hazzard WR, Porte D, Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest. 1973;52:1578–1585. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976;17:536–541. [PubMed] [Google Scholar]
- 16.Augustus AS, Yagyu H, Haemmerle G, Bensadoun A, Vikramadithyan RK, Park SY, Kim JK, Zechner R, Goldberg IJ. Cardiac-specific knockout of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J Biol Chem. 2004;279:25050–25057. doi: 10.1074/jbc.M401028200. [DOI] [PubMed] [Google Scholar]
- 17.Levak-Frank S, Hofmann W, Weinstock PH, Radner H, Sattler W, Breslow JL, Zechner R. Induced mutant mouse lines that express lipoprotein lipase in cardiac muscle, but not in skeletal muscle and adipose tissue, have normal plasma triglyceride and high-density lipoprotein-cholesterol levels. Proc Natl Acad Sci U S A. 1999;96:3165–3170. doi: 10.1073/pnas.96.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Knaub LA, Jensen DR, Young Jung D, Hong EG, Ko HJ, Coates AM, Goldberg IJ, de la Houssaye BA, Janssen RC, McCurdy CE, Rahman SM, Soo Choi C, Shulman GI, Kim JK, Friedman JE, Eckel RH. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58:116–124. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivecrona G, Olivecrona T. Triglyceride lipases and atherosclerosis. Curr Opin Lipidol. 2010;21:409–415. doi: 10.1097/MOL.0b013e32833ded83. [DOI] [PubMed] [Google Scholar]
- 20.Beigneux AP, Davies BS, Bensadoun A, Fong LG, Young SG. GPIHBP1, a GPI-anchored protein required for the lipolytic processing of triglyceride-rich lipoproteins. J Lipid Res. 2009;50 (Suppl):S57–62. doi: 10.1194/jlr.R800030-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havel RJ, Kane JP, Kashyap ML. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J Clin Invest. 1973;52:32–38. doi: 10.1172/JCI107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 285:37976–37986. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamarche B, Uffelman KD, Carpentier A, Cohn JS, Steiner G, Barrett PH, Lewis GF. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J Clin Invest. 1999;103:1191–1199. doi: 10.1172/JCI5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horowitz BS, Goldberg IJ, Merab J, Vanni TM, Ramakrishnan R, Ginsberg HN. Increased plasma and renal clearance of an exchangeable pool of apolipoprotein A-I in subjects with low levels of high density lipoprotein cholesterol. J Clin Invest. 1993;91:1743–1752. doi: 10.1172/JCI116384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galeano NF, Milne R, Marcel YL, Walsh MT, Levy E, Ngu’yen TD, Gleeson A, Arad Y, Witte L, Al-Haideri M, Rumsey SC, Deckelbaum RJ, et al. Apoprotein B structure and receptor recognition of triglyceride-rich low density lipoprotein (LDL) is modified in small LDL but not in triglyceride-rich LDL of normal size. J Biol Chem. 1994;269:511–519. [PubMed] [Google Scholar]
- 26.Zheng C, Khoo C, Furtado J, Sacks FM. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 121:1722–1734. doi: 10.1161/CIRCULATIONAHA.109.875807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zilversmit DB. A proposal linking atherogenesis to the interaction of endothelial lipoprotein lipase with triglyceride-rich lipoproteins. Circ Res. 1973;33:633–638. doi: 10.1161/01.res.33.6.633. [DOI] [PubMed] [Google Scholar]
- 28.Yla-Herttuala S, Lipton BA, Rosenfeld ME, Goldberg IJ, Steinberg D, Witztum JL. Macrophages and smooth muscle cells express lipoprotein lipase in human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88:10143–10147. doi: 10.1073/pnas.88.22.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamo JC, Proctor SD, Smith D. Retention of chylomicron remnants by arterial tissue; importance of an efficient clearance mechanism from plasma. Atherosclerosis. 1998;141 (Suppl 1):S63–69. doi: 10.1016/s0021-9150(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 30.Mahley RW, Innerarity TL, Rall SC, Jr, Weisgraber KH. Lipoproteins of special significance in atherosclerosis. Insights provided by studies of type III hyperlipoproteinemia. Ann N Y Acad Sci. 1985;454:209–221. doi: 10.1111/j.1749-6632.1985.tb11860.x. [DOI] [PubMed] [Google Scholar]
- 31.Duff GL, Brechin DJ, Finkelstein WE. The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. IV. The effect of insulin therapy on the inhibition of atherosclerosis in the alloxan-diabetic rabbit. J Exp Med. 1954;100:371–380. doi: 10.1084/jem.100.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordestgaard BG, Stender S, Kjeldsen K. Reduced atherogenesis in cholesterol-fed diabetic rabbits. Giant lipoproteins do not enter the arterial wall. Arteriosclerosis. 1988;8:421–428. doi: 10.1161/01.atv.8.4.421. [DOI] [PubMed] [Google Scholar]
- 33.Rutledge JC, Mullick AE, Gardner G, Goldberg IJ. Direct visualization of lipid deposition and reverse lipid transport in a perfused artery : roles of VLDL and HDL. Circ Res. 2000;86:768–773. doi: 10.1161/01.res.86.7.768. [DOI] [PubMed] [Google Scholar]
- 34.VanderLaan PA, Reardon CA, Thisted RA, Getz GS. VLDL best predicts aortic root atherosclerosis in LDL receptor deficient mice. J Lipid Res. 2009;50:376–385. doi: 10.1194/jlr.M800284-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordestgaard BG, Stender S, Kjeldsen K. Severe hypertriglyceridemia, large lipoproteins and protection against atherosclerosis. Scand J Clin Lab Invest Suppl. 1987;186:7–12. [PubMed] [Google Scholar]
- 36.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 37.Benlian P, De Gennes JL, Foubert L, Zhang H, Gagne SE, Hayden M. Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. N Engl J Med. 1996;335:848–854. doi: 10.1056/NEJM199609193351203. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Qi R, Xian X, Yang F, Blackstein M, Deng X, Fan J, Ross C, Karasinska J, Hayden MR, Liu G. Spontaneous atherosclerosis in aged lipoprotein lipase-deficient mice with severe hypertriglyceridemia on a normal chow diet. Circ Res. 2008;102:250–256. doi: 10.1161/CIRCRESAHA.107.156554. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein MM, Yin L, Tu Y, Wang X, Wu X, Castellani LW, Walzem RL, Lusis AJ, Fong LG, Beigneux AP, Young SG. Chylomicronemia elicits atherosclerosis in mice--brief report. Arterioscler Thromb Vasc Biol. 2010;30:20–23. doi: 10.1161/ATVBAHA.109.196329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang WY, Schwartz E, Wang Y, Attrep J, Li Z, Reaven P. Elevated concentrations of nonesterified fatty acids increase monocyte expression of CD11b and adhesion to endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:514–519. doi: 10.1161/01.ATV.0000200226.53994.09. [DOI] [PubMed] [Google Scholar]
- 42.Chung BH, Segrest JP, Smith K, Griffin FM, Brouillette CG. Lipolytic surface remnants of triglyceride-rich lipoproteins are cytotoxic to macrophages but not in the presence of high density lipoprotein. A possible mechanism of atherogenesis? Journal of Clinical Investigation. 1989;83:1363–1374. doi: 10.1172/JCI114024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Xian X, Huang W, Chen L, Wu L, Zhu Y, Fan J, Ross C, Hayden MR, Liu G. Expression of LPL in endothelial-intact artery results in lipid deposition and vascular cell adhesion molecule-1 upregulation in both LPL and ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:197–203. doi: 10.1161/01.ATV.0000249683.80414.d9. [DOI] [PubMed] [Google Scholar]
- 45.Tsuda T, Yoshimura H, Hamasaki N. Effect of phosphatidylcholine, phosphatidylethanolamine and lysophosphatidylcholine on the activated factor X-prothrombin system. Blood Coagul Fibrinolysis. 2006;17:465–469. doi: 10.1097/01.mbc.0000240919.72930.ee. [DOI] [PubMed] [Google Scholar]
- 46.Sato N, Kokame K, Miyata T, Kato H. Lysophosphatidylcholine decreases the synthesis of tissue factor pathway inhibitor in human umbilical vein endothelial cells. Thromb Haemost. 1998;79:217–221. [PubMed] [Google Scholar]
- 47.Saraswathi V, Hasty AH. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res. 2006;47:1406–1415. doi: 10.1194/jlr.M600159-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, Naylor S, Rao M, Hubbard B, Farese RV., Jr DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 120:756–767. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, Schaffer JE, Yu YH, Goldberg IJ. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci U S A. 2003;100:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci U S A. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutledge JC, Woo MM, Rezai AA, Curtiss LK, Goldberg IJ. Lipoprotein lipase increases lipoprotein binding to the artery wall and increases endothelial layer permeability by formation of lipolysis products. Circ Res. 1997;80:819–828. doi: 10.1161/01.res.80.6.819. [DOI] [PubMed] [Google Scholar]
- 54.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest. 1999;103:1697–1705. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clee SM, Bissada N, Miao F, Miao L, Marais AD, Henderson HE, Steures P, McManus J, McManus B, LeBoeuf RC, Kastelein JJ, Hayden MR. Plasma and vessel wall lipoprotein lipase have different roles in atherosclerosis. J Lipid Res. 2000;41:521–531. [PubMed] [Google Scholar]
- 56.Semenkovich CF, Coleman T, Daugherty A. Effects of heterozygous lipoprotein lipase deficiency on diet-induced atherosclerosis in mice. J Lipid Res. 1998;39:1141–1151. [PubMed] [Google Scholar]
- 57.Takahashi M, Hiyama Y, Yokoyama M, Yu S, Hu Y, Melford K, Bensadoun A, Goldberg IJ. In vivo arterial lipoprotein lipase expression augments inflammatory responses and impairs vascular dilatation. Arterioscler Thromb Vasc Biol. 2008;28:455–462. doi: 10.1161/ATVBAHA.107.153239. [DOI] [PubMed] [Google Scholar]
- 58.Esenabhalu VE, Cerimagic M, Malli R, Osibow K, Levak-Frank S, Frieden M, Sattler W, Kostner GM, Zechner R, Graier WF. Tissue-specific expression of human lipoprotein lipase in the vascular system affects vascular reactivity in transgenic mice. Br J Pharmacol. 2002;135:143–154. doi: 10.1038/sj.bjp.0704440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katcher HI, Hill AM, Lanford JL, Yoo JS, Kris-Etherton PM. Lifestyle approaches and dietary strategies to lower LDL-cholesterol and triglycerides and raise HDL-cholesterol. Endocrinol Metab Clin North Am. 2009;38:45–78. doi: 10.1016/j.ecl.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Ghandehari H, Le V, Kamal-Bahl S, Bassin SL, Wong ND. Abdominal obesity and the spectrum of global cardiometabolic risks in US adults. Int J Obes (Lond) 2009;33:239–248. doi: 10.1038/ijo.2008.252. [DOI] [PubMed] [Google Scholar]
- 61.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 62.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 63.Albrink MJ, Man EB. Serum triglycerides in coronary artery disease. AMA Arch Intern Med. 1959;103:4–8. doi: 10.1001/archinte.1959.00270010010002. [DOI] [PubMed] [Google Scholar]
- 64.Castelli WP. The triglyceride issue: a view from Framingham. Am Heart J. 1986;112:432–437. doi: 10.1016/0002-8703(86)90296-6. [DOI] [PubMed] [Google Scholar]
- 65.LaRosa JC. Triglycerides and coronary risk in women and the elderly. Arch Intern Med. 1997;157:961–968. [PubMed] [Google Scholar]
- 66.Sprecher DL, Pearce GL, Park EM, Pashkow FJ, Hoogwerf BJ. Preoperative triglycerides predict post-coronary artery bypass graft survival in diabetic patients: a sex analysis. Diabetes Care. 2000;23:1648–1653. doi: 10.2337/diacare.23.11.1648. [DOI] [PubMed] [Google Scholar]
- 67.Harchaoui KE, Visser ME, Kastelein JJ, Stroes ES, Dallinga-Thie GM. Triglycerides and cardiovascular risk. Curr Cardiol Rev. 2009;5:216–222. doi: 10.2174/157340309788970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 69.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 70.Varbo A, Nordestgaard BG, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Benn M. Nonfasting triglycerides, cholesterol, and ischemic stroke in the general population. Ann Neurol. 2011 doi: 10.1002/ana.22384. epub Feb 18. [DOI] [PubMed] [Google Scholar]
- 71.Marcoux C, Hopkins PN, Wang T, Leary ET, Nakajima K, Davignon J, Cohn JS. Remnant-like particle cholesterol and triglyceride levels of hypertriglyceridemic patients in the fed and fasted state. J Lipid Res. 2000;41:1428–1436. [PubMed] [Google Scholar]
- 72.Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AM, Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12:1336–1345. doi: 10.1161/01.atv.12.11.1336. [DOI] [PubMed] [Google Scholar]
- 73.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 74.Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Whitlock G, Woodward M. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation. 2004;110:2678–2686. doi: 10.1161/01.CIR.0000145615.33955.83. [DOI] [PubMed] [Google Scholar]
- 75.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 76.Di AE, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burkhardt R, Toh SA, Lagor WR, Birkeland A, Levin M, Li X, Robblee M, Fedorov VD, Yamamoto M, Satoh T, Akira S, Kathiresan S, Breslow JL, Rader DJ. Trib1 is a lipid- and myocardial infarction-associated gene that regulates hepatic lipogenesis and VLDL production in mice. J Clin Invest. 2010;120:4410–4414. doi: 10.1172/JCI44213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 79.Perron P, Brisson D, Santure M, Blackburn P, Bergeron J, Vohl MC, Despres JP, Gaudet D. Apolipoprotein E and lipoprotein lipase gene polymorphisms interaction on the atherogenic combined expression of hypertriglyceridemia and hyperapobetalipoproteinemia phenotypes. J Endocrinol Invest. 2007;30:551–557. doi: 10.1007/BF03346348. [DOI] [PubMed] [Google Scholar]
- 80.Sousa MO, Alia P, Pinto X, Corbella E, Navarro MA. Interaction between APOA5 -1131T>C and APOE polymorphisms and their association with severe hypertriglyceridemia. Clin Chim Acta. 2008;395:68–71. doi: 10.1016/j.cca.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Orho-Melander M, Melander O, Guiducci C, Perez-Martinez P, Corella D, Roos C, Tewhey R, Rieder MJ, Hall J, Abecasis G, Tai ES, Welch C, Arnett DK, Lyssenko V, Lindholm E, Saxena R, de Bakker PI, Burtt N, Voight BF, Hirschhorn JN, Tucker KL, Hedner T, Tuomi T, Isomaa B, Eriksson KF, Taskinen MR, Wahlstrand B, Hughes TE, Parnell LD, Lai CQ, Berglund G, Peltonen L, Vartiainen E, Jousilahti P, Havulinna AS, Salomaa V, Nilsson P, Groop L, Altshuler D, Ordovas JM, Kathiresan S. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes. 2008;57:3112–3121. doi: 10.2337/db08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rung J, Cauchi S, Albrechtsen A, Shen L, Rocheleau G, Cavalcanti-Proenca C, Bacot F, Balkau B, Belisle A, Borch-Johnsen K, Charpentier G, Dina C, Durand E, Elliott P, Hadjadj S, Jarvelin MR, Laitinen J, Lauritzen T, Marre M, Mazur A, Meyre D, Montpetit A, Pisinger C, Posner B, Poulsen P, Pouta A, Prentki M, Ribel-Madsen R, Ruokonen A, Sandbaek A, Serre D, Tichet J, Vaxillaire M, Wojtaszewski JF, Vaag A, Hansen T, Polychronakos C, Pedersen O, Froguel P, Sladek R. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009;41:1110–1115. doi: 10.1038/ng.443. [DOI] [PubMed] [Google Scholar]
- 83.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, Konig IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Doring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA, Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O’Connell JR, Shuldiner AR. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sacks FM, Alaupovic P, Moye LA, Cole TG, Sussex B, Stampfer MJ, Pfeffer MA, Braunwald E. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 2000;102:1886–1892. doi: 10.1161/01.cir.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 86.Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, Ouwehand W, Watkins H, Samani NJ, Saleheen D, Lawlor D, Reilly MP, Hingorani AD, Talmud PJ, Danesh J. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem. 2005;280:21553–21560. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- 88.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hegele RA, Ban MR, Hsueh N, Kennedy BA, Cao H, Zou GY, Anand S, Yusuf S, Huff MW, Wang J. A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia. Hum Mol Genet. 2009;18:4189–4194. doi: 10.1093/hmg/ddp361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johansen CT, Wang J, Lanktree MB, Cao H, McIntyre AD, Ban MR, Martins RA, Kennedy BA, Hassell RG, Visser ME, Schwartz SM, Voight BF, Elosua R, Salomaa V, O’Donnell CJ, Dallinga-Thie GM, Anand SS, Yusuf S, Huff MW, Kathiresan S, Hegele RA. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet. 42:684–687. doi: 10.1038/ng.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, Ban MR, Zou GY, Cao H, Lin T, Kennedy BA, Anand S, Yusuf S, Huff MW, Pollex RL, Hegele RA. Polygenic determinants of severe hypertriglyceridemia. Hum Mol Genet. 2008;17:2894–2899. doi: 10.1093/hmg/ddn188. [DOI] [PubMed] [Google Scholar]
- 92.Hopkins PN, Heiss G, Ellison RC, Province MA, Pankow JS, Eckfeldt JH, Hunt SC. Coronary artery disease risk in familial combined hyperlipidemia and familial hypertriglyceridemia: a case-control comparison from the National Heart, Lung, and Blood Institute Family Heart Study. Circulation. 2003;108:519–523. doi: 10.1161/01.CIR.0000081777.17879.85. [DOI] [PubMed] [Google Scholar]
- 93.Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 375:1875–1884. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 94.Bays HE, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther. 2008;6:391–409. doi: 10.1586/14779072.6.3.391. [DOI] [PubMed] [Google Scholar]
- 95.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 96.Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 97.Burr ML. Secondary prevention of CHD in UK men: the Diet and Reinfarction Trial and its sequel. Proc Nutr Soc. 2007;66:9–15. doi: 10.1017/S0029665107005241. [DOI] [PubMed] [Google Scholar]
- 98.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 99.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 100.Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 101.Lee JM, Robson MD, Yu LM, Shirodaria CC, Cunnington C, Kylintireas I, Digby JE, Bannister T, Handa A, Wiesmann F, Durrington PN, Channon KM, Neubauer S, Choudhury RP. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54:1787–1794. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 102.Ginsberg HN. REVIEW: Efficacy and mechanisms of action of statins in the treatment of diabetic dyslipidemia. J Clin Endocrinol Metab. 2006;91:383–392. doi: 10.1210/jc.2005-2084. [DOI] [PubMed] [Google Scholar]
- 103.Athyros VG, Kakafika AI, Papageorgiou AA, Tziomalos K, Skaperdas A, Pagourelias E, Pirpasopoulou A, Karagiannis A, Mikhailidis DP. Atorvastatin decreases triacylglycerol-associated risk of vascular events in coronary heart disease patients. Lipids. 2007;42:999–1009. doi: 10.1007/s11745-007-3103-z. [DOI] [PubMed] [Google Scholar]
- 104.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]