Abstract
Loss of epithelial integrity often correlates with the progression of malignant tumors. Sds22, a regulatory subunit of Protein Phosphatase 1 (PP1), has recently been linked to regulation of epithelial polarity in Drosophila. However, its role in tumorigenesis remains obscure. Here, using Drosophila imaginal tissue as an in vivo model system, we show that sds22 is a new potential tumor suppressor gene in Drosophila. Without sds22, cells lose epithelial architecture, and become invasive and tumorigenic when combined with Ras overexpression; conversely, sds22 overexpression can largely suppress tumorigenic growth of RasV12scrib−/ − mutant cells. Mechanistically, we show that sds22 prevents cell invasion and metastasis by inhibiting myosin II and JNK activity downstream of PP1. Loss of this inhibition causes cells to lose epithelial organization and promotes cell invasion. Finally, human Sds22 is focally deleted and down-regulated in multiple carcinomas, and this downregulation correlates with tumor progression, suggesting that sds22 inactivation may contribute to tumorigenesis and metastatic potential in human cancers via a similar mechanism.
Keywords: Tumor suppressor, Epithelial integrity, Cell invasion, sds22, Protein Phosphatase 1
Introduction
Cell invasion is an active process involving dynamic remodeling of the actin cytoskeleton and is a critical step for tumor metastasis, which occurs in 90% of cancer-related human deaths. However, the genetic changes that cause noninvasive tumors to become metastatic are not well understood. A stable epithelial architecture is thought to limit cell proliferation and cell invasion (Dow and Humbert, 2007; Wodarz and Nathke, 2007). Several key molecules have been identified that are required to establish and maintain epithelial integrity, namely the Scribble complex (Scribble (Scrib)/Discs Large (Dlg)/Lethal giant larvae (Lgl)), the Par complex (Par3/Par6/atypical protein kinase C), and the Crumbs complex (Crumbs/Stardust/Patj) (Bilder, 2004). Among these, scrib, dlg, and lgl have been identified as “neoplastic” tumor suppressors, whose loss cause tissue overgrowth accompanied by disruptions in cellular architecture and differentiation (Bilder, 2004). However, clones of scrib, dlg, or lgl survive poorly when surrounded by wild-type cells and are eliminated by cell apoptosis (Agrawal et al., 1995; Brumby and Richardson, 2003; Woods and Bryant, 1991). This phenomenon is reminiscent of the multi-gene requirement for a normal cell to become tumorigenic and progress to malignancy (Hanahan and Weinberg, 2000; Land et al., 1983). Drosophila imaginal discs have become a powerful system to study the effects of multiple genetic changes on discrete populations of cells immediately adjacent to wild-type neighboring cells, which closely resembles the clonal nature of human cancer.
Protein Phosphatase 1 (PP1) is a member of one of the major classes of serine/threonine protein phosphatases, which consists of a catalytic subunit and various regulatory subunits that target the complex to specific locations and regulate substrate specificity (Ceulemans and Bollen, 2004). PP1 expression is reported to be significantly lower in some human cancer cells (www.oncomine.org) and human PP1 interacts with breast cancer susceptibility protein BRCA1 (Winter et al., 2007). Additionally, the PP1 inhibitor okadaic acid has been reported to act as a tumor promoter and can increase migration and invasion of non-metastatic LLC-C8 cells (Suganuma et al., 1988; Young, 1997), indicating that loss of PP1 may contribute to tumor formation and metastasis. However, genetic studies of PP1 function in vivo have been complicated by the presence of multiple homologs and its involvement in a wide range of cellular processes in most organisms. Therefore, PP1 regulatory subunits can provide a key to understanding the role of PP1 in tumor growth and metastasis.
Sds22 is a conserved, leucine-rich repeat protein first identified as a regulatory subunit of PP1 that is required for the completion of mitosis in yeast (Ohkura and Yanagida, 1991). Recently, one group identified Drosophila sds22 as a regulator of epithelial polarity (Grusche et al., 2009). In this report, we show that, in addition to its role in cell polarity, sds22 is critical for maintaining epithelial integrity, and that without sds22 cells become invasive and tumorigenic. Furthermore, sds22 overexpression can largely suppress the tumorigenic growth ofRasV12scrib−/ − cells. Finally, we show that one potential mechanism by which sds22 prevents cell invasion and metastasis is through inhibition of myosin II and JNK activity downstream of PP1. Together, these results highlight the importance of sds22 as a novel member of the neoplastic tumor suppressor gene class that links changes in epithelial integrity with signaling pathways driving tumor metastasis.
Results
sds22 behaves as a new potential Drosophila tumor suppressor gene
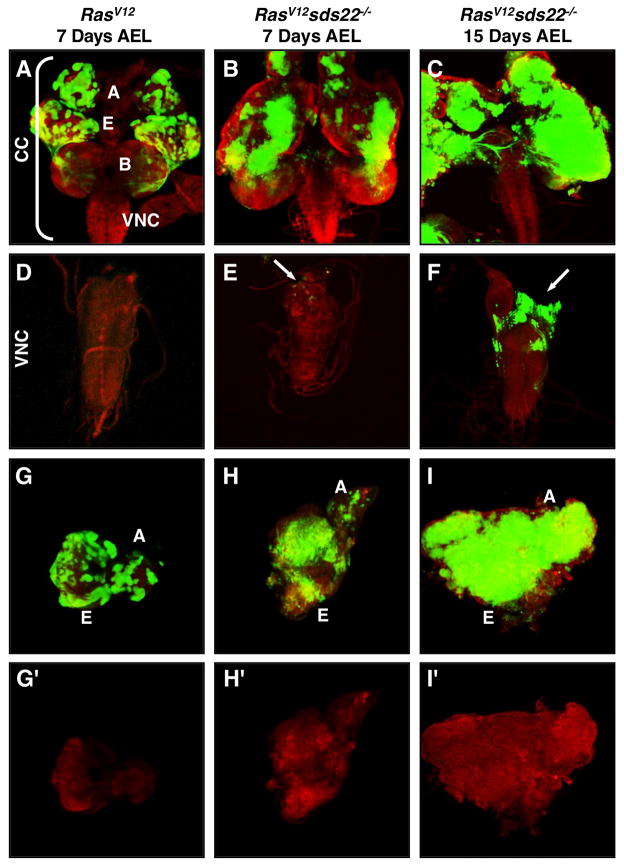
A previous study showed that sds22 is important for epithelial cell shape and polarity (Grusche et al., 2009). Given that loss of cell polarity often synergizes with activated Ras (RasV12) to induce tumor growth and invasion as seen in scrib/dlg/lgl mutants (Brumby and Richardson, 2003; Pagliarini and Xu, 2003), we first tested whether loss of sds22 will have a similar effect. We generated null alleles of sds22 by imprecise excision of a nearby P-element insertion (P {EPgy2} EY06161) in Drosophila, which also deleted another gene named CREG (cellular repressor of E1A-stimulated genes) (Figure S1A). The lethality and mutant phenotypes can be fully rescued by a genomic rescue construct and a UAS-sds22 transgene, suggesting that sds22 is the gene responsible for the observed phenotypes (Figure S1C–E). sds22 homozygotes die at or prior to the first larva instar. To test whether loss of sds22 promotes tumor growth and metastasis of RasV12 expressing cells, we expressed RasV12 in sds22 mutant cells using the eyFLP/MARCM system, in which 30% of the eye is typically composed of mutant tissue (Lee and Luo, 2001). Consistent with previous reports, RasV12 overexpression alone induces benign overgrowth but cells never invade into the nearby ventral nerve cord (VNC) or other tissues (Figure 1A,D). When RasV12 overexpression is combined with homozygous loss of sds22, such animals can grow as larvae for up to 15 days after egg laying (AEL) and die prior to pupation or as early pupae (wild-type animals normally pupate 5 days AEL at 25°C). In contrast, animals expressing RasV12 alone can only grow as larvae for up to 9 days AEL and then die as early pupae. At 7 days AEL, we observe extensive hyperproliferation in eye discs of RasV12sds22−/ − animals (Figure 1B,H) but GFP-positive cells are seen in the VNC at only low frequency (Figure 1E, white arrow). At 15 days AEL we find significant numbers of ectopic GFP-positive cells spreading from a primary tumor in the brain into the VNC (Figure 1F). In addition, as RasV12sds22−/ − tumors grow, the two eye-antennal discs appear to fuse into one large mass (Figure 1I). Together, these results suggest that loss of sds22 can cooperate with RasV12 to promote tumor growth and invasive behavior in a time-dependent manner.
Figure 1. Loss of sds22 promotes tumor growth and metastasis of RasV12 cells.
eyFLP-induced mutant clones (green) of RasV12 cells 7 days after egg laying (AEL) (A,D,G), and RasV12sds22−/ − cells either 7 days AEL (B,E,H) or 15 days AEL (C,F,I) from third-instar larvae are double labeled with GFP and Texas-Red-conjugated phalloidin (red). (A–F) Upper panels show the cephalic complex (CC), including eye (E) and antennal (A) discs, brain (B), and ventral nerve cord (VNC). Lower panels show representative VNCs. RasV12sds22−/ − animals show progressive growth and migration of tumor cells. White arrows indicate ectopic GFP cells in the VNC (E,F), where they are normally absent in RasV12 mutants (D). (G–I) Compared to the control RasV12 eye-antennal disc (G), RasV12sds22−/ − (H,I) mutant discs show strong overproliferation as indicated by the intensity and size of GFP clones and appear to fuse into one large mass at day 15 AEL (I).
Next, we asked whether the sds22 mutation alone is sufficient to cause tumor growth or metastasis. Similar to cells mutant for the neoplastic tumor suppressor genes scrib, dlg or lgl, we find that sds22 mutant clones are more sensitive to cell competition, exhibit cell apoptosis, and do not over proliferate or metastasize (Figure S2A–G and S3A). The role of Ras signaling in promoting cell survival has been well documented (Bonni et al., 1999; Kurada and White, 1998). To test whether the cooperative effect between loss of sds22 and Ras overexpression is linked to cell survival, we coexpressed the baculovirus caspase inhibitor p35 in sds22 mutant cells using the eyFLP/MARCM system to block cell death. Interestingly, these ‘undead’ cells (Martin et al., 2009) induce both cell-autonomous and non-cell autonomous cellular proliferation and result in a massively overgrown and folded eye disc and enlarged tumor-like adult eyes (Figure S3C–C‴), suggesting that loss of sds22 confers tumor growth when cell death is inhibited. Overexpression of p35 alone does not cause any obvious growth defects (Figure S3B). However, we do not find GFP-labeled mutant cells outside of the eye-antennal disc/optic lobe region (data not shown), suggesting that blocking cell death is not sufficient to promote metastasis of sds22−/ − cells. Combined with the overgrowth phenotype in cooperation with oncogenic Ras, these results suggest that sds22 mutant cells induce uncontrolled proliferation when combined with a second genetic change or “hit” that promotes cell survival. Given that tumor suppressor mutations often require a second hit to manifest their full phenotypes, these data suggest that sds22 is a new Drosophila tumor suppressor gene.
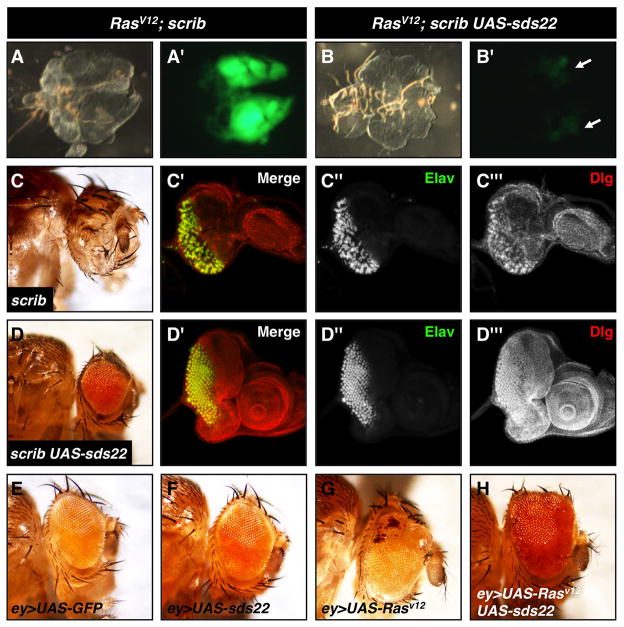
To further investigate the possible contribution of sds22 to tumor suppression, we next tested if sds22 gain-of-function is capable of suppressing tumor growth using the previously established Drosophila tumor model RasV12scrib−/ − (Pagliarini and Xu, 2003). Coexpression of RasV12 in scrib mutant cells using the eyFLP/MARCM system induces strong tumor growth at 7 days AEL (Figure 2A). RasV12scrib−/ − animals keep growing as larvae until 13 days AEL and die before pupation. We find that coexpression of sds22 strongly suppresses the tumor growth phenotype in all clones observed at 7 days AEL compared to RasV12scrib−/ − alone (Figure 2A,B). Most of these animals can pupate but die as early pupae, while RasV12scrib−/ − animals rarely pupate. These results suggest that overexpression of sds22 can suppress the tumor-like growthof RasV12scrib−/ − cells. To determine the mechanism by which overexpression of sds22 activity suppresses RasV12scrib−/ − overproliferation, we tested if sds22 overexpression can suppress RasV12 or scrib phenotypes individually. We observe strong suppression of scrib phenotypes in both larval and adult stages by overexpression of sds22 in scrib mutant eye discs (Figure 2C,D). However, overexpression of sds22 does not suppress the enlarged eye phenotype caused by overexpression of RasV12 using ey-GAL4 (Figure 2E–H). Thus, we conclude that sds22 can suppress tumor growth in part through its interaction with the cell polarity gene scrib.
Figure 2. Overexpression of sds22 suppresses the RasV12scrib−/ − tumor growth phenotype.
(A,B) Cephalic complexes dissected from third instar larvae at 7 days AEL. The same complex is shown as both bright field (left) and fluorescent (right) images. (A–A′) The RasV12scrib−/ − genotype leads to tumorigenic growth as indicated by the large size of GFP-marked clones. (B–B′) Overexpression of sds22 in RasV12scrib−/ − cells substantially suppresses the tumor growth phenotype, indicated by the reduced size and intensity of GFP clones (white arrows indicate the paired eye-antennal discs). (C) Loss of scrib generated by EGUFcl causes defects in differentiation (Elav, green, C″) and disc morphology (Dlg, red, C‴t) in the third instar eye disc. Most of these animals die as pupae and the few escapers found are eyeless (C). (D) Overexpression of sds22 suppresses scrib mutant defects both in adults (D) and larvae (D′–D‴). (E–H) Light microscopy of adult eyes from animals carrying ey-GAL4 and: (E) UAS-GFP, (F) UAS-sds22, (G) UAS-RasV12, and (H) UAS-RasV12, UAS-sds22. Compared to the control (E), overexpression of RasV12 induces eye overgrowth (G) that is not suppressed by overexpressionof sds22 (H). Note that overexpression of sds22 alone does not cause external eye defects (F).
Loss of sds22 leads to cell invasive behavior
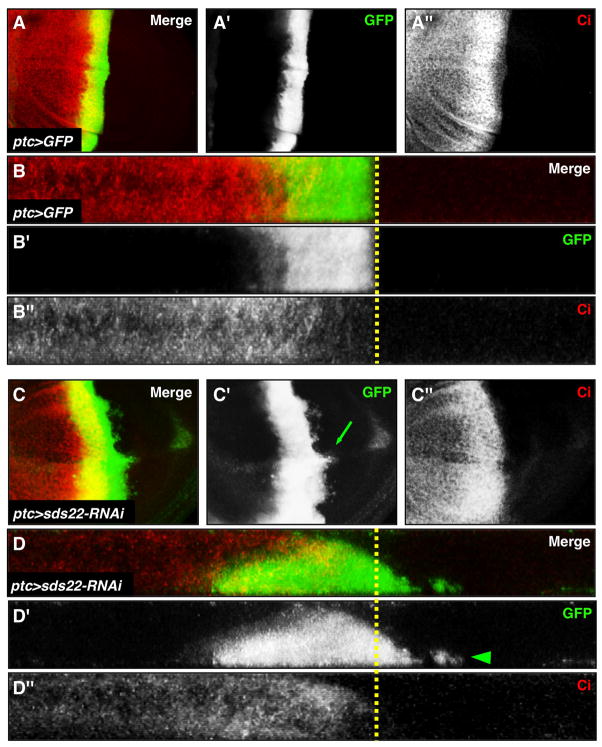
The metastatic capability of RasV12sds22−/ − cells but not RasV12 alone may result from a potential acquired role of sds22 in preventing cellular invasion. To test this possibility, we used patched-GAL4 (ptc-GAL4)/UAS-GFP system to knock down sds22 using RNAi in a defined region along the anterior/posterior (A/P) compartment boundary of the wing disc, a well used system to study cell migratory behavior in Drosophila (Singh et al., 2010; Speck et al., 2003; Srivastava et al., 2007; Vidal et al., 2006). Compared to controls where GFP-marked wild-type cells are localized within a straight stripe (Figure 3A,B), GFP-positive sds22 deficient cells are basally extruded and migrate away from the ptc-GAL4 expression domain into the posterior compartment (Figure 3C,D), resulting in an abnormal apical folding of the disc epithelium along the A-P boundary (Figure 6A,B). The A-P compartment boundary remains relatively smooth and regular based on expression of the anterior compartment-specific marker Cubitus interruptus (Ci) (Figure 3C″), indicating that the invasion-like behavior of sds22−/ − cells is unlikely to result from disruption of AP compartmentalization.
Figure 3. sds22 deficient cells are basally excluded and migrate toward the posterior compartment.
Confocal projections of third instar wing discs expressing either UAS-GFP alone (A), or UAS-GFP and sds22-RNAi (B), both driven by ptc-GAL4 along the anterior-posterior (A-P) compartment boundary. Here and in all subsequent figures with wing discs, anterior is to the left and dorsal is up. GFP (green) marks mutant cells and Ci (red) marks anterior cells. (A,B) All GFP-marked cells form a cohesive stripe in the ptc expression region. The posterior edge of GFP expression is coincident with the low Ci expression, which marks the A-P boundary (indicated by a yellow dash line). (C,D) In contrast to controls, sds22 deficient cells migrate away from the A-P boundary into the wild-type posterior compartment (green arrow in C′) and are localized basally from X/Z sections (white arrowhead in D′). Note the A-P boundary is straight and smooth, suggesting the A-P compartment may not be disturbed in mutant cells.
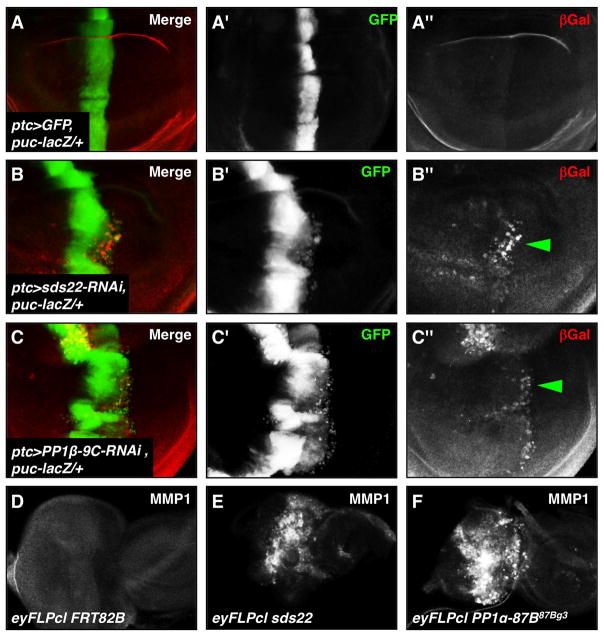
Figure 6. Loss of either sds22 or PP1 leads to increased JNK signaling and MMP1 expression.
(A–C) ptc-GAL4-expressing cells are visualized by GFP (green) and puc-lacZ reporter expression is revealed by anti-βGal (red). Transcription of a puc-lacZ reporter gene is normally low or absent in the wing disc (A). Increased puc-lacZ expression in sds22 or PP1β mutant migrating cells (B″,C″, green arrowheads) suggests that loss of either sds22 or PP1β activates puc transcription in response to JNK pathway activation. (D–F) Third instar eye-antennal discs are labeled with MMP1, another downstream target of JNK signaling. Compared to the control disc (D), sds22 (E) or PP1α (F) mutant discs show strong activation of MMP1 expression.
To test whether the invasion-like phenotype caused by loss of sds22 is specific to the wing epithelium, we generated sds22 mutant cells using the eyFLPcl technique, which removes ~90% of gene function in the eye disc (Newsome et al., 2000). We find that loss of sds22causes severely reduced and disorganized photoreceptor differentiation (Figure 4A,B). Additionally, we find ectopic neurons in the optic stalk (Figure 4B′, green arrow), where they are normally never seen (Figure 4A′, green arrow). This invasion-like phenotype is also observed in sds22 mitotic clones near the posterior margin of the eye disc (Figure S4B). To test whether these ectopic cells are sds22 mutant or wild-type, we used the hsFLP/MARCM technique to positively mark mutant cells with GFP. We find that the Elav-positive neurons in the optic stalk are also GFP-positive (Figure S4C, white arrows), suggesting that sds22 mutant cells are migrating away from the eye disc. In addition to photoreceptor cells, we also find undifferentiated cells and cone cells in the eye disc are mislocalized in the optic stalk (data not shown), indicating that the migratory behavior is not simply due to photoreceptor axon extension. Another possibility is that the basal migration by sds22 mutant cells might be a secondary consequence of cell death. To test this, we blocked cell death by overexpression of p35 in sds22 mutant cells. Elav-positive mutant neurons are still mislocalized in the optic stalk (Figure S4D″, white arrow), indicating that cell invasion is not a secondary consequence of cell death induced by loss of sds22. Together, these results suggest that sds22 is required for maintaining proper cellular position in both the wing and eye disc.
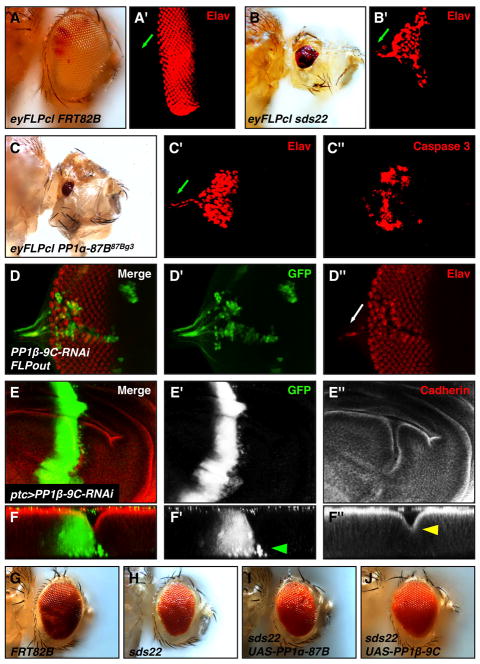
Figure 4. Loss of PP1 causes similar phenotypes as loss of sds22, and overexpression of PP1β can suppress sds22 mutant phenotypes.
(A–C) Adult mosaic heads and corresponding third instar eye discs generated by using the eyFLPcl/FRT system: (A–A′) FRT82, (B–B′) sds22, and (C–C″) PP1α. Anti-Elav (red) staining reveals photoreceptor cells. Eye morphogenesis is disrupted and eye size is reduced in either sds22 or PP1α mutants compared to controls. Additionally, photoreceptor cells are mislocalized in the optic stalk of mutant discs but not in controls (green arrows in A′,B′,C′). Loss of PP1α induces strong cell death as revealed by activated Caspase-3 staining (C″). (D) PP1β deficient cells were made using RNAi knockdown and the FLPout technique in which mutant cells are positively marked by GFP (green). Mutant Elav-positive cells (red and green) are disorganizedin the clone and are mislocalized in the optic stalk (white arrow in D″). (E) PP1β deficient cells generated using ptc-GAL4 migrate away from the boundary into the wild-type posterior compartment. X/Z sections from the wing pouch domain show these migrating cells are localized basally (green arrowhead in F′). Note that mutant cells have a disorganized pattern of Cadherin expression and exhibit an abnormal folding (yellow arrowhead in F″). (G–J) Light microscopy of adult mosaic heads generated by the eyFLP/MARCM technique: (G) FRT82B, (H) sds22e00975, (I) sds22e00975 UAS-PP1a-87B, and (J) sds22e00975 UAS-PP1β -9C. Note that coexpression of PP1β -9C, but not PP1a-87B, along with loss of sds22 results in a largely restored adult eye, suggesting that PP1β acts downstream of sds22.
sds22 is an essential regulator of PP1
Sds22 physically binds to Protein Phosphatase 1 (PP1) and regulates PP1 activity in yeast and mammalian cells (Ceulemans et al., 2002; MacKelvie et al., 1995; Stone et al., 1993). Binding of the Drosophila homolog of Sds22 to PP1 subunits has also been confirmed in a yeast two-hybrid system and Drosophila S2 cells (Bennett et al., 2006; Grusche et al., 2009). However, the functional significance of this interaction has not been studied in vivo and the role of PP1 in epithelial integrity and cellular invasion is not clear. To explore the mechanism of how loss of sds22 induces cell invasion-like behavior, we first asked whether loss of PP1 activity causes a similar phenotype as loss of sds22. Drosophila has four PP1 isoforms, named after theirsubtype and chromosome location: PP1β9C, PP1α13C, PP1α87B, and PP1α96A (Dombradi et al., 1990; Dombradi et al., 1993). Of these, PP1α13C and PP1α96A are not essential based on loss-of-function studies (Kirchner et al., 2007a; Smith, 1999) and therefore were not included in this study. We find that loss of PP1α87B or PP1β9C share many features with loss of sds22, including loss of tissue architecture and differentiation, increased cell death and cell invasive behavior (Figure 4C–F). Since loss of sds22 phenotypes in yeast can be suppressed by high dosage of PP1 (Ohkura and Yanagida, 1991), we tested whether a similar relationship exists in Drosophila. Strikingly, overexpression of PP1β9C, but not PP1α87B, can significantly suppress sds22 phenotypes (Figure 4G–J). Overexpression of individual PP1 isoforms alone does not cause an obvious phenotype (data not shown). Together, these results suggest sds22 functions as an essential positive regulator of PP1 to maintain epithelial organization and to block cell invasion.
Myosin II activity is responsible for changes caused by loss of sds22
Nonmuscle myosin II (referred to as myosin II hereafter) is an actin-based motor protein complex which plays a crucial role in cytoskeleton and tissue organization (Vicente-Manzanares et al., 2009). The myosin II regulatory light chain Spaghetti Squash (Sqh) is a direct target of PP1β9C and dephosphorylation of Sqh inactivates Myosin II (Vereshchagina et al., 2004). Phosphorylation of Sqh (p-Sqh) is increased in sds22 mutant follicle cells (Grusche et al., 2009), suggesting that Sqh hyperphosphorylation may play a role in mediating phenotypes caused by loss of sds22. To test this hypothesis, we first ectopically expressed a phosphomimetic (activated) form of Sqh (UAS-sqhDD) in the eye disc using either the FLPout technique or ey-GAL. In each case, neurons expressing activated Sqh become mislocalized in the optic stalk (Figure 5A,5I), closely phenocopying sds22-mediated cell migratory behavior. In addition, knockdown of myosin II activity by coexpression of an RNAi construct against the myosin IIheavy chain (zip) or the regulatory light chain (sqh) in sds22 mutant cells suppresses the sds22 migratory behavior (Figure 5D′,E′,F′). Moreover, reducing myosin II activity can largely rescue the cell morphology defects of sds22 mutant cells (Figure 5E″,F″). Knockdown of zip or sqh alone does not cause any invasion-like phenotype (Figure 5B and data not shown). Taken together, these results suggest that myosin II is essential for sds22-mediated cell morphology defects and cell invasion behavior. Interestingly, the phenotypes resulting from myosin II hyperactivity are less severe than those caused by knockdown of either sds22 or PP1 (Figure 5G–I), raising the possibility that Sds22/PP1 regulates additional substrates other than Sqh.
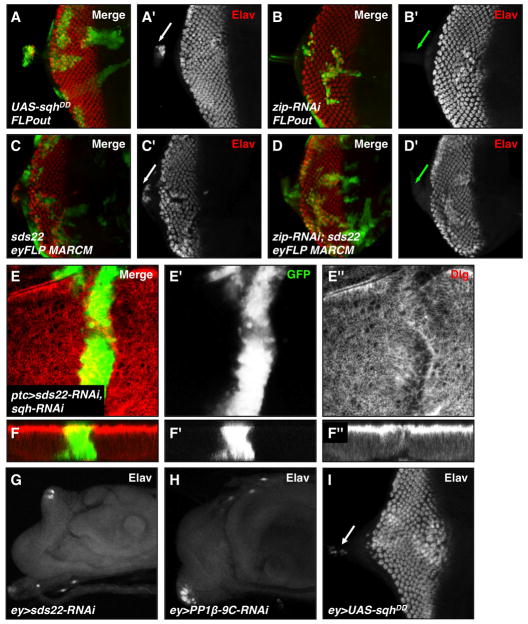
Figure 5. Nonmuscle myosin II activity is required for cell invasion and tissue integrity changes caused by loss of sds22.
(A–D) Third instar eye discs stained with GFP (green, positively marks clones) and Elav (red, marks neurons) are shown. (A) Increasing myosin II activity by overexpression of a phosphomimetic form of Sqh (sqhDD) using the FLPout method induces cell invasion into the optic stalk (A′, white arrow). (B) Reducing myosin II activity by knockdown of zip activity using the FLPout technique causes differentiation defects without inducing cell invasion (lack of Elav, red channel) into the optic stalk (B′, green arrow). (C) Loss of sds22 using the eyFLP/MARCM method causes a cell invasion phenotype (C′, white arrow). (D) Coexpression of an RNAi construct against zip can suppress the cell invasion phenotype caused by loss of sds22 (indicated by lack of Elav-positive cells in the optic stalk; D′, green arrow), suggesting that myosin II acts downstream of sds22 to mediate cell invasion. (E–F) Reducing myosin II activity by knockdown of sqh activity using the ptc-GAL4 driver suppresses both the cell invasion and apical abnormal folding phenotypes of sds22 deficient cells. GFP (green) marks mutant cells and Dlg (red) marks the tissue morphology. (G–I) knockdown of either sds22 (G) or PP1β (H) byey-GAL4 causes a dramatic loss of differentiation as indicated by a nearly complete loss of Elav staining. However, overexpression of UAS-sqhDD (I) by ey-GAL4 causes a less severe phenotype with Elav-positive cells mislocalized in the optic stalk (white arrow).
JNK signaling is required for loss of sds22-mediated cell invasion and apoptosis
The Jun N-terminal kinase (JNK) signaling pathway is an important mediator of tumor invasion (Huang et al., 2003; Igaki et al., 2006; Uhlirova et al., 2005). In addition, activated JNK signaling induces cell apoptosis (Behrens et al., 1999). Since loss of sds22 causes cell invasion and increased cell death, it seems likely that modulation of JNK pathway activity is involved in these phenotypes. To test this hypothesis, we examined transcription levels of puc, which encodes a JNK-specific phosphatase and acts as both a downstream target and a feedback inhibitor of the JNK signaling pathway (Martin-Blanco et al., 1998; McEwen and Peifer, 2005). Consistent with our hypothesis, puc-lacZ reporter expression is increased in sds22 deficient migrating cells (Figure 6B). Loss of PP1β also increases puc-lacZ expression (Figure 6C), suggesting an increase in JNK-dependent transcription in sds22 deficient cells is likely through regulation of PP1 activity by sds22. Next, we tested whether active JNK is responsible for the changes observed in sds22 mutant cells. Increasing JNK signaling alone by overexpression of eiger (a ligand for the Drosophila JNK pathway) using ptc-GAL4 is sufficient to cause massive cell migration and cell death (Figure S5C). Importantly, blocking JNK activity by overexpression of puc (a JNK activity inhibitor) in sds22 mutant cells suppresses both cell migration and cell death caused by loss of sds22 (Figure 7C,D and S5D–F). Overexpression of puc alone does not causeany obvious defects in the cytoskeleton or cell invasion (Figure S5G). Finally, blocking JNK activity also fully suppresses tumor growth and metastasis of RasV12sds22−/ − cells (Figure S6A–D). Collectively, these results suggest that increased JNK signaling plays a substantial role in cell invasion and cell death induced by loss of sds22.
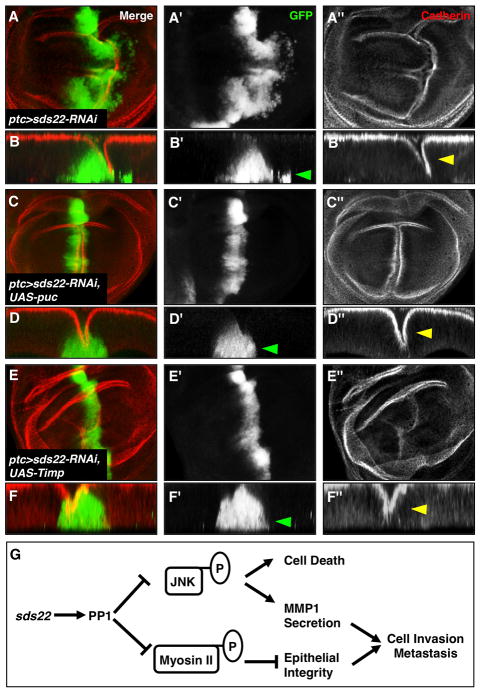
Figure 7. JNK activity is required for sds22-mediated cell invasion and overgrowth.
(A–D) Blocking the JNK pathway by coexpression of puc (a feedback inhibitor of JNK) in sds22 mutant cells suppresses the cell invasion phenotype caused by loss of sds22 (loss of GFP-positive cells in the posterior compartment, D′ compared to B′, green arrowheads). Note that disruption of the cell adhesion marker Cadherin (red) is not suppressed by puc expression (D″ compared to B′, yellow arrowheads). (E,F) Overexpression of Timp, an inhibitor of MMPs, also largely suppresses the cell invasive behavior of sds22 mutant cells (F′, green arrowhead). Tissue integrity indicated by Cadherin staining is not suppressed (F″, yellow arrowhead). (G) A model for sds22 as a tumor suppressor gene in Drosophila. sds22 functions as an essential positive regulator of PP1 to restrict two important signaling events that drive tumor metastasis: nonmuscle myosin II (myosin II) activity and the JNK signaling pathway. Hyperphosphorylation of myosin II leads to cytoskeleton reorganization and loss of epithelial integrity that is a criticaldeterminant of cell invasion and metastasis. Increased JNK signaling is required for sds22-mediated cell death and cell invasion likely through inducing MMP1 level.
Preventing basement membrane degradation suppresses invasiveness of sds22 mutant cells
JNK functions in part by modulating expression of Matrix metalloprotease 1 (MMP1) to promote tumor cell motility (Jasper et al., 2001; Uhlirova and Bohmann, 2006). MMP1 is essential for degradation of the basement membrane (BM) (Deryugina and Quigley, 2006), and is therefore required for metastatic potential of Drosophila tumors (Beaucher et al., 2007; Uhlirova and Bohmann, 2006). Consistent with this view, we find dramatically increased expression of MMP1 in both sds22 and PP1 mutant eye discs compared to controls (Figure 6E,F). To test whether MMPs play a role in sds22-mediated cell invasion, we blocked MMP function in sds22 mutant clones by ectopic expression of Timp, which encodes a Drosophila homolog of the Tissue inhibitor of metalloproteases (Llano et al., 2000; Page-McCaw et al., 2003; Pohar et al., 1999). We observe that overexpression of Timp using ptc-GAL4 strongly suppresses the invasive behavior of sds22 deficient cells in the wing disc (Figure 7E,F, green arrowhead), while overexpression of Timp alone causes no obvious defects (data not shown). These data suggest that MMP activity is critical for the cell invasive behavior caused by loss of sds22. In addition, we find that epithelial organization defects, including an abnormal apical folding along the A-P boundary of the wing disc, are not rescued by overexpression of either puc or Timp (Figure 7D″,F″, yellow arrowheads), suggesting that hyperactivity of myosin II may be sufficient to mediate this epithelial integrity defect.
Discussion
Stable epithelial integrity is required for normal tissue morphogenesis during development, and its loss is often associated with cancer. The importance of sds22 in regulating epithelial morphology has been recently reported (Grusche et al., 2009). However, the detailed mechanism of sds22 function and its role in tumor suppression have not been studied. By generating new, null alleles of sds22 in Drosophila, we show for the first time that sds22 is a new potential tumor suppressor gene that plays a key role in the metastatic process. Consistent with the work of Grusche et al., our results show that sds22 mutant cells lose epithelial organization, fail to differentiate normally, and undergo cell death. Beyond this, we show that sds22 mutant cells become invasive and migrate into neighboring regions, likely by increasing Matrix metalloprotease 1 (MMP1) secretion to degrade the basement membrane. Importantly, sds22 mutant cells undergo uncontrolled proliferation when cell death is blocked or in cooperation with activated Ras. Conversely, overexpression of sds22 can substantially delay tumor growth of RasV12scrib−/ − cells and suppress the scrib phenotype in vivo, consistent with sds22 functioning as a tumor suppressor gene. Finally, our genetic evidence leads us to propose a novel model in which sds22 functions as an essential positive regulator of PP1 to restrict myosin II and JNK activity, thereby maintaining epithelial integrity and preventing proliferation and metastasis (see model in Figure 7G), which provides significant new mechanistic insights into tumor suppressor pathways.
Tumor suppressive properties of sds22 mutant cells in epithelial tissues
Most human tumors are derived from epithelial tissues and loss of epithelial integrity has been linked to tumor growth and invasion (Brumby and Richardson, 2003; Igaki et al., 2006; Pagliarini and Xu, 2003). Here, we provide evidence that sds22 is a regulator of epithelial integrity and cell invasion, two key characteristics of malignant epithelial cells (Hanahan and Weinberg, 2000). We have considered the possibility that the invasion-like behavior of sds22−/ − cells might be secondary to defects in cell death or cell adhesion. However, not all invasive sds22−/ − cells are Caspase-3 positive and blocking cell death does not suppress cell invasion behavior. Additionally, we find loss of sds22 always causes directional migration, while defects in cell adhesion often cause cells to disperse into surrounding wild type cells (Knox and Brown, 2002). Moreover, loss of sds22 is sufficient to induce metastatic behavior of RasV12 cells, while loss of cell adhesion molecules, such as E-cadherin, does not (Pagliarini and Xu, 2003). Finally, loss of sds22 can induce MMP1 secretion downstream of JNK signaling, which is known to be activated by invading cells. Taken together, these data support the view that sds22−/ − cells actively invade surrounding tissue.
Why does loss of sds22 alone not cause tumor-like growth? In human cancer, it is rare that mutation of a single gene is sufficient to cause malignant transformation. Instead, multiple mutations are most often required for tumorigenesis (Brumby and Richardson, 2005; Land et al., 1983). Similar to the tumor suppressor scrib, loss of sds22 induces massive cell death, presumably as a result of stresses induced by loss of epithelial integrity. However, when cell death is blocked by expression of the caspase inhibitors p35, sds22−/ − cells can grow to form large, tumor-like masses. Additionally, loss of sds22 in combination with expression of oncogenic Ras promotes tumor growth and metastasis, similar to studies of other tumor suppressors involved in maintenance of cell polarity (Pagliarini and Xu, 2003). Interestingly, blocking cell death in sds22 mutant cells is not sufficient to induce tumor metastasis, suggesting that there must be an additional mechanism of Ras function other than promoting cell survival to account for tumor invasion.
A new role for PP1 in epithelial organization and cell invasion through regulation of myosin II and JNK
Both Drosophila and humans have multiple genes encoding PP1c isoforms, which has complicated analysis of their biological roles in vivo. In this study, we provide the first in vivo evidence that PP1 plays essential roles in controlling epithelial organization and cell invasion. Our studies suggest that sds22 functions as a key regulatory subunit of PP1 to inhibit myosin II and JNK signaling. In addition to the previously identified target myosin II (Grusche et al., 2009), we find that JNK signaling is also regulated by sds22/PP1. How sds22 regulates JNK signaling, which mediates both cell invasion and cell apoptosis, remains unclear. The fact that not all sds22 deficient cells induce active JNK indicates that sds22/PP1 may regulate JNK activity indirectly through regulation of upstream components. Genetic studies suggest that Drosophila PP1β can regulate JNK through myosin II (Kirchner et al., 2007b). However, blocking myosin II activity in our study does not abolish the sds22/PP1-mediated JNK activation (data not shown). Alternatively, the JNK pathway can be activated by disruption of cell polarity genes (Brumby and Richardson, 2003; Ryoo et al., 2004; Zhu et al., 2010), suggesting that JNK could be a common downstream signal induced by the absence of these tumor suppressors. The role of cell polarity genes in mediating JNK activation downstream of sds22/PP1 will require further investigation.
Relationship between Sds22/PP1 and cell polarity genes
Although the cell invasion and death phenotypes caused by loss of sds22 can be fully suppressed by reducing myosin II and JNK activity, epithelial defects are not fully rescued, suggesting that additional targets of the Sds22/PP1 complex might be involved. Phosphorylation of cell polarity regulators, including Baz and Lgl, must be tightly regulated for their normal subcellular localization and function (Betschinger et al., 2003; Morais-de-Sa et al., 2010). Although much is known regarding the roles of their kinases such as Par-1 and aPKC, the mechanism of their dephosphorylation is unclear. Recently, sds22 was identified in a geneticinteraction screen with Baz (Shao et al., 2010), a key regulator of apical membrane polarity and a substrate of PP1 in mouse cell culture (Traweger et al., 2008), suggesting that sds22/PP1 may act directly on critical components of the cell polarity machinery to maintain epithelial integrity and prevent metastasis. Consistent with this interpretation, we find that overexpression of sds22 can largely suppress the loss-of-function phenotypes of the cell polarity gene scrib. Further research will be necessary to clarify the mechanism of the interplay between Sds22/PP1 and cell polarity genes.
Sds22/PP1 function in mammals
The proteins Sds22, PP1, and components of myosin II and the JNK signaling pathway are highly conserved between Drosophila and humans. This raises the possibility that human Sds22 may play a role in regulating PP1 to maintain proper epithelial integrity and prevent cell invasion via a mechanism similar to that reported in Drosophila. Indeed, the human sds22 homolog, PPP1R7, also regulates cell shape and myosin II light chain phosphorylation (Grusche et al., 2009). In support of a tumor suppressive role for PPP1R7 in cancer, a survey of the Turmorscape portal (http://www.broadinstitute.org/tumorscape/) for copy number alterations in cancer (Beroukhim et al., 2010) shows that PPP1R7 (2q37.3), is frequently deleted in six cancer subtypes that include breast, ovarian, and melanoma among others (Figure S7A). This finding is consistent with published reports indicating PPP1R7 deletion in oral and cervical cancer (Cengiz et al., 2007; Narayan et al., 2003). Consistent with its genomic loss, PPP1R7 RNA expression is also significantly down-regulated in multiple cancer types (Rhodes et al., 2004). Among those cancers is melanoma, where PPP1R7 expression is down-regulated in primary tumors versus normal skin and benign nevi (Talantov et al., 2005) and in melanoma metastases versus primary tumor specimens (Kabbarah et al., 2010) (Figure S7B). Collectively, these findings support a role for PPP1R7 in tumor suppression in mammals and emphasize the importance of epithelial regulators in tumor progression.
In conclusion, the data presented here add new information about the role of sds22 during normal epithelial tissue organization and tumor cell invasion. Our studies show that the interaction of Sds22 with PP1 regulates a subset of the proteins normally controlled by PP1 activity and affects signaling pathways involved in apoptosis, cell migration, and cytoskeleton control, and whose misregulation leads to enhanced invasive behavior and transforms cells from a nonmetastatic to a metastatic state. Importantly, we also find that sds22 interacts with the known neoplastic tumor suppressor scrib, and can cooperate with activated Ras to promote tissue neoplasia and metastasis. Together, our results raise the interesting possibility that dephosphorylation of key molecules that normally control cell polarity and cell migration through sds22/PP1 activity could be a previously unrecognized tumor suppression mechanism.
Experimental Procedures
Drosophila Stocks
Fly cultures were raised at 25°C using standard media. The following stocks were used: P{EPgy2}EY06161, PP1a-87B87Bg3, UAS-PP1a-87B, and UAS-PP1β-9C (Bloomington Stock Center), sds22-RNAi, PP1β9C-RNAi, zip-RNAi (VDRC), UAS-sqhDD (Yasuyoshi Nishida), UAS-eiger and UAS-RasV12 (Tian Xu), UAS-puc, and puc-lacZ (Andreas Bergmann), scrib2 (Georg Halder), UAS-Timp (Dirk Bohmann). sds22Δ1.8, sds22Δ1.83, sds22Δ2.2, sds22Δ2.3 and sds22Δ2.8 were derived from imprecise excision of the P-element P{EPgy2}EY06161 using Δ2–3 transposase following conventional methods. All these alleles fail to complement each other and yield the same lethal phase and adult eye phenotypes described in this study.
Generation of Rescue Constructs
For rescue experiments, UAS-sds22 contains a full-length cDNA from EST clone GH07711 between the EcoRI and XhoI sites of pUAST-attB. An sds22 construct that contains only the sds22 genomic locus rescues all known sds22 mutant phenotypes. Details of this construct are available upon request.
Clonal Analysis
Mitotic or RNAi FLPout clones were generated by the FRT/FLP technique (Xu and Rubin, 1993) by applying a 1 hr heat shock (37°C) to induce hs-FLP 48 hr AEL. The eyFLP/MARCM system (Lee and Luo, 2001) was used to induce clones positively marked by GFP in eye/antennal discs.
Immunohistochemistry
Antibody stainings of imaginal discs were performed as previously described (Pepple et al., 2008). The following antibodies were used: rat anti-Elav (1:400, DSHB), chicken-anti-GFP (1:1000, Sigma), rat anti-DE cadherin (1:50, DSHB), Mouse anti-MMP1 (1:150, from Andreas Bergmann), rabbit anti-β-Galactosidase (1:1000, Cappel), mouse anti-Dlg (1:100, DSHB), and Rabbit anti-Caspase-3 (1:100, Cell Signaling), rhodamine-conjugated phalloidin (1:50, Molecular Probes). Secondary antibodies were anti-rat-Alexa 488, anti- guinea pig-Cy3, anti-chicken-Alexa 488, anti-rat-Cy3, anti-mouse-Cy3, anti-rabbit-Cy3 (1:1000 each, Molecular Probes), and anti-mouse-Cy5 (1:1000, Jackson Immunochemicals). Images were captured with a Zeiss LSM 510 confocal microscope and processed with ImageJ and Adobe Photoshop software. The images of compared genotypes are at the same magnification.
Supplementary Material
Acknowledgments
We thank Andreas Bergmann, Yasuyoshi Nishida, Tian Xu, the Bloomington Stock Center, VDRC, and the DSHB for stocks and antibodies. We especially thank Georg Halder, Rui Chen, Mardelle Atkins, Umesh Karandikar, and Nicholas Justice for helpful comments on the manuscript. This work was supported by the National Eye Institute (R01 EY011232 [G.M.] and EY016853 [R.C.]) and the Retina Research Foundation (G.M.).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Agrawal N, Kango M, Mishra A, Sinha P. Neoplastic transformation and aberrant cell-cell interactions in genetic mosaics of lethal(2)giant larvae (lgl), a tumor suppressor gene of Drosophila. Dev Biol. 1995;172:218–29. doi: 10.1006/dbio.1995.0017. [DOI] [PubMed] [Google Scholar]
- Beaucher M, Hersperger E, Page-McCaw A, Shearn A. Metastatic ability of Drosophila tumors depends on MMP activity. Dev Biol. 2007;303:625–34. doi: 10.1016/j.ydbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–9. doi: 10.1038/6854. [DOI] [PubMed] [Google Scholar]
- Bennett D, Lyulcheva E, Alphey L, Hawcroft G. Towards a comprehensive analysis of the protein phosphatase 1 interactome in Drosophila. J Mol Biol. 2006;364:196–212. doi: 10.1016/j.jmb.2006.08.094. [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–30. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–25. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–39. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- Cengiz B, Gunduz M, Nagatsuka H, Beder L, Gunduz E, Tamamura R, et al. Fine deletion mapping of chromosome 2q21–37 shows three preferentially deleted regions in oral cancer. Oral Oncol. 2007;43:241–7. doi: 10.1016/j.oraloncology.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84:1–39. doi: 10.1152/physrev.00013.2003. [DOI] [PubMed] [Google Scholar]
- Ceulemans H, Vulsteke V, De Maeyer M, Tatchell K, Stalmans W, Bollen M. Binding of the concave surface of the Sds22 superhelix to the alpha 4/alpha 5/alpha 6-triangle of protein phosphatase-1. J Biol Chem. 2002;277:47331–7. doi: 10.1074/jbc.M206838200. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Dombradi V, Axton JM, Brewis ND, da Cruz e Silva EF, Alphey L, Cohen PT. Drosophila contains three genes that encode distinct isoforms of protein phosphatase 1. Eur J Biochem. 1990;194:739–45. doi: 10.1111/j.1432-1033.1990.tb19464.x. [DOI] [PubMed] [Google Scholar]
- Dombradi V, Mann DJ, Saunders RD, Cohen PT. Cloning of the fourth functional gene for protein phosphatase 1 in Drosophila melanogaster from its chromosomal location. Eur J Biochem. 1993;212:177–83. doi: 10.1111/j.1432-1033.1993.tb17648.x. [DOI] [PubMed] [Google Scholar]
- Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- Grusche FA, Hidalgo C, Fletcher G, Sung HH, Sahai E, Thompson BJ. Sds22, a PP1 phosphatase regulatory subunit, regulates epithelial cell polarity and shape [Sds22 in epithelial morphology] BMC Dev Biol. 2009;9:14. doi: 10.1186/1471-213X-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–23. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16:1139–46. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Jasper H, Benes V, Schwager C, Sauer S, Clauder-Munster S, Ansorge W, et al. The genomic response of the Drosophila embryo to JNK signaling. Dev Cell. 2001;1:579–86. doi: 10.1016/s1534-5807(01)00045-4. [DOI] [PubMed] [Google Scholar]
- Kabbarah O, Nogueira C, Feng B, Nazarian RM, Bosenberg M, Wu M, et al. Integrative genome comparison of primary and metastatic melanomas. PLoS One. 2010;5:e10770. doi: 10.1371/journal.pone.0010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner J, Gross S, Bennett D, Alphey L. Essential, overlapping and redundant roles of the Drosophila protein phosphatase 1 alpha and 1 beta genes. Genetics. 2007a;176:273–81. doi: 10.1534/genetics.106.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner J, Gross S, Bennett D, Alphey L. The nonmuscle myosin phosphatase PP1beta (flapwing) negatively regulates Jun N-terminal kinase in wing imaginal discs of Drosophila. Genetics. 2007b;175:1741–9. doi: 10.1534/genetics.106.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285–8. doi: 10.1126/science.1067549. [DOI] [PubMed] [Google Scholar]
- Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–29. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Llano E, Pendas AM, Aza-Blanc P, Kornberg TB, Lopez-Otin C. Dm1-MMP, a matrix metalloproteinase from Drosophila with a potential role in extracellular matrix remodeling during neural development. J Biol Chem. 2000;275:35978–85. doi: 10.1074/jbc.M006045200. [DOI] [PubMed] [Google Scholar]
- MacKelvie SH, Andrews PD, Stark MJ. The Saccharomyces cerevisiae gene SDS22 encodes a potential regulator of the mitotic function of yeast type 1 protein phosphatase. Mol Cell Biol. 1995;15:3777–85. doi: 10.1128/mcb.15.7.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FA, Perez-Garijo A, Morata G. Apoptosis in Drosophila: compensatory proliferation and undead cells. Int J Dev Biol. 2009;53:1341–7. doi: 10.1387/ijdb.072447fm. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–70. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DG, Peifer M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 2005;132:3935–46. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–23. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–24. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Narayan G, Pulido HA, Koul S, Lu XY, Harris CP, Yeh YA, et al. Genetic analysis identifies putative tumor suppressor sites at 2q35-q36.1 and 2q36.3-q37.1 involved in cervical cancer progression. Oncogene. 2003;22:3489–99. doi: 10.1038/sj.onc.1206432. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–60. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Yanagida M. S. pombe gene sds22+ essential for a midmitotic transition encodes a leucine-rich repeat protein that positively modulates protein phosphatase-1. Cell. 1991;64:149–57. doi: 10.1016/0092-8674(91)90216-l. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Serano J, Sante JM, Rubin GM. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev Cell. 2003;4:95–106. doi: 10.1016/s1534-5807(02)00400-8. [DOI] [PubMed] [Google Scholar]
- Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–31. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- Pepple KL, Atkins M, Venken K, Wellnitz K, Harding M, Frankfort B, et al. Two-step selection of a single R8 photoreceptor: a bistable loop between senseless and rough locks in R8 fate. Development. 2008;135:4071–9. doi: 10.1242/dev.028951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohar N, Godenschwege TA, Buchner E. Invertebrate tissue inhibitor of metalloproteinase: structure and nested gene organization within the synapsin locus is conserved from Drosophila to human. Genomics. 1999;57:293–6. doi: 10.1006/geno.1999.5776. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Shao W, Wu J, Chen J, Lee DM, Tishkina A, Harris TJ. A modifier screen for Bazooka/PAR-3 interacting genes in the Drosophila embryo epithelium. PLoS One. 2010;5:e9938. doi: 10.1371/journal.pone.0009938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Aaronson SA, Mlodzik M. Drosophila Abelson kinase mediates cell invasion and proliferation through two distinct MAPK pathways. Oncogene. 2010;29:4033–45. doi: 10.1038/onc.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DP. Drosophila odor receptors revealed. Neuron. 1999;22:203–4. doi: 10.1016/s0896-6273(00)81078-8. [DOI] [PubMed] [Google Scholar]
- Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–7. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci U S A. 2007;104:2721–6. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Yamano H, Kinoshita N, Yanagida M. Mitotic regulation of protein phosphatases by the fission yeast sds22 protein. Curr Biol. 1993;3:13–26. doi: 10.1016/0960-9822(93)90140-j. [DOI] [PubMed] [Google Scholar]
- Suganuma M, Fujiki H, Suguri H, Yoshizawa S, Hirota M, Nakayasu M, et al. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci U S A. 1988;85:1768–71. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, et al. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11:7234–42. doi: 10.1158/1078-0432.CCR-05-0683. [DOI] [PubMed] [Google Scholar]
- Traweger A, Wiggin G, Taylor L, Tate SA, Metalnikov P, Pawson T. Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3. Proc Natl Acad Sci U S A. 2008;105:10402–7. doi: 10.1073/pnas.0804102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc Natl Acad Sci U S A. 2005;102:13123–8. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereshchagina N, Bennett D, Szoor B, Kirchner J, Gross S, Vissi E, et al. The essential role of PP1beta in Drosophila is to regulate nonmuscle myosin. Mol Biol Cell. 2004;15:4395–405. doi: 10.1091/mbc.E04-02-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Winter SL, Bosnoyan-Collins L, Pinnaduwage D, Andrulis IL. The interaction of PP1 with BRCA1 and analysis of their expression in breast tumors. BMC Cancer. 2007;7:85. doi: 10.1186/1471-2407-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–24. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–64. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–37. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Young MR. Protein phosphatases-1 and -2A regulate tumor cell migration, invasion and cytoskeletal organization. Adv Exp Med Biol. 1997;407:311–8. doi: 10.1007/978-1-4899-1813-0_46. [DOI] [PubMed] [Google Scholar]
- Zhu M, Xin T, Weng S, Gao Y, Zhang Y, Li Q, et al. Activation of JNK signaling links lgl mutations to disruption of the cell polarity and epithelial organization in Drosophila imaginal discs. Cell Res. 2010;20:242–5. doi: 10.1038/cr.2010.2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.