Abstract
Objective
Calcification and fibrosis reduce vascular compliance in arteriosclerosis. To better understand the role of osteopontin (OPN), a multifunctional protein upregulated in diabetic arteries, we evaluated contributions of OPN in male LDLR−/− mice fed high fat diets (HFD).
Methods and Results
OPN had no impact on HFD-induced hyperglycemia, dyslipidemia, or body composition. However, OPN−/−;LDLR−/− mice exhibited an altered time-course of aortic calcium accrual -- reduced during initiation but increased with progression -- vs. OPN+/+;LDLR−/− controls. Collagen accumulation, chondroid metaplasia, and mural thickness were increased in aortas of OPN−/−;LDLR−/− mice. Aortic compliance was concomitantly reduced. Vascular re-expression of OPN (SM22-OPN transgene) reduced aortic Col2A1 and medial chondroid metaplasia, but did not impact atherosclerotic calcification, Col1A1 expression, collagen accumulation, or arterial stiffness. Dosing with the pro-inflammatory OPN fragment SVVYGLR upregulated aortic Wnt and osteogenic gene expression, increased aortic β-catenin, and restored early-phase aortic calcification in OPN−/−;LDLR−/− mice.
Conclusions
OPN exerts stage-specific roles in arteriosclerosis in LDLR−/− mice. Actions phenocopied by the OPN metabolite SVVYGLR promote osteogenic calcification processes with disease initiation. OPN limits vascular chondroid metaplasia, endochondral mineralization and collagen accumulation with progression. Complete deficiency yields a net increase in arteriosclerotic disease, reducing aortic compliance and conduit vessel function in LDLR−/− mice.
Keywords: Arteriosclerosis, Calcification, Chondroid Metaplasia, Diabetes, Osteopontin
INTRODUCTION
Atherosclerosis, calcification, mural fibrosis, endothelial dysfunction, and elastin matrix senescence cause arteriosclerosis, the age-associated conduit vessel stiffening that impairs Windkessel physiology necessary for efficient distal tissue perfusion1, 2. Vascular remodeling processes also increase wall thickness and reduce conduit artery lumen area, thus worsening arterial compliance and its impact2. In the musculoskeletal system, lower extremities experience the brunt of arteriosclerotic disease. Claudication and amputation are salient manifestations that diminish mobility and increase morbidity, but hip fracture is also increased3. Importantly, the presence and extent of arterial calcification conveys risk for progression of arteriosclerosis to critical limb ischemia requiring amputation4, 5. Osteochondrocytic gene programs are elaborated by calcifying arteries of patients with arteriosclerosis, indicating that active osteogenic processes contribute to arterial calcium accrual6. The increasing prevalence of type II diabetes (T2DM) – a risk factor for arterial calcification7, 8 – will increase arteriosclerotic disease burden in the future. A fundamental understanding of pro-calcific and pro-fibrotic arterial remodeling is required to develop new strategies to better address this burgeoning clinical need9, 10.
In our studies of arteriosclerotic calcification, we have emphasized the LDLR−/− mouse model. When male LDLR−/− mice are fed high fat diets (HFD) characteristic of Westernized caloric composition, animals develop obesity, insulin-resistant diabetes, hypertriglyceridemia and hypercholesterolemia with progressively severe arterial calcification11. While patchy arterial medial calcification predominates in the model at early stages11, atherosclerotic calcification with or without ectopic ossification (endochondral bone formation) increasingly accounts for vascular calcium load over time11, 12. At very early stages of HFD-induced disease, active osteogenic gene regulatory programs are elaborated in the artery wall. Within a few weeks of HFD challenge and the initiation of “diabesity,” the osteoblast transcription factor Msx2 is upregulated along with osteopontin (OPN)13, a polyphosphorylated matrix cytokine and integrin ligand first identified in bone14. We’ve shown that mesenchymal Msx2 expression promotes cardiovascular calcification by activating osteogenic Wnt/β-catenin signals13. However, the contributions of OPN to vascular biology are more complex14. OPN is a SIBLING (small integrin binding ligand N-glycosylated) family member. Like all SIBLINGS, OPN is a highly processed multifunctional protein14. Full-length phosphorylated OPN is relatively protease-resistant, promotes cell attachment and migration, and is an inhibitor of calcium deposition14, 15. Giachelli first identified that OPN is expressed in calcifying vascular segments14 and that intact phosphorylated OPN limits the extent of atherosclerotic calcium deposition16. Thrombin-processed OPN -- viz. the N-terminal fragment that contain the C-terminal epitope SVVYGLR (SLAYGLR in mouse) 14 -- is pro-inflammatory17, pro-angiogenic18, pro-osteogenic19, and supports aortic MMP9 activation20. Indeed, in calcifying human aortic valves the SVVYGLR pro-inflammatory OPN “N-half” epitope is enriched at sites of calcification, correlating with activation of thrombin21. OPN is pivotal in MMP-dependent vascular remodeling during aortic aneurysm formation22 and arteriogenic collateralization responses to limb ischemia23. Given the distinct bioactivities of OPN metabolites and isoforms14, OPN excess or insufficiency will impact multiple aspects of diabetic vascular biology.
To better understand the role of OPN in the arteriosclerosis of T2DM24, we have examined the effects of OPN deficiency in the LDLR−/− model of HFD-induced disease. We find that OPN SVVYGLR signals promote calcification and osteogenic signaling with disease initiation, while other OPN actions limit arterial fibrosis, vascular chondroid metaplasia seen in advanced human disease24, and vascular calcium accrual with disease progression. Complete OPN deficiency yields a net increase in arteriosclerotic disease and reduces aortic compliance in diabetic LDLR−/− mice.
METHODS
Reagents and animals
Biochemicals, histochemical stains, and molecular biology reagents were obtained from Fisher, Sigma, or Invitrogen. Taqman Gene Expression Assays for analysis of aortic mRNAs by RT-qPCR were purchased from Applied Biosystems. The custom synthetic OPN heptapeptide SVVYGLR was obtained from Invitrogen. All procedures for maintaining and handling mice were approved by the Washington University Animal Studies Committee. All other reagents and protocols11, 13, 25 are detailed in the online supplement.
Statistics
Statistical analyses were performed using GraphPad Instat Software (version 3.06), implementing standard parametric or non-parametric (Mann-Whitney U-test for histological image analysis scores) methods as indicated. Post-hoc analysis for significance between groups in one-way ANOVA was carried out using Student-Neuman-Keuls multiple comparison testing. Differences were deemed significant for p < 0.05. Graphic data are presented as the mean ± SEM.
RESULTS
OPN differentially regulates initiation and progression phases of aortic calcification in diabetic LDLR−/− mice
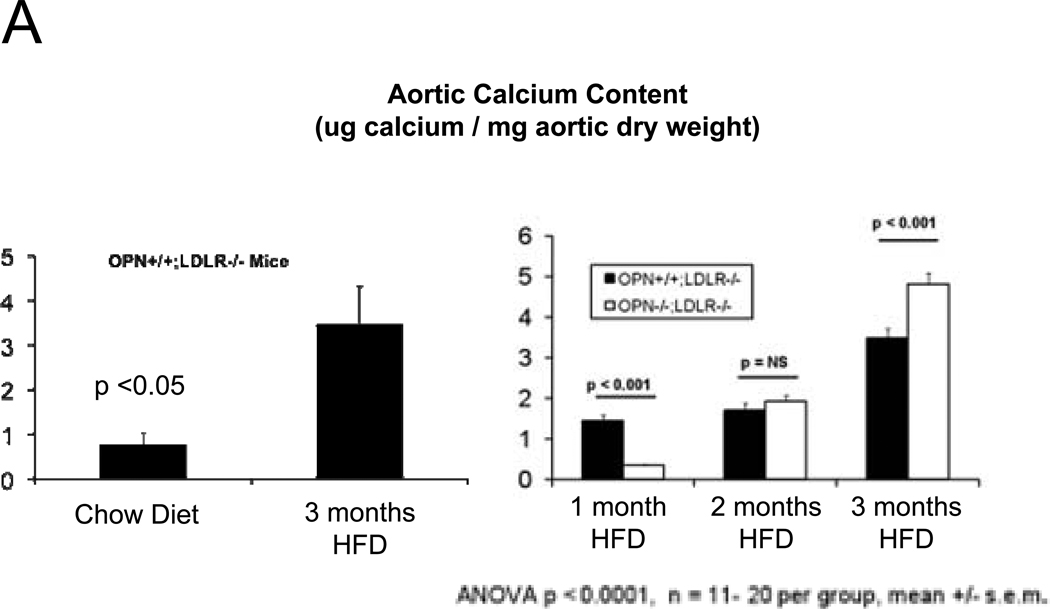
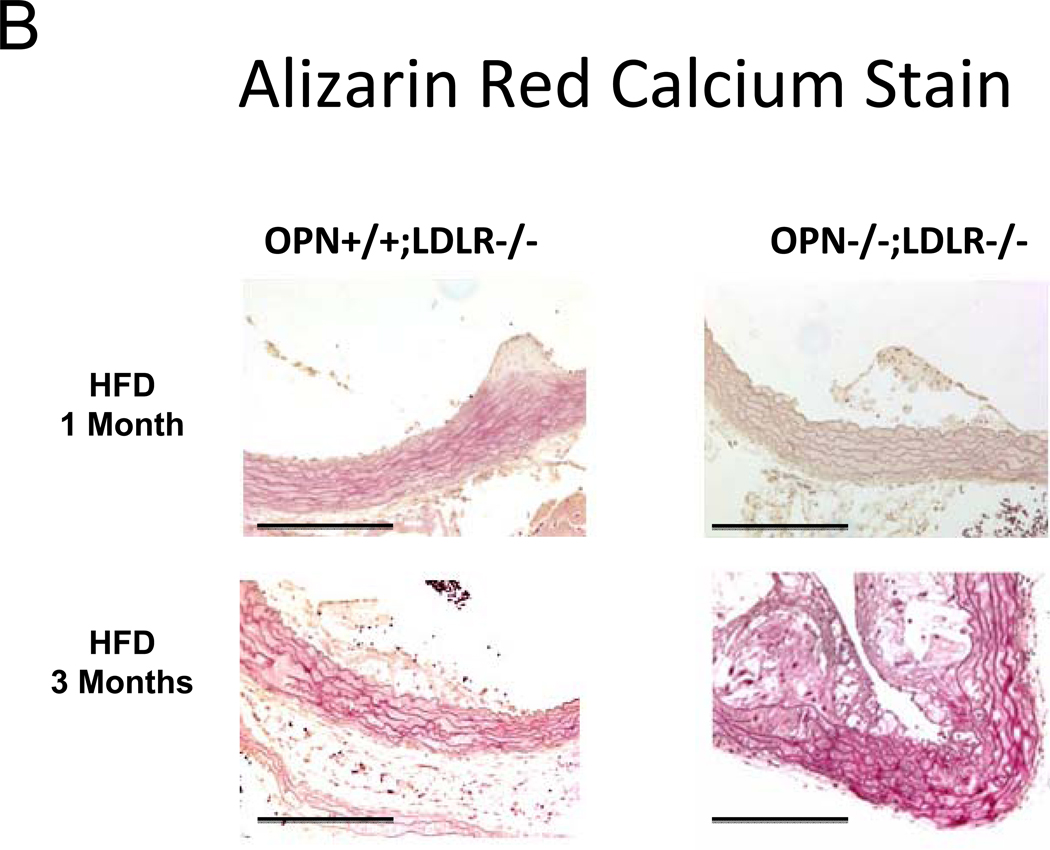
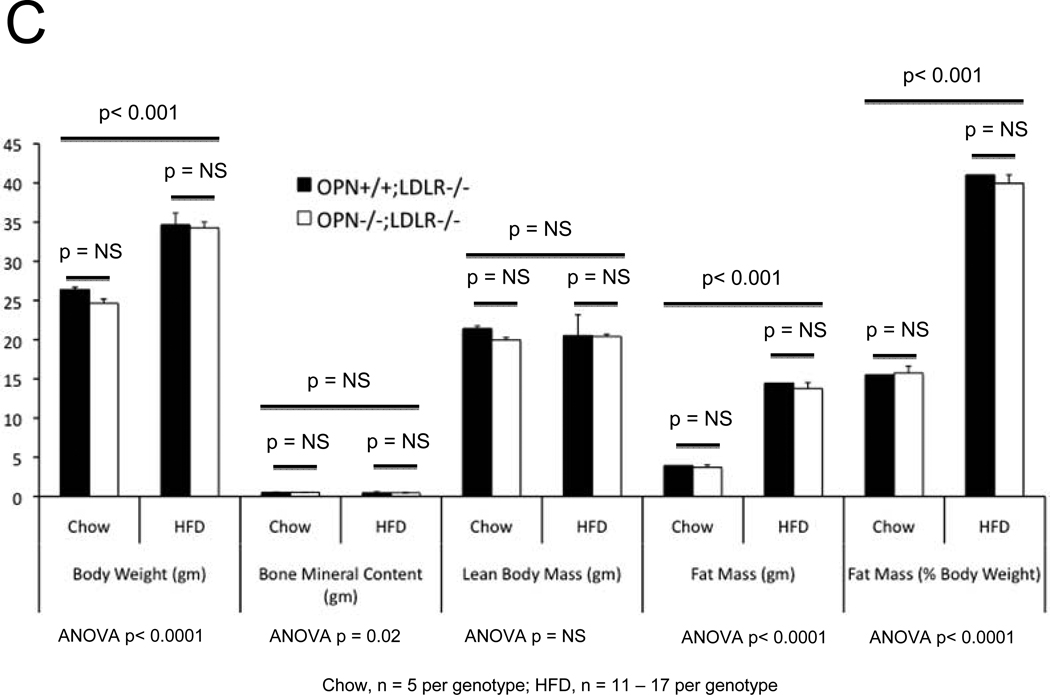
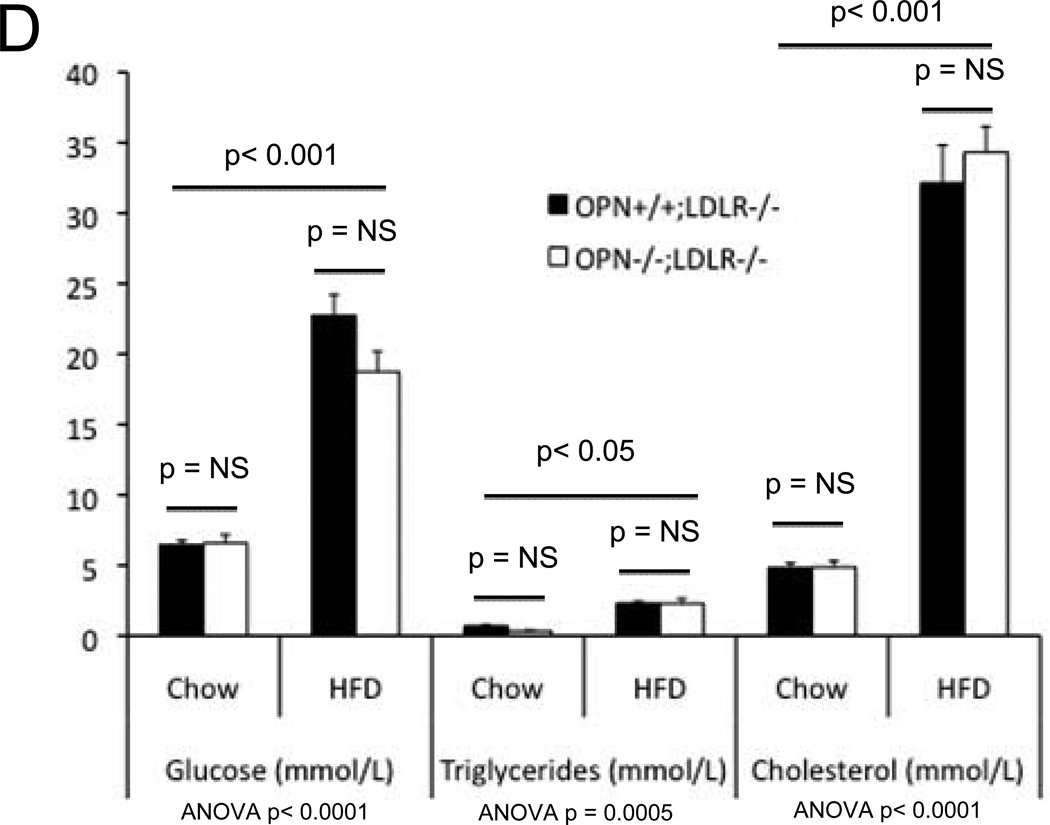
In the MGP- and male apoE- deficient models of arterial calcification, concomitant OPN deficiency clearly increases arterial calcium accrual26, 27. However, apoE-28 and MGP29-deficient mice do not develop the diet-induced obesity and diabetes that commonly afflicts patients with vasculopathy8. Since OPN is a multifunctional modulator of migration and inflammation14, we hypothesized that OPN may exert distinct contributions to the initiation and progression of diabetic arteriosclerosis. To test this notion and better understand the role of OPN, we studied the impact of OPN deficiency on arterial calcification in the male LDLR−/− mouse fed diabetogenic HFD. HFD increases aortic calcium accrual 3-fold by 3 months in male LDLR−/− mice (Figure 1A). Thus, beginning at 1.5 to 2 months of age, cohorts of male OPN+/+;LDLR−/− and OPN−/−;LDLR−/− mice were challenged with HFD feeding for 1, 2, and 3 months duration (supplement Figure S1) to assess the impact of OPN on vascular calcium load during disease initiation and progression. After 1 month of HFD, OPN−/−;LDLR−/− mice exhibited significantly reduced aortic calcium content (ug / mg aorta) as compared with OPN+/+;LDLR−/− mice (Figure 1A). This reduction was not related to increased aortic weight in OPN−/−;LDLR−/− mice (trended lighter, p = NS). Of note, at this early disease stage, OPN−/−;LDLR−/− mice exhibit significantly diminished oxidative stress20 and reduced aortic expression of Msx2 and Sox2 (Figure S2) -- transcriptional regulators of self renewing osteoprogenitors30, 31. Alizarin red calcium staining localized the early reduction in aortic calcium content in OPN−/−;LDLR−/− animals to the tunica media (Figure 1B, upper panels). By contrast, by 3 months of HFD challenge – a stage at which atherosclerotic calcification increasingly contributes to aortic calcium accrual11 -- OPN−/−;LDLR−/− mice exhibit significantly increased aortic calcification vs. OPN+/+; LDLR−/− cohorts (Figure 1A). This was confirmed by Alizarin red staining (Figure 1B, lower panels), and reflected in the aortic expression of osteochondrocytic transcription factors including Msx2 (Figure S2, lower panel). At the intermediate stage of 2 months on HFD, no difference was noted in aortic calcium load between genotypes (Figure 1A). Importantly, OPN deficiency did not significantly impact HFD-induced changes in body composition (Figure 1C) or in fasting serum glucose, triglycerides, or total cholesterol (Figure 1D). Thus, OPN supports the early phase of aortic calcification in diabetic LDLR−/− mice, but limits the extent of aortic calcium accrual with disease progression.
Figure 1. OPN deficiency alters the kinetics of vascular calcification in the aortas of diabetic LDLR−/− mice fed high fat diabetogenic diets (HFD).
Panel A: HFD increases aortic calcification in LDLR−/− mice. The time course of aortic calcification differs in diabetic OPN−/−:LDLR−/− mice, with significant reductions during disease initiation (1 month) but with significant increases during disease progression (3 months) vs. OPN+/+;LDLR−/− controls. Panel B, Alizarin staining confirmed reductions in medial calcification at early stages, but increased calcification with advanced disease in diabetic OPN−/−;LDLR−/− cohorts. All scale bars are 100 micron. Body composition (panel C) and fasting serum metabolic profiles (panel D) did not differ by genotype. Data for the 3 month time point are shown. The trend for HFD to reduce bone mineral content66 detected by ANOVA did not reach significance on post-hoc testing. Chow, n = 5 per genotype; HFD, n = 11 – 17 per genotype.
Aortic chondroid metaplasia, elastin fragmentation, mural thickness and fibrosis are increased in OPN−/−;LDLR−/− mice
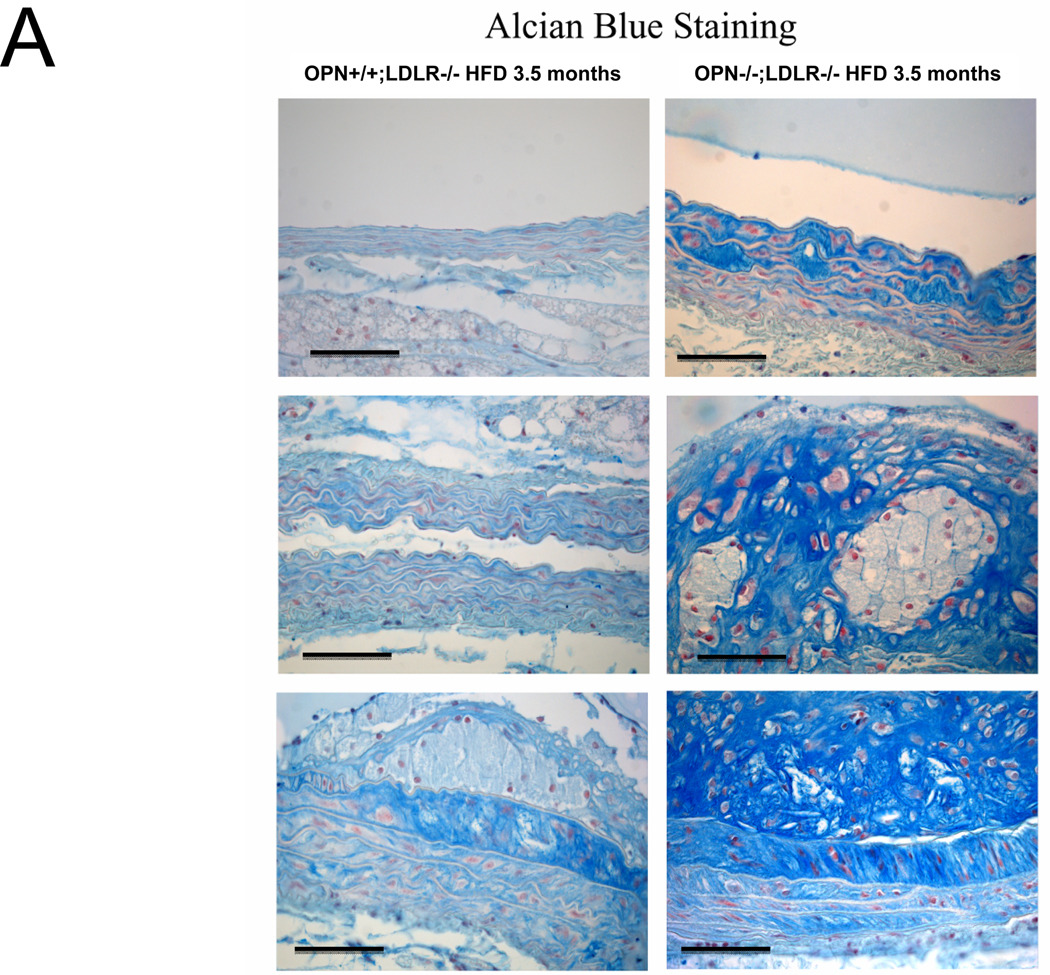
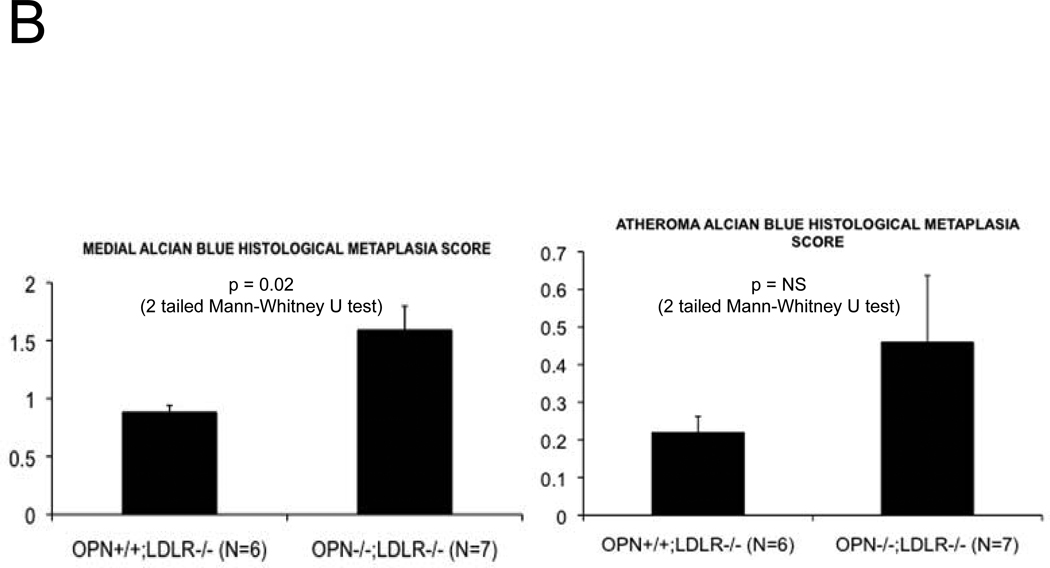
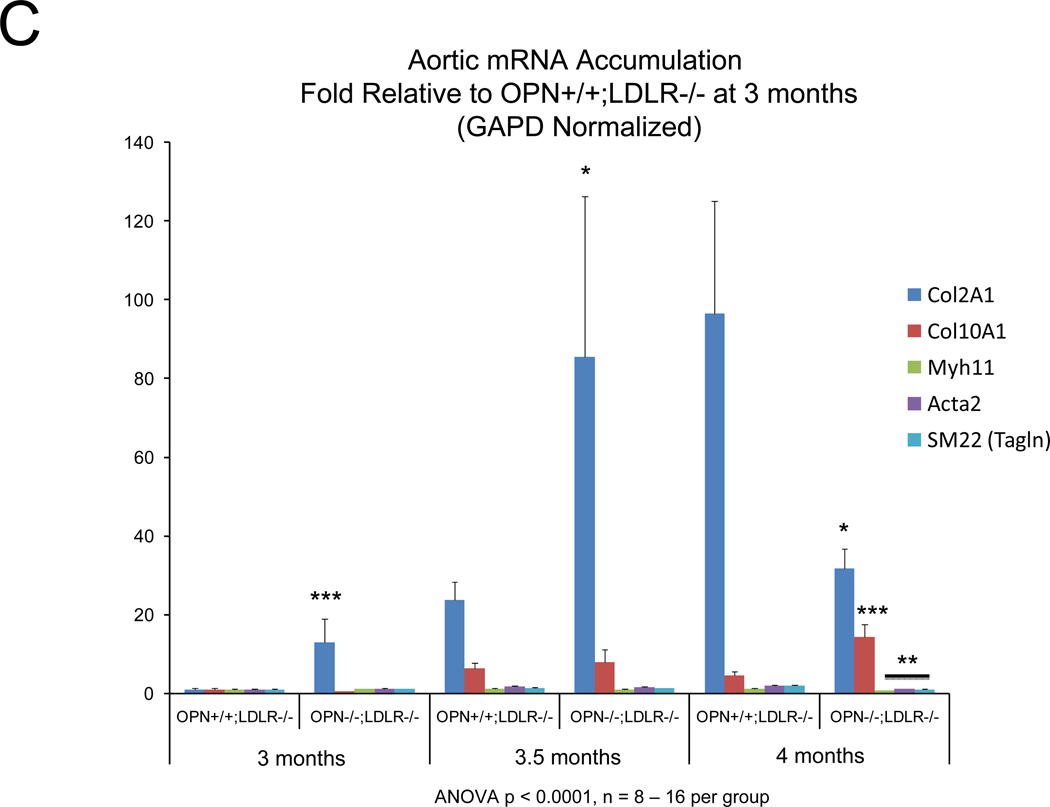
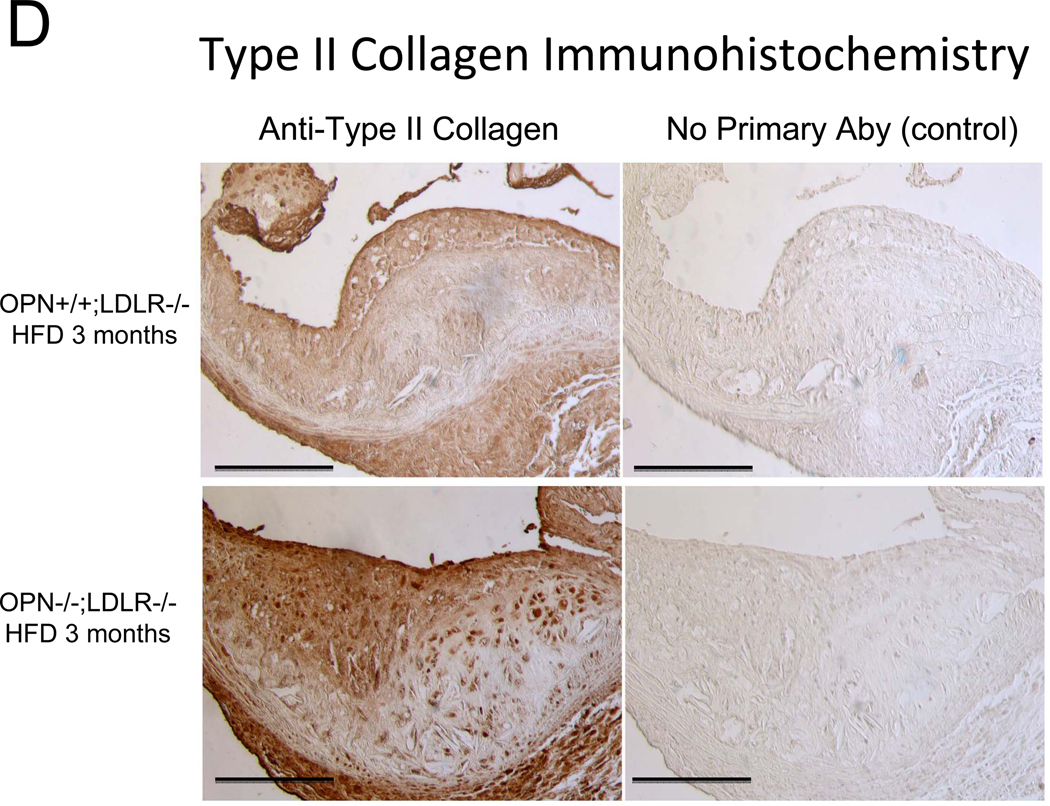
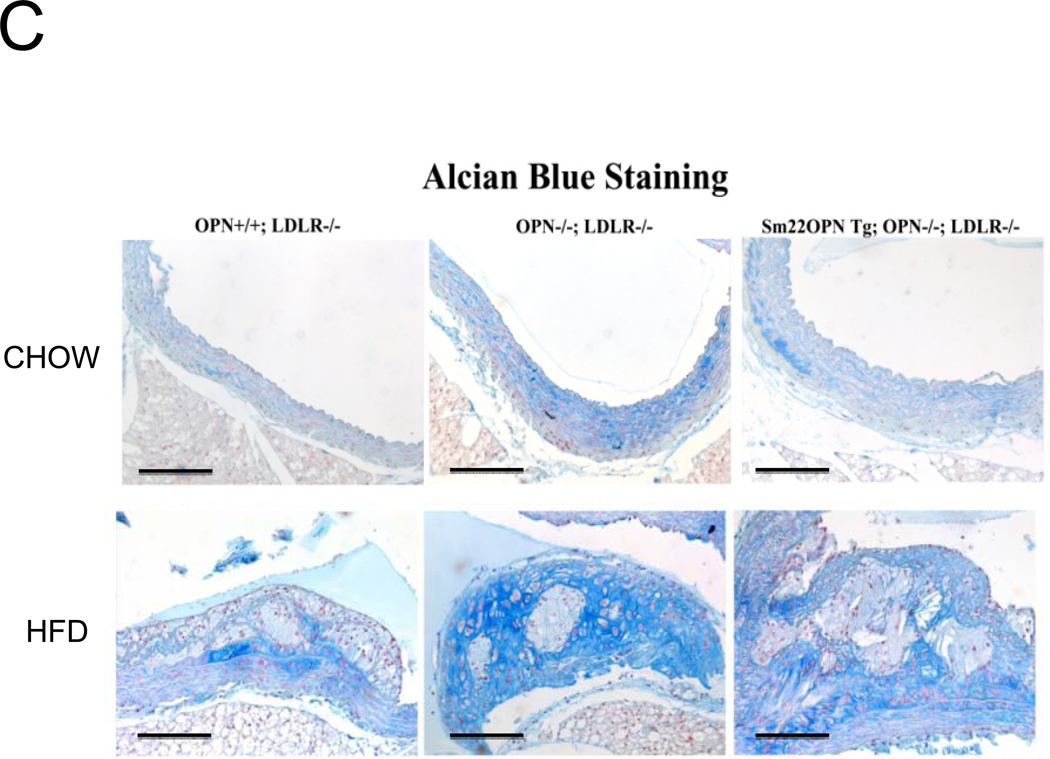
Alizarin and picrosirius red stains suggested that arterial chondroid metaplasia might be increased in OPN−/−;LDLR−/− mice as compared with OPN+/+;LDLR−/− cohorts (Figure S3A). Metaplasia was histologically confirmed by Alcian blue staining24 for cartilage glycosaminoglycans (Figure 2A). Chondroid metaplasia was much more prominent in the tunica media of OPN−/−;LDLR−/− mice, and was also more pronounced in the large atheroma elaborated by these animals (Figure 2A). In comparison to calcium (Alizarin red) and inorganic phosphate (von Kossa stain) deposits, chondroid metaplastic changes (Alcian stain) were much more extensive and observed in areas not yet involved with mineral deposition (Figure S3B). Histological scoring of the intensity and extent of Alcian blue staining confirmed increased medial chondroid metaplasia in OPN−/−;LDLR−/− vs. OPN+/+;LDLR−/− mice on HFD (Figure 2B; p= 0.02, U-test), and dependent upon HFD challenge (data not shown). A non-significant trend for atheroma staining was also observed in OPN-null cohorts (Figure 2B). Differences in metaplasia did not relate to differences in lesion monocyte / macrophage content as quantified by immunohistochemical enumeration (Figure S4). Aortic Col2A1 – a marker of early chondrocyte differentiation6 – was dramatically upregulated in OPN−/−;LDLR−/− mice vs. OPN+/+;LDLR−/− cohorts after 3 months of HFD (Figure 2C). Immunohistochemistry confirmed the increased intensity and extent of type II collagen protein accumulation in aortas of OPN−/−;LDLR−/− mice (Figure 2D). Aortic endochondral ossification24 is histologically evident between 3– 4 months of HFD (Figure S3B)12. During this later phase, Col2A1 decreased in OPN−/−;LDLR−/− aortas while Col10A1 – a hypertrophic chondrocyte marker upregulated with endochondral bone mineralization 32 – concomitantly increased (Figure 2C), thereby confirming endochondral ossification with disease progression33. VSMC markers Myh11, Acta2, and SM22 were significantly reduced by 40%–45% in aortas of OPN−/−;LDLR−/− mice after 4 months of HFD (Figure 2C, Figure S5). Thus, OPN deficiency promotes chondroid metaplasia in diabetic LDLR−/− mice on HFD.
Figure 2. OPN deficiency increases aortic chondroid metaplasia in diabetic LDLR−/− mice.
Panel A, representative Alician Blue histochemistry of aortic chondroid metaplasia in OPN+/+;LDLR−/− and OPN−/−;LDLR−/− mice on HFD. Panel B, histological scoring of the extent and intensity of staining demonstrated a statistically significance increase in OPN−/−;LDLR−/− mice. Panel C, OPN−/−;LDLR−/− mice exhibit augmented aortic expression of Col2A1 vs. OPN+/+;LDLR−/− controls. While Col10A1 is significantly increased, aortic VSMC markers are significantly decreased in OPN−/−;LDLR−/− vs. OPN+/+;LDLR−/− between 3 and 4 months of HFD. Panel D, immunohistochemistry reveals increased medial and atherosclerotic staining for type II collagen in OPN−/−;LDLR−/− mice vs. OPN+/+;LDLR−/− cohorts.
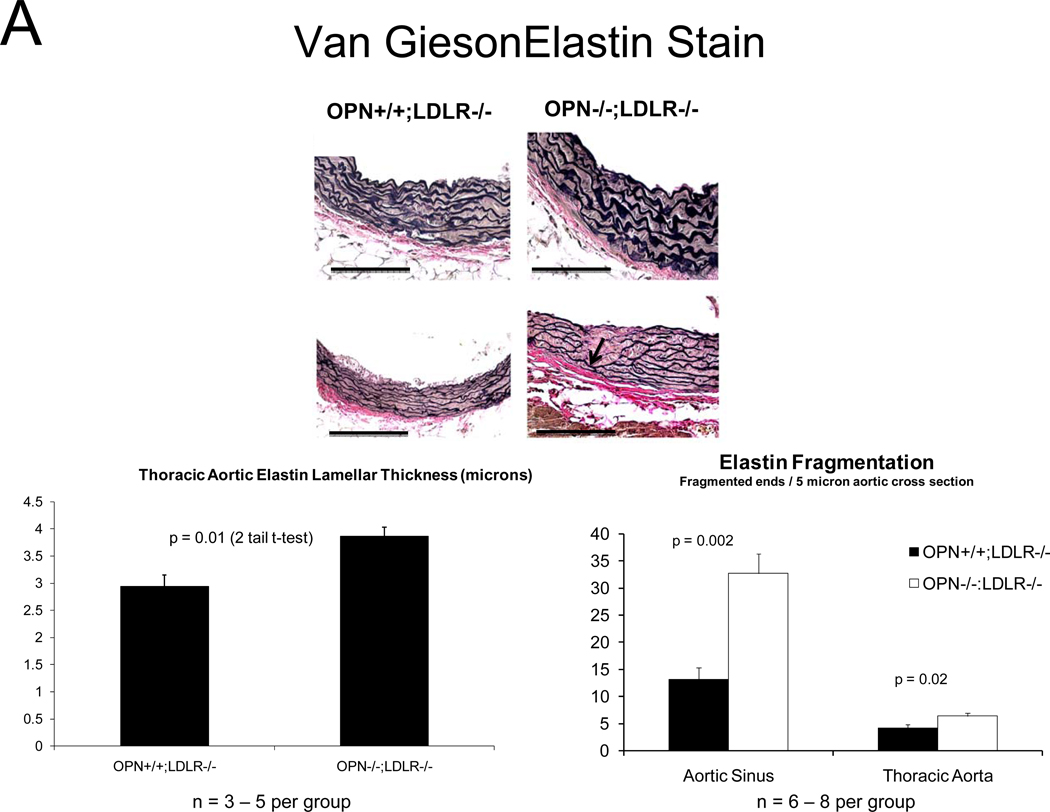
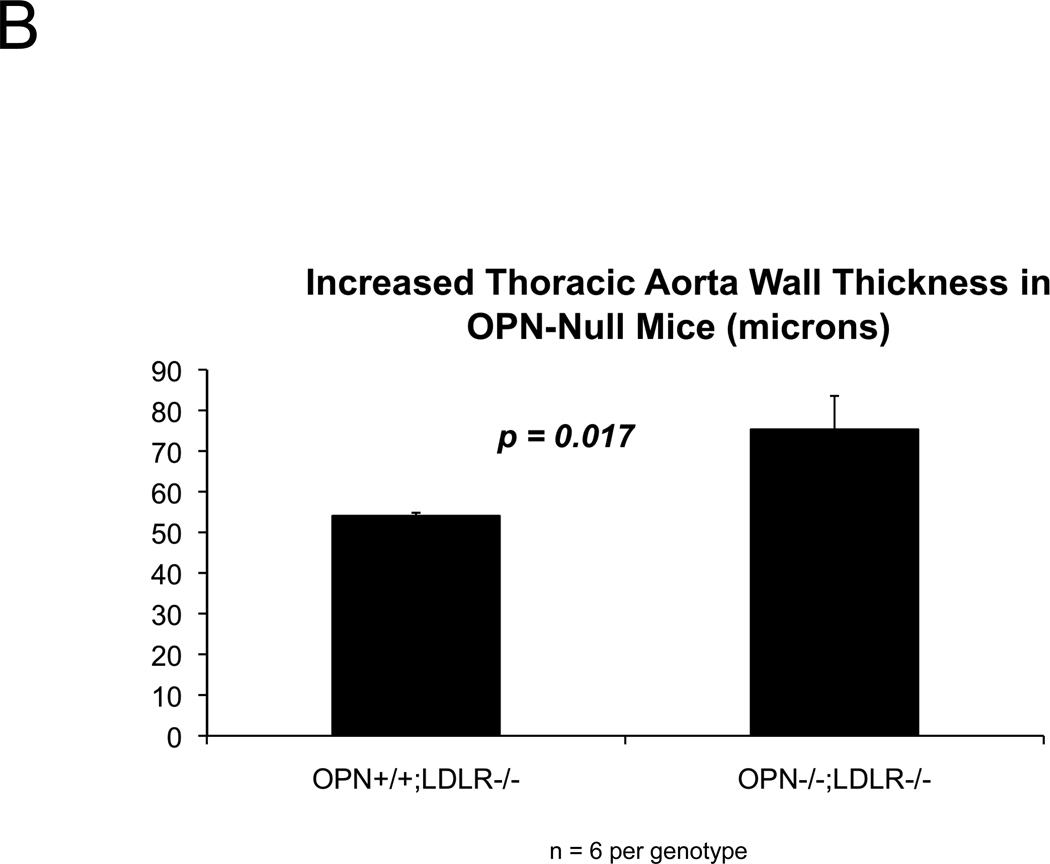
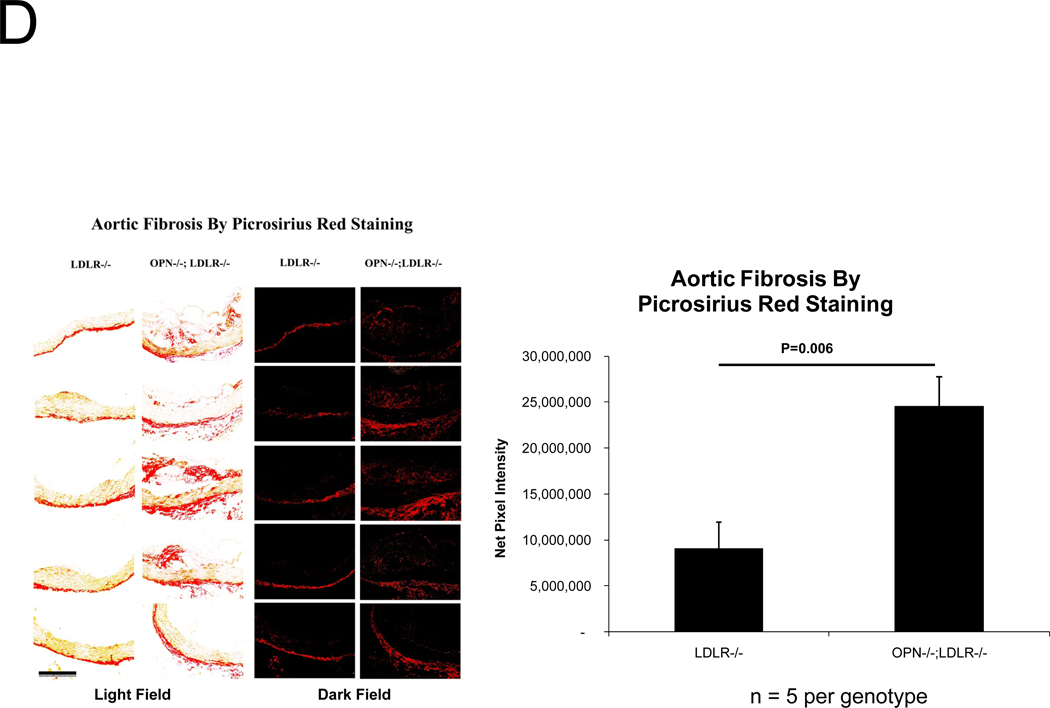
Because vascular OPN supports aortic collagenase activity and is associated with elastin fibers 34, we examined collagen and elastin matrix characteristics. Elastin morphology was abnormal in diabetic OPN−/−;LDLR−/− animals; histology revealed frequent fragmentation of thickened lamellae (Figure 3A), and aortic wall thickness was increased (Figure 3B). Even prior to HFD challenge, aortic collagen content was 70% greater in OPN−/−;LDLR−/− animals (p < 0.001), but elastin initially did not differ between the two genotypes (data not shown). However, with HFD- induced disease both total aortic collagen and elastin protein content were significantly greater in OPN−/−;LDLR−/− animals vs. OPN+/+;LDLR−/− cohorts (Figure 3C; 3–4 months of diabetogenic HFD). Picrosirius red staining with dark-field illumination analysis25 confirmed mural collagen accumulation, localizing fibrosis to the tunica adventitia with medial and atheromatous contributions (Figure 3D). Serum P1NP, a global marker of type I collagen biosynthesis, was also increased35 (Figure S6). Thus, OPN deficiency increases aortic collagen deposition, elastin fragmentation, and wall thickness in diabetic LDLR−/− mice.
Figure 3. Aortic collagen accumulation and elastin morphology are perturbed in diabetic OPN−/−;LDLR−/− mice.
Panel A, OPN−/−;LDLR−/− mice exhibit increased lamellar fragmentation and thickness (arrow) as assessed by histomorphometry. Panel B, total thoracic aortic wall thickness is increased in OPN−/−;LDLR−/− mice. Panel C, diabetic OPN−/−;LDLR−/− mice accrue greater aortic collagen and elastin content vs. OPN+/+;LDLR−/− cohorts. Figure D, histochemical staining of aortic sections with picrosirius red and darkfield digital image analysis25 confirms increased aortic fibrosis in OPN−/−;LDLR−/− mice.
Aortic compliance is reduced in diabetic OPN−/−;LDLR−/− mice
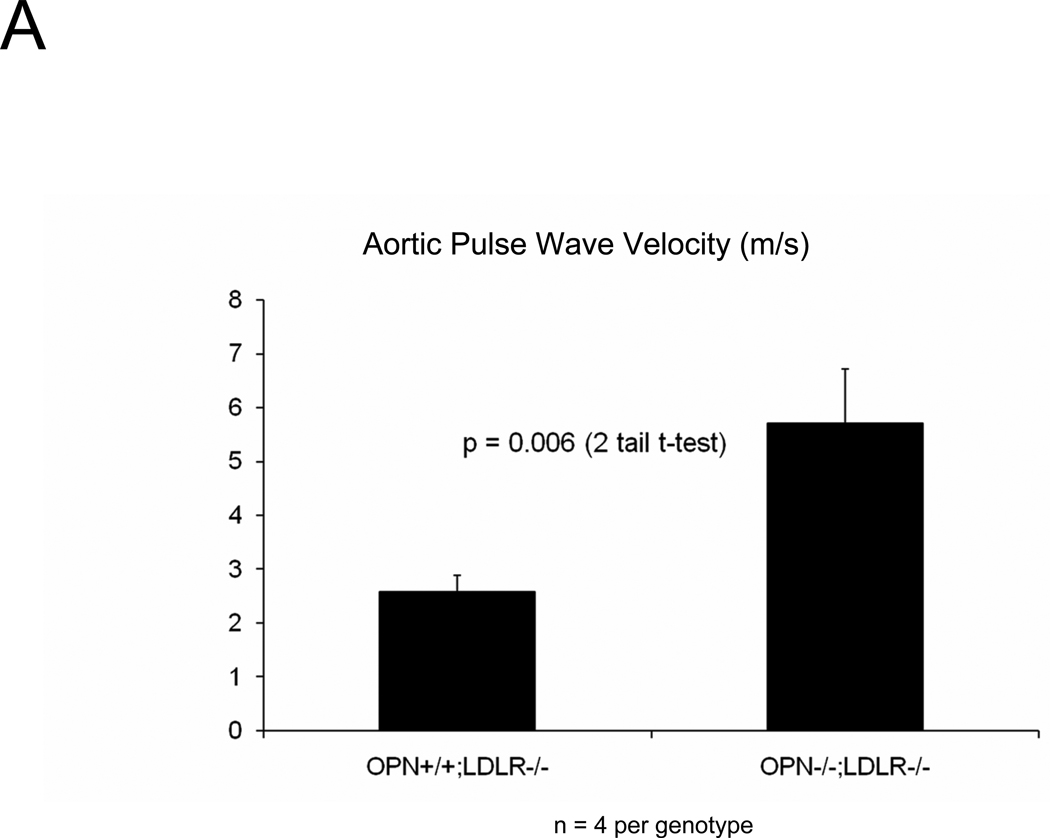
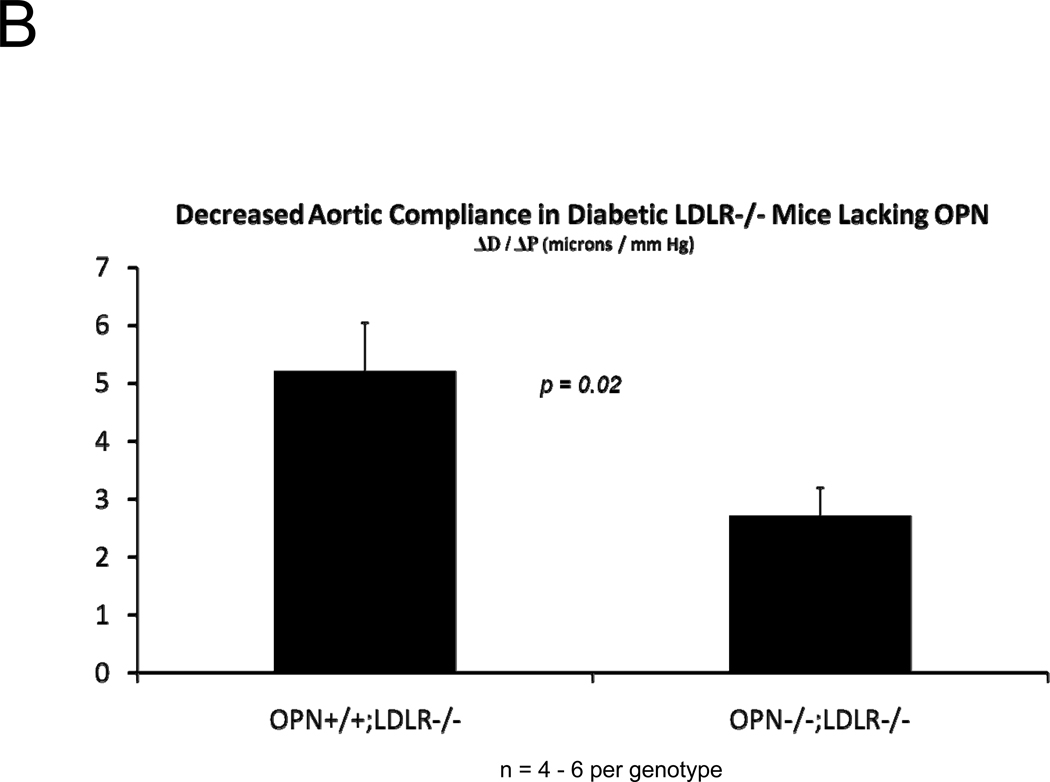
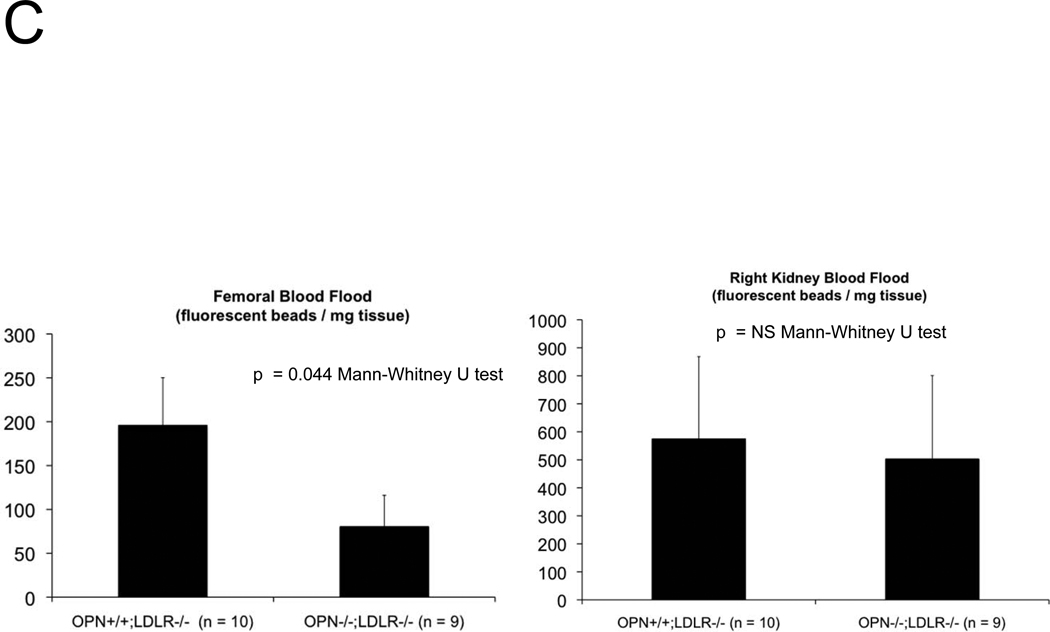
The presence of aortic fibrosis with elastin fragmentation and wall thickening strongly suggested that vessel mechanical properties would be altered by OPN deficiency2, 36. We first examined aortic arch pulse wave velocity (PWV)37 – an in vivo parameter that increases with reduced arterial compliance38 – as an assessment of aortic stiffness. As shown in Figure 4A, aortic PWV is significantly increased in OPN−/−;LDLR−/− mice vs. OPN+/+;LDLR−/− cohorts. We then implemented ex vivo video aortic plethysmography36 (see supplement) to characterize the diameter-pressure relationships of thoracic segments isolated from OPN+/+;LDLR−/− and OPN−/−;LDLR−/− mice. Following 4 months on HFD, the change in vessel diameter per mm Hg hydrostatic pressure load was significantly reduced in OPN−/−;LDLR−/− mice vs. OPN+/+;LDLR−/− cohorts (Figure 4B), confirming reduced aortic compliance. Similar reductions in pressure-diameter relationships were noted at baseline in OPN−/−;LDLR−/− mice even in the absence of HFD challenge (data not shown). Reduced arterial compliance is predicted to impair conduit vessel Windkessel functions and distal tissue perfusion39. Indeed, when assessed by fluorescent microsphere analysis40, blood flow to the femur was reduced by ~65% in OPN−/−;LDLR−/− mice as compared to OPN+/+;LDLR−/− cohorts (Figure 4C). Thus, OPN deficiency reduces aortic compliance and femur blood flow in LDLR−/− mice.
Figure 4. Aortic compliance is reduced in diabetic OPN−/−;LDLR−/− mice.
Panel A, aortic arch pulse wave velocity (PWV, m/s) was increased in OPN−/−:LDLR−/− mice vs. OPN+/+;LDLR−/− controls. Panel B, ex vivo aortic video plethysmography also demonstrated reduced aortic compliance. Note reductions in aortic diameter (D; microns) as a function of intravascular pressure (P; mm Hg). Panel C, femur blood flow as assessed by fluorescent microsphere analysis40 is significantly reduced in arteriosclerotic OPN−/−;LDLR−/− mice.
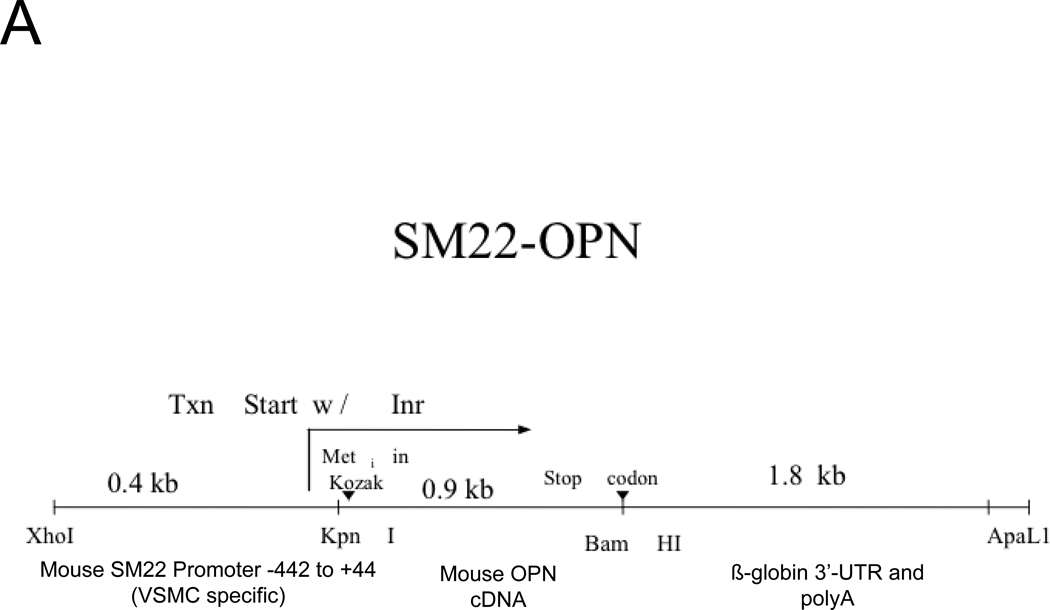
A SM22 promoter-driven OPN transgene diminishes medial chondroid metaplasia but does not does restore vascular compliance in arteriosclerotic OPN−/−;LDLR−/− mice
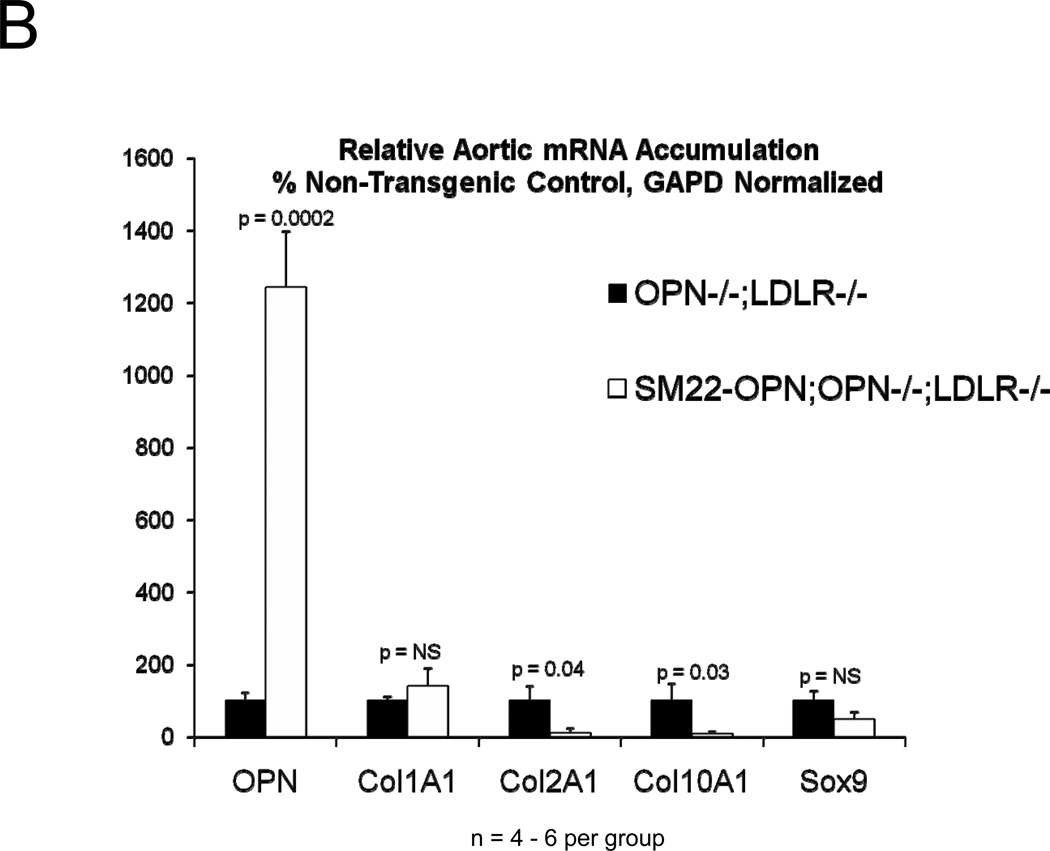
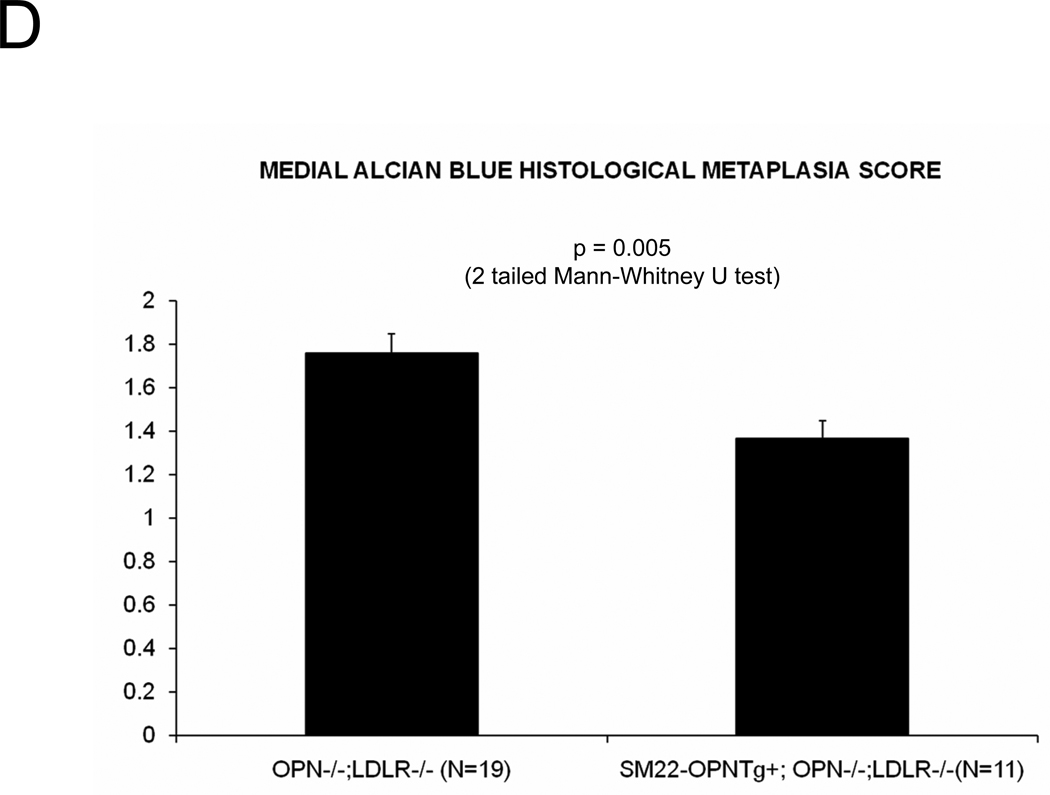
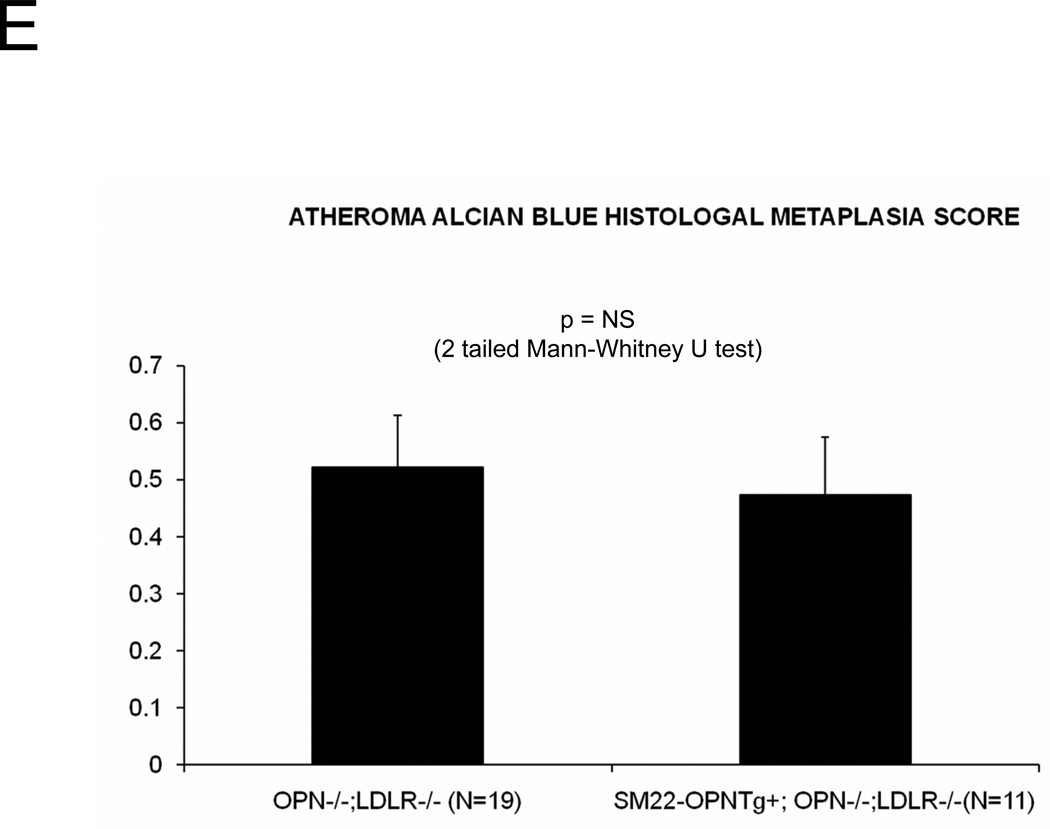
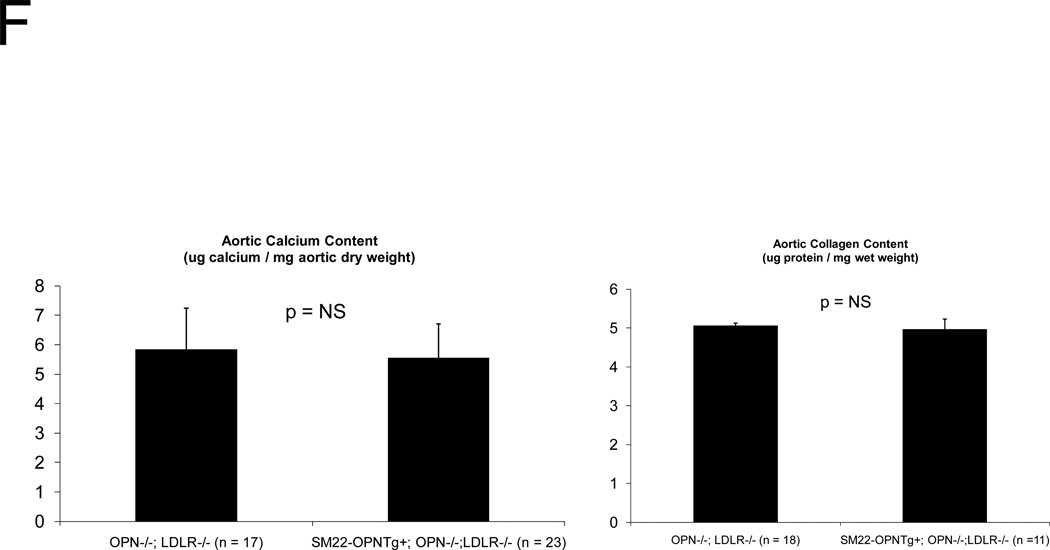
In diabetic vessels, both VSMCs and macrophages express OPN41, and OPN may exert different actions in these two cell types. We wished to better understand the cell-type specific mechanisms whereby VSMC OPN regulates the latter phases of arteriosclerotic disease. Thus, we generated SM22-OPN transgenic mice expressing the OPN protein from the VSMC-specific 0.5 kb SM22 promoter (Figure 5A), and examined the impact upon osteochondrocytic gene expression, chondroid metaplasia, and collagen accumulation in OPN−/−;LDLR−/− animal. As compared to OPN−/−;LDLR−/− siblings, SM22-OPN;OPN−/−;LDLR−/− mice exhibited significant aortic OPN message (Figure 5B). Col1A1 mRNA was unaffected, but aortic expression of Col2A1 and Col10A1 were significantly suppressed (Figure 5B). Histological scoring of Alcian blue stained sections (Figure 5C) confirmed that the medial chondroid metaplasia of OPN deficiency was partially reversed by the SM22-OPN transgene (Figure 5D). However, no significant change was observed in Alcian staining of adjacent atheroma (Figure 5E). Furthermore, no significant alteration in total aortic collagen content or calcium content was observed in response to the SM22-OPN transgene (Figure 5F), and aortic compliance quantified by ex vivo plethysmography was not improved (2.0 +/− 0.3 vs. 1.3 +/− 0.5 microns/mmHg load, p= 0.24, OPN−/−;LDLR−/− vs. SM22-OPN;OPN−/−;LDLR−/−). Thus, while partially mitigating medial chondroid metaplasia, the SM22-OPN transgene cannot reverse arteriosclerotic calcification and fibrosis in OPN−/−;LDLR−/− mice.
Figure 5. A SM22-OPN transgene down-regulates aortic chondrocyte collagens and chondroid metaplasia without inhibiting fibrosis and calcification diabetic OPN−/−;LDLR−/− mice.
Panel A, schematic of the SM22-OPN transgene. Panel B, relative aortic gene expression SM22-OPN;OPN−/−;LDLR−/− vs. OPN−/−;LDLR−/− siblings. Col2A1 and Col10A1 are significantly reduced by the transgene. Panel C, medial Alcian blue staining of OPN+/+;LDLR−/−, OPN−/−;LDLR−/− and SM22-OPN;OPN−/−:LDLR−/− mice. Panel D, histological scoring confirmed significant reduction of medial chondroid metaplasia24 in SM22-OPN;OPN−/−;LDLR−/− mice vs. OPN−/−;LDLR−/− siblings. Panel E, no significant change was observed in Alcian blue scoring of atheroma. Panel F, total aortic collagen protein and calcium accumulation were unaltered by the transgene.
OPN SVVYGLR signaling promotes early phase osteogenic mineralization in OPN−/−;LDLR−/− mice on HFD
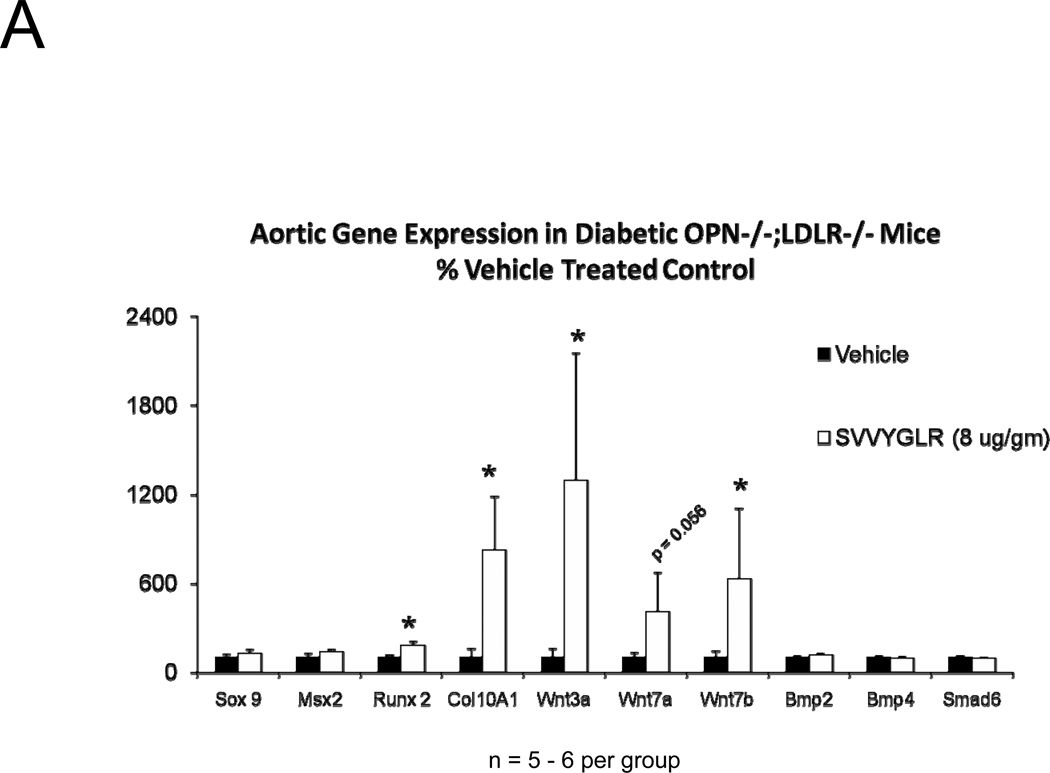
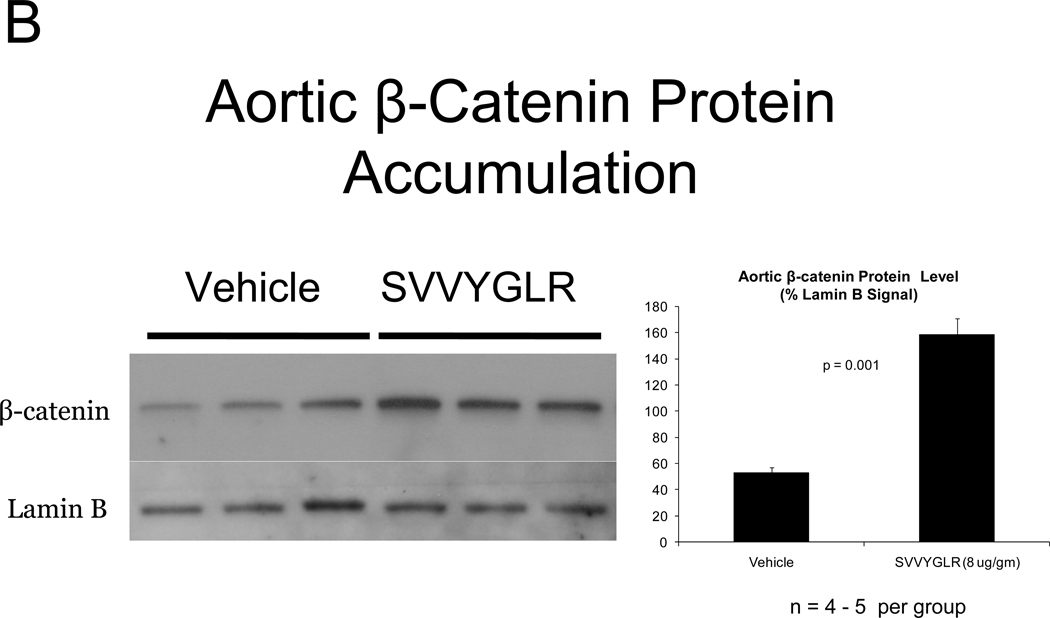
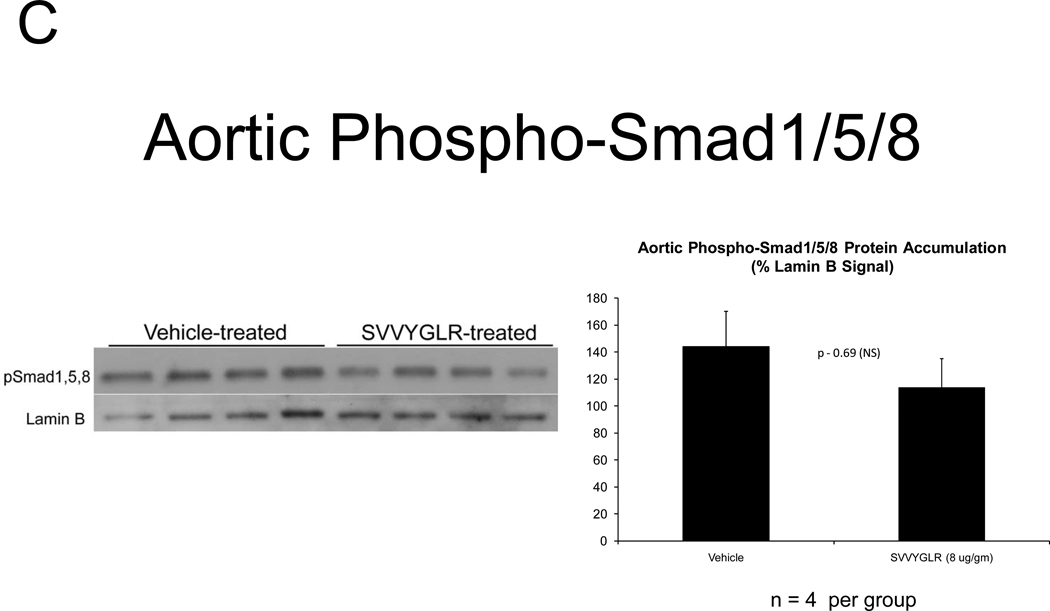
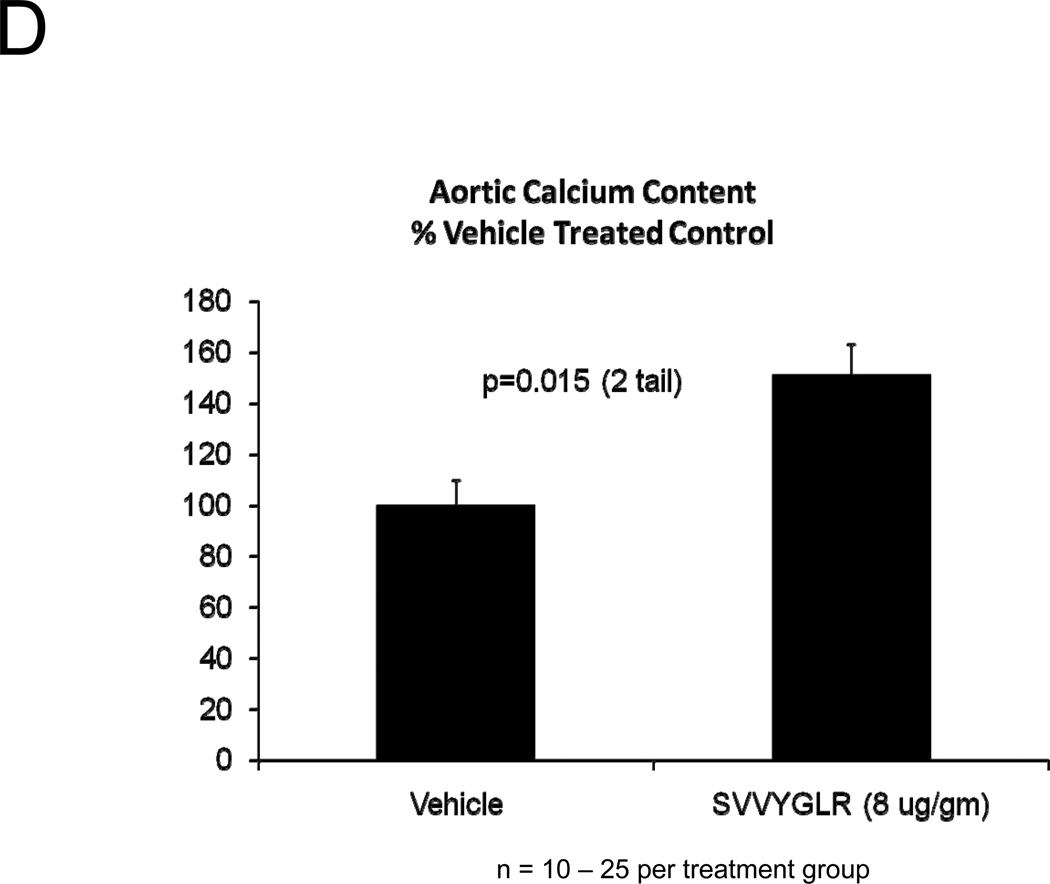
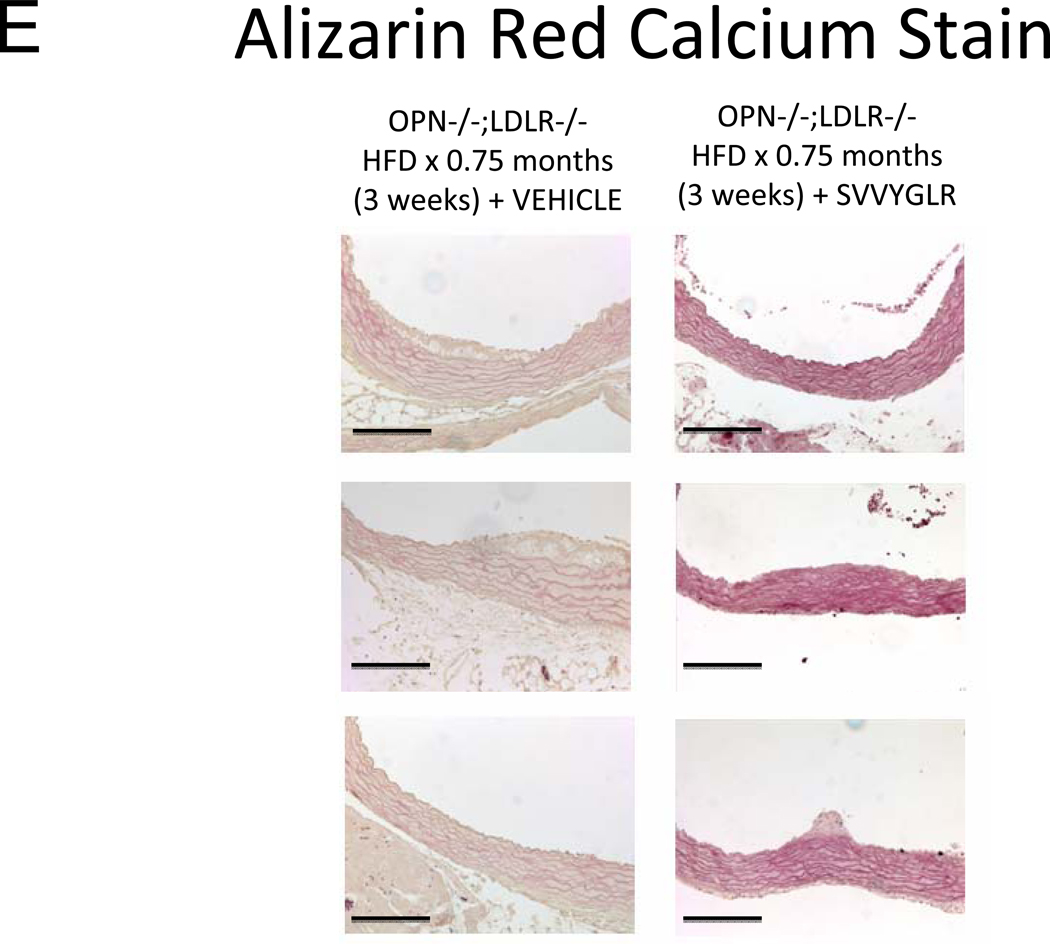
OPN deficiency reduces aortic oxidative stress (ROS) and MMP activation20 – two key contributors to aortic calcification responses42, 43 -- in newly diabetic LDLR−/− mice20. Dosing with the OPN N-half peptide metabolite SVVYGLR restores early aortic ROS production and MMP activation in OPN−/−;LDLR−/− animals20. HFD feeding increases urinary N-terminal OPN fragments with the murine epitope in LDLR−/− mice (Figure S7). This OPN metabolite is significantly enriched at sites of aortic calcification in humans21. Therefore, we hypothesized that this OPN-dependent pro-inflammatory signal, lacking in OPN-null mice, supports early osteogenic vascular mineralization at the initiation of diabetic arteriosclerosis. To test this notion, we quantified the impact of SVVYGLR OPN peptide dosing on aortic osteogenic gene expression and calcification in OPN−/−;LDLR−/− mice fed HFD. Eight days of daily SVVYGLR administration to OPN−/−;LDLR−/− mice upregulated aortic expression of COL10A1, Wnt3a, and Wnt7 (Figure 6A), and increased aortic β-catenin protein accumulation 3-fold (Figure 6B) – hallmarks of osteochondrocytic differentiation and Wnt/β-catenin signaling44. A smaller but significant 1.8-fold increase in Runx2 was also noted. A shorter, independent experiment indicated that SVVYGLR (thrice daily) increased total aortic β-catenin within 3 days, with concomitant increases in β-catenin Ser-675 phosphorylation that further confirms activation (see supplement; Figure S8A–B). The expression of aortic BMP2 and BMP4 -- and BMP targets Msx2, Sox9 and Smad6 -- were unaltered by SVVYGLR (Figure 6A). Moreover, in OPN−/−;LDLR−/− mice on HFD, levels of aortic phospho-Smad1/5/845 were not regulated by SVVYGLR dosing (Figure 6C). Finally, the early stage of aortic calcium accumulation deficient in OPN−/−;LDLR−/− mice (Figure 1A and 1B, upper panel) was substantially restored by SVVYGLR administration (Figure 6D). Alizarin red staining reflected this and localized SVVYGLR-induced aortic calcification to the tunica media (Figure 6E). Thus, the OPN metabolite SVVYGLR preferentially augments aortic Wnt/β-catenin signaling and restores early-stage medial artery calcium deposition in OPN−/−;LDLR−/− mice.
Figure 6. The OPN peptide SVVYGLR increases aortic calcification and osteochondrocytic gene expression in diabetic OPN−/−;LDLR−/− mice.
Panel A, dosing with SVVYGLR upregulates aortic expression of Runx2, Col10A1, and key osteogenic Wnt ligands in diabetic OPN−/−;LDLR−/− mice. Panel B, aortic β-catenin, a marker of canonical Wnt signaling, is increased by SVVYGLR. Panel C, aortic phospho-Smad1/5/8 was not regulated by SVVYGLR. Panel D, administration of OPN SVVYGLR to OPN−/−;LDLR−/− mice on HFD increases aortic calcification. Panel E, Alizarin red staining confirms increased medial calcification in SVVYGLR-treated mice.
MMP activity contributes to β-catenin signaling and calcium accumulation in OPN−/−;LDLR−/− mice treated with SVVYGLR
Recent studies have highlighted the crucial role for proteases in vascular mineralization43, 46. Moreover, extracellular MMP activities promote activation of β-catenin signaling by cleaving N-cadherin47. Since the OPN SVVYGLR peptide upregulates vascular MMP9 activity20, we assessed whether MMP activity contributed to SVVYGLR actions in vivo. Co-administration of doxycycline, a prokaryotic protein synthesis antagonist and inhibitor of multiple eukaryotic MMPs43, down-regulated aortic β-catenin accumulation elicited by SVVYGLR (Supplement Figure S9A–B). A smaller but statistically significant decrease in aortic calcium accumulation was also observed (Figure S9C). Thus, at early stages of HFD challenge, OPN SVVYGLR signaling supports aortic Wnt/β-catenin signaling and calcification in OPN−/−;LDLR−/− mice, dependent in part upon doxycycline-sensitive MMP activity.
DISCUSSION
OPN plays multiple modulatory roles in post-natal physiology14, 48. OPN regulates the hematopoietic niche, controls T-cell programming by dendritic cells, promotes migration and activities of the monocyte/macrophage (M/Mφ) lineage, helps eradicate certain pathogens, supports tumor metastasis, and facilitates vascular remodeling49. Skeletal calcium mobilization is also impaired by OPN deficiency50. Giachelli was the first to identify that OPN participates in vascular disease processes14. Implementing elegant murine models of genetically programmed spontaneous arterial chondroid metaplasia, they highlighted the importance of OPN as an inducible inhibitor of vascular mineralization27. Our study demonstrates that OPN in fact plays both pathogenic and protective roles in the diet-induced diabetic arteriosclerosis of LDLR−/− mice. Endogenous OPN supports calcium accrual with disease initiation via actions phenocopied by exogenous OPN SVVYGLR peptide dosing. These mechanistic insights are consistent with recent observations of Breyne et al21; they demonstrated increased N-half OPN SVVYGLR epitope levels in calcified vs. non-calcified zones of human aortic valves, arising from tissue factor-related thrombin activation21. The cryptic OPN peptide motif -- SVVYGLR in humans, SLAYGLR in mice -- is liberated by the thrombin-mediated proteolysis and promotes inflammation, angiogenesis, and calcification 14, 19, 21. Our data show that the in vivo aortic calcium accrual promoted by SVVYGLR dosing represents, in part, ectopic recapitulation of an osteogenic repair processes19. The upregulation of β-catenin and vascular calcification by SVVYGLR is antagonized by doxycycline, an inhibitor of both MMP bioactivity and mRNA accumulation51. In pre-clinical models of Marfan aortopathy, doxycycline and angiotensin receptor 1 antagonists synergize to improve aortic structure and function52. Furthermore, reductions in MMP activation by doxycycline reduce vascular inflammation53, a key contributor to vascular calcification8. Thus, a better understanding of the interactions between OPN-linked extracellular proteolytic cascades (thrombin, MMP activation), inflammation, and the intracellular transcription factors (β-catenin; Runx, Sox, Msx family members) that control osteogenic mineralization33 promises to yield novel strategies for mitigating diabetic arteriosclerosis24.
Yet, OPN clearly plays a protective role in the advanced phase of diabetic arteriosclerosis24, limiting adventitial and mural fibrosis in addition to chondroid metaplasia atherosclerotic calcium accrual as first described in non-diabetic models27. With respect to fibrosis, the effects of OPN deficiency in the remodeling conduit arteries differ unexpectedly from those observed in myocardial fibrosis54. Implementing SM22-OPN transgenic mice, we demonstrate that chondroid metaplasia of mural VSMCs is diminished by OPN in vivo (Figure 5) and is cell-autonomous in vitro (not shown). However, the advanced fibrosis and endochondral calcification observed in OPN−/−;LDLR−/− mice was not inhibited by the SM22-OPN transgene. Thus, cell types other than the VSMC primarily mediate OPN-dependent inhibition of vascular collagen and calcium55 accrual and the tempo of disease progression. The monocyte/macrophage (M/Mφ) lineage plays a critical role in the adventitial, medial, and intimal remodeling necessary for arteriogenesis56,55. Thus, it is tempting to speculate that the changes in time-course and severity of arteriosclerosis arising in OPN−/−;LDLR−/− mice relates in part to altered vascular M/Mφ activities (Figure S10)16, 23. Future studies will directly test this notion, and the impact of SLAYGLR-mutated OPN transgenes on arteriosclerotic disease kinetics.
The earliest phase of osteochondrogenic mineral deposition occurs in conjunction with acidic phospholipid vesicles and multiple annexin complexes with little inorganic phosphate57, 58. This may help explain the frequent disconnect observed between arteriosclerotic vascular Alizarin (calcium) and von Kossa (inorganic phosphate) stains first addressed by Puchtler four decades ago59. While type II collagen is unable to support such mineralization58, type I collagen58, type X collagen60, and elastin61, 62 can. Activation of MMPs and osteogenic β-catenin signals by OPN SVVYGLR rapidly promotes aortic calcium accrual -- demonstrated by biochemical assay and Alizarin histochemistry -- without initially enhancing von Kossa inorganic phosphate staining characteristic calcium phosphate deposition and ossification (Figure 6, and data not shown). This suggests that the early medial calcification processes supported by OPN during disease initiation in LDLR−/− mice on HFD differ from the vascular ossification processes that ensue following chondroid metaplasia with advanced disease24. Future studies will examine whether SVVYGLR and Wnt/β-catenin signals regulate vascular annexin-phospholipid58, 63 or other mineralizing lipoprotein complexes58. Combination of sensitive near infrared fluorescent bisphosphonate probes for calcium64 with annexin immunofluorescence and von Kossa phosphate staining may help temporally and spatially resolve the forms of vascular calcium biochemically quantified during disease initiation and progression. Once vascular calcium phosphate crystals accumulate, however, autocrine processes involving both BMP2 and OPN signaling 65 are likely to rapidly drive progressive mineralization.
There are, of course, limitations to our study. The mechanisms whereby complete OPN deficiency perturbs elastin morphology and promotes fragmentation in diabetic LDLR−/− mice have not been established – and may differ dependent upon the specific disease model27, 28. Furthermore, in the cardiomyocyte OPN excess increases myocardial fibrosis via T-cell mediated mechanisms54. However, we demonstrate OPN deficiency increases aortic adventitial and mural fibrosis in diabetic LDLR−/− mice. Given the cell-type specific contributions of OPN14, we speculate that OPN actions in M/Mφ vs. striated muscle vs. VSMC lineages will differentially impact collagen accumulation. Reductions in OPN-dependent collagenase activity likely play a role in adventitial collagen accumulation14, but the precise mechanisms whereby global OPN deficiency increases aortic fibrosis in LDLR−/− mice have yet to be delineated. P1NP levels indicate that increased collagen synthesis contributes as well22. Nevertheless, our study demonstrates the differential impact of OPN on the initiation and progression phases of arteriosclerosis with diet-induced diabetes in LDLR−/− mice. At the initiation of diabetes and vascular disease, OPN promotes calcification via actions recapitulated by the pro-inflammatory OPN SVVYGLR metabolite --- but OPN limits mural fibrosis, chondroid metaplasia, and arterial calcium accrual with disease progression. Complete OPN deficiency thus yields a net increase in arteriosclerotic disease severity in the LDLR−/− mouse. Strategies that selectively inhibit OPN pro-inflammatory SVVYGLR actions14, 17, 20 -- yet preserve OPN production in cells that limit arterial fibrosis and calcium accrual55 -- may improve arterial compliance and distal tissue perfusion in diabetic vascular disease.
Supplementary Material
Acknowledgments
We thank Dr. Susan Rittling (The Forsyth Institute, Boston, MA) and Dr. David Denhardt (Rutgers University, Piscataway, NJ) for kindly providing the N10.OPN−/− line used to generate OPN−/−;LDLR−/− mice.
Funding Sources – Supported by NIH grants HL88651 and HL69229 to D.A.T., and the Barnes-Jewish Hospital Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures – None.
REFERENCES
- 1.O'Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12:329–341. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 3.Collins TC, Ewing SK, Diem SJ, Taylor BC, Orwoll ES, Cummings SR, Strotmeyer ES, Ensrud KE. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009;119:2305–2312. doi: 10.1161/CIRCULATIONAHA.108.820993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51:1967–1974. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 6.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 7.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 8.Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension. 2010;55:579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajamannan NM. Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler Thromb Vasc Biol. 2009;29:162–168. doi: 10.1161/ATVBAHA.107.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 12.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 15.Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- 16.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharif SA, Du X, Myles T, Song JJ, Price E, Lee DM, Goodman SB, Nagashima M, Morser J, Robinson WH, Leung LL. Thrombin-activatable carboxypeptidase B cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis. Arthritis Rheum. 2009;60:2902–2912. doi: 10.1002/art.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamada Y, Yuki K, Okazaki M, Fujitani W, Matsumoto T, Hashida MK, Harutsugu K, Nokihara K, Daito M, Matsuura N, Takahashi J. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dent Mater J. 2004;23:650–655. doi: 10.4012/dmj.23.650. [DOI] [PubMed] [Google Scholar]
- 19.Egusa H, Kaneda Y, Akashi Y, Hamada Y, Matsumoto T, Saeki M, Thakor DK, Tabata Y, Matsuura N, Yatani H. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials. 2009;30:4676–4686. doi: 10.1016/j.biomaterials.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, Schumacher A, Loewy AP, Denhardt DT, Rittling SR, Towler DA. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98:1479–1489. doi: 10.1161/01.RES.0000227550.00426.60. [DOI] [PubMed] [Google Scholar]
- 21.Breyne J, Juthier F, Corseaux D, Marechaux S, Zawadzki C, Jeanpierre E, Ung A, Ennezat PV, Susen S, Van Belle E, Le Marec H, Vincentelli A, Le Tourneau T, Jude B. Atherosclerotic-like process in aortic stenosis: activation of the tissue factor-thrombin pathway and potential role through osteopontin alteration. Atherosclerosis. 2010;213:369–376. doi: 10.1016/j.atherosclerosis.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 22.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duvall CL, Weiss D, Robinson ST, Alameddine FM, Guldberg RE, Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arterioscler Thromb Vasc Biol. 2008;28:290–295. doi: 10.1161/ATVBAHA.107.158485. [DOI] [PubMed] [Google Scholar]
- 24.Qiao JH, Mertens RB, Fishbein MC, Geller SA. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum Pathol. 2003;34:402–407. doi: 10.1053/hupa.2003.72. [DOI] [PubMed] [Google Scholar]
- 25.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res. 2010;107:271–282. doi: 10.1161/CIRCRESAHA.110.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Morimoto J, Kimura C, Kon S, Denhardt D, Kitabatake A, Uede T. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1029–1034. doi: 10.1161/01.ATV.0000074878.29805.D0. [DOI] [PubMed] [Google Scholar]
- 27.Speer MY, McKee MD, Guldberg RE, Liaw L, Yang HY, Tung E, Karsenty G, Giachelli CM. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–1055. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002;282:E207–E214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 29.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 30.Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 31.Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 2010;17:1345–1353. doi: 10.1038/cdd.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amizuka N, Davidson D, Liu H, Valverde-Franco G, Chai S, Maeda T, Ozawa H, Hammond V, Ornitz DM, Goltzman D, Henderson JE. Signalling by fibroblast growth factor receptor 3 and parathyroid hormone-related peptide coordinate cartilage and bone development. Bone. 2004;34:13–25. doi: 10.1016/j.bone.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MM., Jr The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–2706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 34.Contri MB, Boraldi F, Taparelli F, De Paepe A, Ronchetti IP. Matrix proteins with high affinity for calcium ions are associated with mineralization within the elastic fibers of pseudoxanthoma elasticum dermis. Am J Pathol. 1996;148:569–577. [PMC free article] [PubMed] [Google Scholar]
- 35.Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther. 2008;12:157–170. doi: 10.1007/BF03256280. [DOI] [PubMed] [Google Scholar]
- 36.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1209–H1217. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- 37.Patten RD, Aronovitz MJ, Bridgman P, Pandian NG. Use of pulse wave and color flow Doppler echocardiography in mouse models of human disease. J Am Soc Echocardiogr. 2002;15:708–714. doi: 10.1067/mje.2002.118912. [DOI] [PubMed] [Google Scholar]
- 38.Wang YX, Halks-Miller M, Vergona R, Sullivan ME, Fitch R, Mallari C, Martin-McNulty B, da Cunha V, Freay A, Rubanyi GM, Kauser K. Increased aortic stiffness assessed by pulse wave velocity in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2000;278:H428–H434. doi: 10.1152/ajpheart.2000.278.2.H428. [DOI] [PubMed] [Google Scholar]
- 39.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 40.Serrat MA. Measuring bone blood supply in mice using fluorescent microspheres. Nat Protoc. 2009;4:1779–1758. doi: 10.1038/nprot.2009.190. [DOI] [PubMed] [Google Scholar]
- 41.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 42.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–1516. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 44.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res. 2009;81:178–186. doi: 10.1093/cvr/cvn278. [DOI] [PubMed] [Google Scholar]
- 48.Rittling SR, Denhardt DT. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp Nephrol. 1999;7:103–113. doi: 10.1159/000020591. [DOI] [PubMed] [Google Scholar]
- 49.Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ihara H, Denhardt DT, Furuya K, Yamashita T, Muguruma Y, Tsuji K, Hruska KA, Higashio K, Enomoto S, Nifuji A, Rittling SR, Noda M. Parathyroid hormone-induced bone resorption does not occur in the absence of osteopontin. J Biol Chem. 2001;276:13065–13071. doi: 10.1074/jbc.M010938200. [DOI] [PubMed] [Google Scholar]
- 51.Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, Sicard GA, Thompson RW. Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg. 2000;31:325–342. doi: 10.1016/s0741-5214(00)90163-0. [DOI] [PubMed] [Google Scholar]
- 52.Yang HH, Kim JM, Chum E, van Breemen C, Chung AW. Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in Marfan syndrome. J Thorac Cardiovasc Surg. 2010;140:305–312. doi: 10.1016/j.jtcvs.2009.10.039. e302. [DOI] [PubMed] [Google Scholar]
- 53.Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation. 2009;119:2209–2216. doi: 10.1161/CIRCULATIONAHA.108.806505. [DOI] [PubMed] [Google Scholar]
- 54.Renault MA, Robbesyn F, Reant P, Douin V, Daret D, Allieres C, Belloc I, Couffinhal T, Arnal JF, Klingel K, Desgranges C, Dos Santos P, Charpentier F, Gadeau AP. Osteopontin expression in cardiomyocytes induces dilated cardiomyopathy. Circ Heart Fail. 2010;3:431–439. doi: 10.1161/CIRCHEARTFAILURE.109.898114. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Speer MY, Yang H, Bergen J, Giachelli CM. Vitamin D receptor activators induce an anticalcific paracrine program in macrophages: requirement of osteopontin. Arterioscler Thromb Vasc Biol. 2010;30:321–326. doi: 10.1161/ATVBAHA.109.196576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80:59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- 57.Genge BR, Wu LN, Wuthier RE. Mineralization of annexin-5-containing lipid-calcium-phosphate complexes: modulation by varying lipid composition and incubation with cartilage collagens. J Biol Chem. 2008;283:9737–9748. doi: 10.1074/jbc.M706523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen NX, O'Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23:1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puchtler H, Meloan SN, Terry MS. On the history and mechanism of alizarin and alizarin red S stains for calcium. J Histochem Cytochem. 1969;17:110–124. doi: 10.1177/17.2.110. [DOI] [PubMed] [Google Scholar]
- 60.Kirsch T, Nah HD, Shapiro IM, Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanimura A, McGregor DH, Anderson HC. Calcification in atherosclerosis. I. Human studies. J Exp Pathol. 1986;2:261–273. [PubMed] [Google Scholar]
- 62.Price PA, Chan WS, Jolson DM, Williamson MK. The elastic lamellae of devitalized arteries calcify when incubated in serum: evidence for a serum calcification factor. Arterioscler Thromb Vasc Biol. 2006;26:1079–1085. doi: 10.1161/01.ATV.0000216406.44762.7c. [DOI] [PubMed] [Google Scholar]
- 63.Genge BR, Wu LN, Wuthier RE. In vitro modeling of matrix vesicle nucleation: synergistic stimulation of mineral formation by annexin A5 and phosphatidylserine. J Biol Chem. 2007;282:26035–26045. doi: 10.1074/jbc.M701057200. [DOI] [PubMed] [Google Scholar]
- 64.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 65.Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79:414–422. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang MS, Lu J, Ivanov Y, Sage AP, Tseng W, Demer LL, Tintut Y. Hyperlipidemia impairs osteoanabolic effects of PTH. J Bone Miner Res. 2008;23:1672–1679. doi: 10.1359/JBMR.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.