Abstract
Objective
The role of plasminogen activator inhibitor-1 (PAI-1) in vein graft (VG) remodeling is undefined. We examined the effect of PAI-1 on VG intimal hyperplasia (IH) and tested the hypothesis that PAI-1 regulates VG thrombin activity.
Methods and Results
VGs from wild-type (WT), Pai1−/−, and PAI-1-transgenic (Tg) mice were implanted into WT, Pai1−/−, or PAI-1-Tg arteries. VG remodeling was assessed 4 wks later. IH was significantly greater in PAI-1-deficient mice than in WT mice. The proliferative effect of PAI-1 deficiency was retained in vitronectin (VN)-deficient mice, suggesting that PAI-1’s anti-proteolytic function plays a key role in regulating IH. Thrombin-induced proliferation of PAI-1-deficient venous smooth muscle cells (SMC) was significantly greater than that of WT SMC, and thrombin activity was significantly higher in PAI-1-deficient VGs than in WT VGs. Increased PAI-1 expression, which has been associated with obstructive VG disease, did not increase IH.
Conclusion
Decreased PAI-1 expression 1) promotes IH by pathways that do not require VN, and 2) increases thrombin activity in VG. PAI-1 over-expression, while promoting SMC migration in vitro, did not increase IH. These results challenge the concept that PAI-1 drives non-thrombotic obstructive disease in VG and suggest that PAI-1’s anti-proteolytic function, including its anti-thrombin activity, inhibits IH.
Keywords: vein graft disease, plasminogen activator inhibitor-1, thrombin, vascular smooth muscle cell
Internal thoracic arteries and saphenous veins are used to perform coronary artery bypass grafting (CABG) in patients with advanced coronary artery disease. However, the development of obstructive disease is significantly more common in venous than arterial grafts, with approximately 40% of vein grafts occluding within 10 years after CABG.1 The initial pathophysiological process in adverse vein graft (VG) remodeling is intimal hyperplasia. While some degree of intimal hyperplasia in VGs is an adaptive response to arterial blood pressure and flow, excessive intimal hyperplasia is common and constitutes the substrate for the development of VG atherosclerosis. The molecular and cellular processes that regulate intimal hyperplasia within VGs are poorly understood and likely exhibit significant differences from those that regulate intimal hyperplasia in native arteries. Hence, additional studies are needed to define the factors that regulate intimal hyperplasia in VGs.
Plasminogen activator inhibitor-1 (PAI-1) is the main physiological inhibitor of tissue-type plasminogen activator (t-PA) and urinary-type PA (u-PA).2 PAI-1 is present in plasma, platelets, endothelial cells, vascular smooth muscle cells (SMC), and extracellular matrix (ECM). PAI-1 binds and is stabilized by its cofactor, vitronectin (VN), which is present in plasma and ECM.3 In addition to regulating fibrinolysis, PAI-1 stimulates migration of vascular smooth muscle cells (SMC) by binding to low density lipoprotein receptor related protein (LRP) present on SMC.4 However, PAI-1 can also inhibit SMC migration by binding to VN in the ECM, thereby blocking VN binding to integrin and non-integrin receptors present on SMC.5 In addition, PAI-1 inhibits thrombin.6 Given that thrombin stimulates SMC proliferation and is hypothesized to stimulate intimal hyperplasia independently of its prothrombotic effects,7–9 it is possible that PAI-1 could regulate intimal hyperplasia by inhibiting thrombin. However, little is known about the roles of PAI-1 in regulating VG intimal hyperplasia and thrombin activity in vivo. Elevated plasma PAI-1 concentration is associated with VG occlusion in humans,10 and PAI-1 expression is up-regulated in obstructed human VGs.11 However, it is unknown whether PAI-1 actively regulates VG intimal hyperplasia, or is simply a biomarker associated with VG disease. Given the potential for PAI-1 to produce both stimulatory and inhibitory effects on cell migration in vitro, it is important to determine the net effects of enhanced and reduced PAI-1 expression on VG intimal hyperplasia in vivo. Consequently, the main objective of this study was to determine the impact of primary alterations in PAI-1 expression, both localized and systemic, on the development of VG intimal hyperplasia. A secondary objective was to examine the potential role of PAI-1 as a thrombin inhibitor in vivo in the VG wall. To accomplish these objectives we studied wild-type, PAI-1-deficient (Pai1−/−), and PAI-1-over-expressing mice in a model of vein bypass grafting.
Materials and Methods
A detailed methods section describing mouse strains, morphometric and immunohistochemical assessments of VGs, measurement of plasma PAI-1, reverse-transcriptase polymerase chain reaction (RT-PCR) analysis of PAI-1 gene expression, isolation and functional assessment of venous SMC, and statistical methods is provided in a supplemental data file, available online at http://atvb.ahajournals.org.
Vein grafting surgery
Surgery was performed as described.12 In brief, the right common carotid artery of a male mouse was ligated proximally and distally and transected at its mid-portion. The transected ends were each passed through polyethylene cuffs, everted back over them, and secured with ligatures. A segment (1 cm) of inferior vena cava (IVC) was excised from a different (donor) male mouse. The cuffed ends of the transected artery were inserted into each end of the VG and secured with sutures. Blood flow was restored by removing the ligatures, after which the surgical wound was sutured closed. All surgical procedures and histological analyses were performed with the investigator blinded to mouse genotype.
Measurement of thrombin activity in VGs
Five days after vein graft insertion mice were anesthetized. After exposing the heart and lacerating the liver, ice-cold 0.9% NaCl (10 mL) was injected into the left ventricle to flush blood from the vasculature. The VG was exposed, excised, and incised longitudinally. Two VGs retrieved from mice with identical donor/graft genotypes were placed in 0.9% NaCl at 4°C and pulverized with a Pellet Pestle (Kimble Kontes). The homogenate was centrifuged at 9000×g for 15 minutes. The supernatant (extract) was collected and its total protein concentration was determined with the BCA reagent. Each extract derived from two VGs was counted as a single sample for statistical analyses. To measure thrombin activity, aliquots of each extract (20 µg) were diluted in assay buffer (50 mM Tris·HCl, pH 8.3, containing 0.2% BSA, 0.13 mM NaCl, 75 KIU/l aprotinin) that lacked or contained hirudin (1.3 mg/mL). After 1 min, chromogenic thrombin substrate H-D-Phe-Pip-Arg-paranitroanilide (200 µM; S-2238; Chromogenix) was added and reactions were incubated at 37°C for 2 hrs. Absorbance of reaction mixtures at 405 nm (A405) was measured in a microplate spectrophotometer. The difference in A405 of identical samples analyzed in the presence and absence of hirudin was defined as the thrombin activity of the sample.
Results
PAI-1 and VN deficiency exert distinct effects on VG remodeling
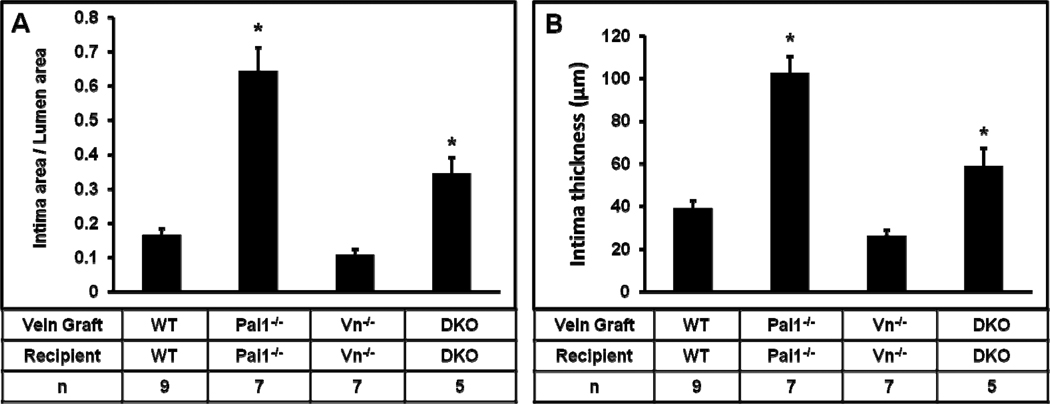
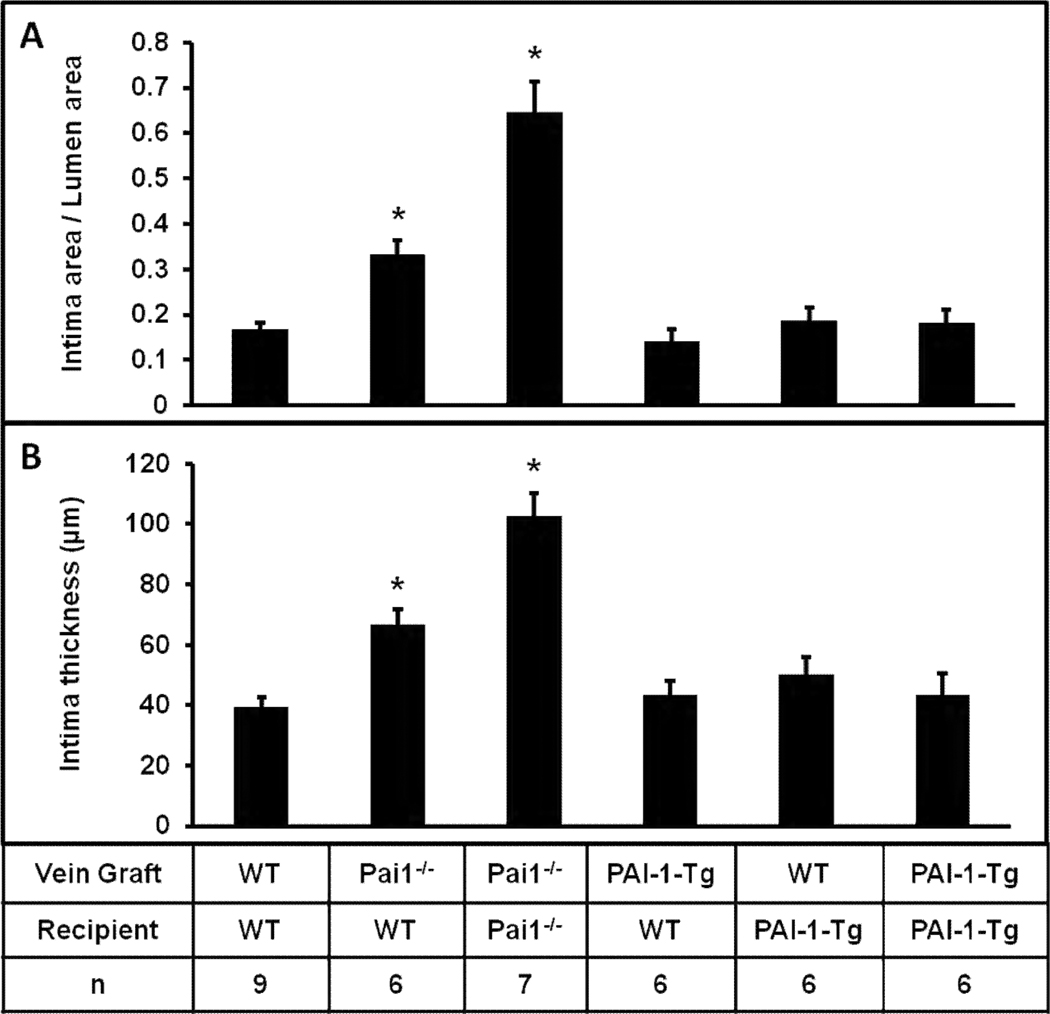
To examine the role of PAI-1 in VG remodeling, we compared VG intimal hyperplasia in WT and PAI-1-deficient mice. For these experiments, the genotype of the recipient mouse was the same as that of the VG donor. At 4 weeks after VG implantation, mean intimal thickness and intimal area indexed to lumen area were significantly greater in Pai1−/− mice than in WT mice (Fig. 1), suggesting that PAI-1 deficiency promotes VG intimal hyperplasia. Enhanced VG neointima formation in PAI-1-deficient mice could result not only from loss of direct effects of PAI-1 on VG remodeling, but also on changes in VN function induced by PAI-1 deficiency, given that PAI-1 and VN regulate each other’s functions. To address this issue, we studied VG neointima formation in Vn−/− mice and mice with combined PAI-1 and VN deficiency (DKO mice), with the genotypes of all VGs being identical to those of recipient mice. DKO mice exhibited significantly greater intimal hyperplasia than Vn−/− mice (Fig. 1). These results, in conjunction with the data derived from WT and Pai1−/− mice, suggested that PAI-1 deficiency promotes intimal hyperplasia whether or not VN was present—i.e. that the effect of PAI-1 deficiency on intimal hyperplasia is not solely mediated indirectly via VN. They also suggested that net effects of deficiency of PAI-1 or VN on VG intimal hyperplasia differ significantly, and supported the hypothesis that PAI-1 deficiency promotes the capacity of VN to support intimal hyperplasia, as the proliferative effect of PAI-1 deficiency was significantly less in mice also lacking VN.
Figure 1.
Complete deficiency of PAI-1 (in both vein graft and recipient mouse) promotes intimal hyperplasia, assessed by measuring intima area/lumen area ratio (A) and intima thickness (B) 4 weeks after surgery. Effect of PAI-1 deficiency was observed both in mice with intact vitronectin (VN) expression (first two bars of each graph) and mice with complete VN deficiency (latter two bars of each graph). DKO, double knock-out (i.e. mice with complete deficiency of PAI-1 and VN). Differences of mean values between all groups were statistically significant (P<0.001). For pair-wise comparisons “*” denotes P≤0.05 vs. all other groups.
Local and systemic expression of PAI-1 regulate its concentration in VGs
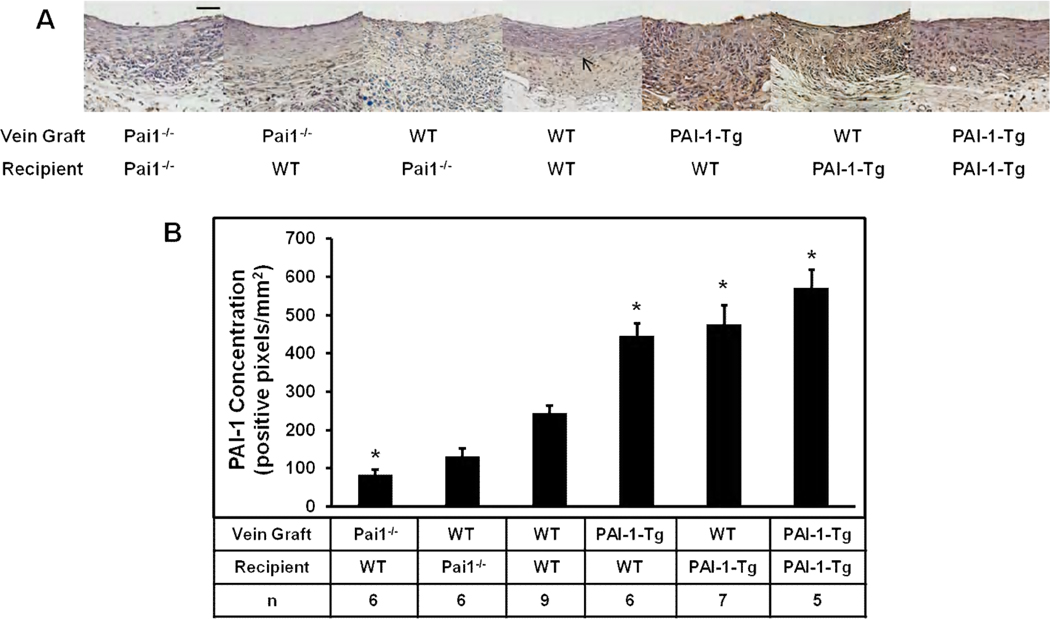
PAI-1 is present in the VG wall,11 though the origin of this pool is poorly defined. To examine this issue we conducted experiments involving various combinations of VG donors and recipients. VG PAI-1 content was assessed by quantitative immunohistochemistry 4 weeks after surgery. Undetectable or extremely low levels of immunostaining, consistent with background signal, were detected in VGs of Pai1−/−graft/Pai1−/−recipient mice (n=7, Fig. 2A), and plasma PAI-1 antigen and activity were undetectable in this group (Table). PAI-1 immunostaining was significantly less in VGs of Pai1−/−graft/WTrecipient mice (n=6) than in those of WTgraft/WTrecipient mice (Fig. 2B), while plasma PAI-1 antigen and activity levels did not differ significantly between these groups. PAI-1 immunostaining was significantly higher in VGs of PAI-1-Tggraft/WTrecipient mice (n=6) than in VGs of WTgraft/WTrecipient controls (n=9), while plasma PAI-1 concentration did not differ significantly between these groups. Together, these results suggested that localized vascular expression is an important source of PAI-1 within VG neointima, as PAI-1-Tg and PAI-1-deficient VGs implanted in WT mice had significantly higher and lower concentrations of intimal PAI-1, respectively, compared to WT VGs, despite identical recipient genotypes and a lack of significant difference in plasma PAI-1 concentration between groups. PAI-1 concentration in VGs of WTgraft/Pai1−/−recipient mice were similar to those of WTgraft/WTrecipient mice (Fig. 2B), further supporting the important role of local synthesis in determining VG PAI-1 concentration. Control experiments revealed significantly higher PAI-1 concentration in PAI-1-Tg IVC than WT IVC, and real-time RT-PCR analysis revealed an approximately 4-fold increase of PAI-1 gene expression in PAI-1-Tg IVC compared to WT IVC (Supplemental Fig. II). These results confirmed that PAI-1 expression was increased in PAI-1-Tg veins at the time of initial graft implantation. To assess the impact of increased systemic PAI-1 expression on VG PAI-1 concentration, we compared WTgraft/PAI-1-Tgrecipient mice (n=7) to WTgraft/WTrecipient controls. The former group exhibited significantly higher VG PAI-1 concentration than the latter (Fig. 2B). Plasma PAI-1 antigen and activity concentrations were significantly higher in WTgraft/PAI-1-Tgrecipient mice than in WTgraft/WTrecipient mice (Table). PAI-1-Tggraft/PAI-1-Tgrecipient mice exhibited the highest concentration of PAI-1 in VG, though results did not differ significantly from those of WTgraft/PAI-1-Tgrecipient mice. Plasma PAI-1 levels did not differ significantly between PAI-1-Tggraft/PAI-1-Tgrecipient and WTgraft/PAI-1-Tgrecipient mice. As a whole, these results suggested that local and systemic PAI-1 expression are both important determinants of VG PAI-1 concentration, while plasma PAI-1 concentration, as expected, is not significantly influenced by VG PAI-1 expression.
Figure 2.
Immunohistochemical analysis of PAI-1 expression in vein grafts. (A). Representative images from each experimental group. Distance bar (50 µm). Arrow indicates boundary between intima and media+adventitia. (B) Quantitative analysis of all mice in each group. Differences of mean values between groups were statistically significant (P<0.001). Pairwise comparisons were as follows: *P<0.05 vs. WTgraft/WTrecipient mice. Differences between PAI-1-Tggraft/WTrecipient, WTgraft/PAI-1-Tgrecipient, and PAI-1-Tggraft/PAI-1-Tgrecipient mice did not achieve statistical significance.
Table.
Plasma PAI-1 concentrations
| Group | Plasma PAI-1 Antigen, pg/mL |
Plasma PAI-1 Activity, pg/mL |
|---|---|---|
| WTgraft/WTrecipient (n=6) | 59±7 | Undetectable |
| Pai1−/−graft/WTrecipient (n=6) | 55±5 | Undetectable |
| Pai1−/−graft/Pai1−/−recipient (n=4) | Undetectable | Undetectable |
| PAI-1-Tggraft/WTrecipient (n=6) | 69.5±9 | Undetectable |
| WTgraft/PAI-1-Tgrecipient (n=6) | 630±50* | 245±39* |
| PAI-1-Tggraft/PAI-1-Tgrecipient (n=6) | 542±41† | 228±46† |
P<0.005 vs. other groups, except PAI-1-Tggraft/PAI-1-Tgrecipient mice
P<0.005 vs. other groups, except WTgraft/PAI-1-Tgrecipient mice
Localized and systemic PAI-1 deficiency increase VG intimal hyperplasia
We examined the effects of localized and systemic decreases in PAI-1 expression on venous remodeling by examining VGs at 4 weeks after surgery. Mean intimal thickness and intimal area indexed to lumen area were significantly greater in Pai1−/−graft/WTrecipient mice than in WTgraft/WTrecipient controls (Fig. 3), suggesting that a decrease in PAI-1 expression localized to the VG increases intimal hyperplasia. Mean intimal thickness and indexed intimal area were significantly greater in Pai1−/−graft/Pai1−/−recipient mice than in Pai1−/−graft/WTrecipient mice, suggesting that systemic PAI-1 deficiency promotes VG intimal hyperplasia beyond that observed with localized VG PAI-1 deficiency.
Figure 3.
PAI-1 deficiency promotes intimal hyperplasia. The mice constituting the WTgraft/WTrecipient and Pai1−/−graft/Pai1−/−recipient groups are the same as those shown in Fig. 1. Differences of mean values between groups were statistically significant (P<0.001). *P<0.05 vs. all other groups (pairwise comparisons).
Localized and systemic PAI-1 over-expression do not increase VG intimal hyperplasia
We compared intimal hyperplasia in PAI-1-Tggraft/WTrecipient mice and WTgraft/WTrecipient mice to determine the effects of a primary increase in VG PAI-1 expression on early venous remodeling. We also compared WTgraft/PAI-1-Tgrecipient mice and WTgraft/WTrecipient mice to assess the impact of increased systemic PAI-1 expression on VG intimal hyperplasia. However, neither local nor systemic PAI-1 over-expression had any significant effect on mean intimal thickness or mean intimal area indexed to lumen area (Fig. 3), suggesting that PAI-1 over-expression does not promote VG intimal hyperplasia. Consistent with these data, intimal hyperplasia in mice with combined over-expression of PAI-1 both locally and systemically (i.e. PAI-1-Tggraft/PAI-1-Tgrecipient mice) did not differ significantly from that of WTgraft/WTrecipient mice (Fig. 3). We did not analyze intimal hyperplasia of WT or PAI-1-Tg VGs transplanted into PAI-1-deficient mice because of the anticipated immune reaction to VG PAI-1 in PAI-1-deficient mice.
PAI-1 regulates cellular composition of VG neointima
We analyzed the composition of VG neointima. Quantitative smooth muscle alpha-actin (SMAA) immunostaining revealed a significant increase in the % SMAA-positive area in Pai1−/−graft/Pai1−/−recipient VGs compared to WTgraft/WTrecipient VGs and all other experimental groups (Supplemental Fig. III), suggesting that PAI-1 deficiency increased not only neointima area, but also the density of SMC in neointima, which is consistent with a report that PAI-1 increases ECM accumulation within developing neointima.13 Differences between other groups and WTgraft/WTrecipient mice did not achieve statistical significance. There was no significant difference in neointima macrophage concentration between experimental groups (data not shown).
PAI-1 regulates venous SMC proliferation and migration
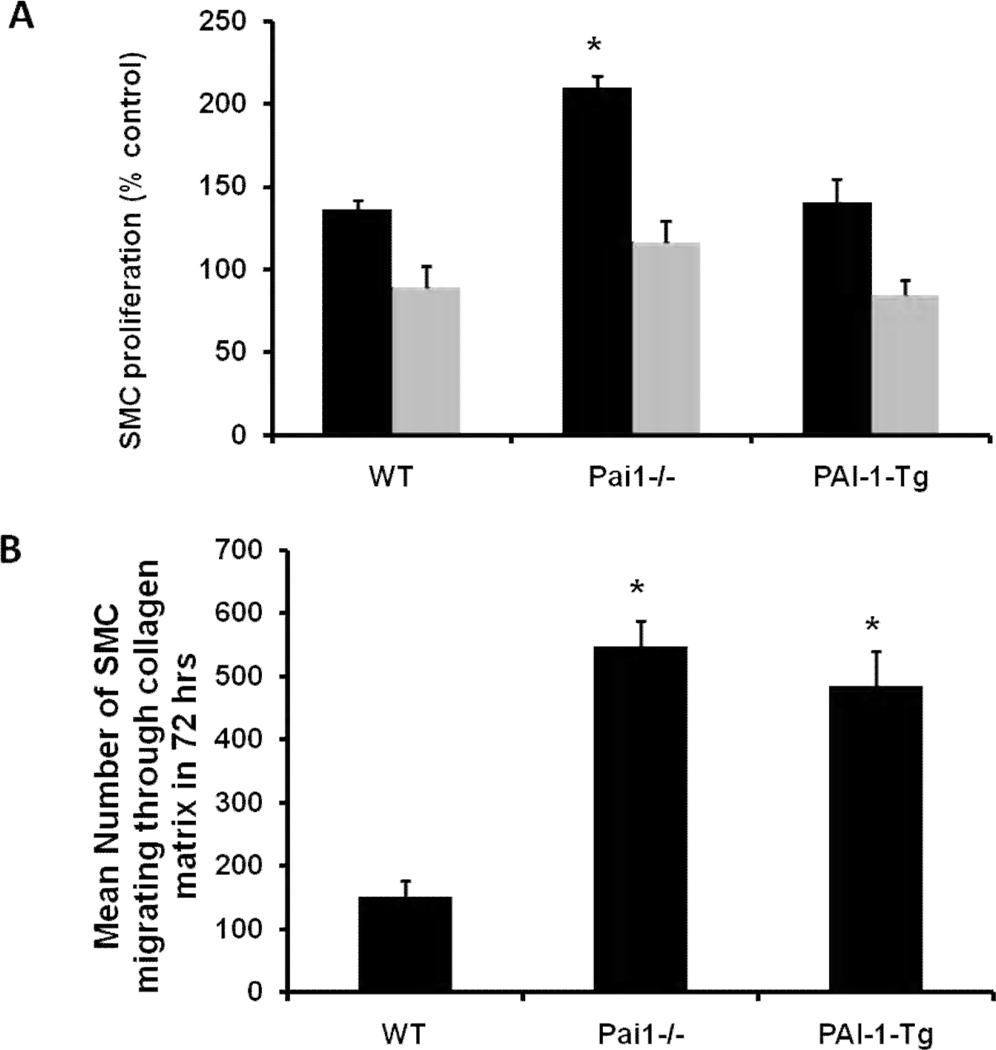
SMC express PAI-1 and play a central role in intimal hyperplasia.14 To identify potential mechanisms underlying our in vivo findings, we isolated WT, PAI-1-deficient, and PAI-1-Tg SMC from mouse vena cava, established cultured lines, and compared their proliferation and migration in vitro. To study proliferation, we cultured SMC with thrombin (1 U/mL) and measured cell proliferation by the BrdU incorporation method. Thrombin-induced proliferation of PAI-1-deficient SMC was significantly greater than that of WT SMC, while no significant differences were observed between these groups in the presence of hirudin, a specific thrombin inhibitor (Fig. 4A). These results suggested that basal rates of venous SMC proliferation are not affected by PAI-1 deficiency, but that deficient expression of PAI-1 by venous SMC enhances thrombin-induced proliferation. However, we found no significant difference between WT and PAI-1-Tg venous SMC in thrombin-induced proliferation (Fig. 4A), including in experiments (not shown) involving thrombin concentrations as low as 0.125 U/mL. Consistent with these in vitro data, we found that the % of actively proliferating (i.e. PCNA-positive) cells in VG neointima was significantly higher in Pai1−/−graft/Pai1−/−recipient mice than in WTgraft/WTrecipient mice, but did not differ significantly between PAI-1-Tggraft/PAI-1-Tgrecipient and WTgraft/WTrecipient mice (Supplemental Fig. IV). To study the effect of SMC PAI-1 expression on migration, we added SMC to the surface of 3-dimensional collagen gels and triggered migration of cells through gels with PDGF-BB. PAI-1-Tg and PAI-1-deficient venous SMC both migrated significantly faster than WT SMC (Fig. 4B), results consistent with a previous study of ours involving arterial SMC.15
Figure 4.
Effect of genotype on proliferation and migration of venous SMC in vitro. Data are mean of at least 3 independent experiments. (A) Thrombin-induced SMC proliferation. Black and gray bars represent cell proliferation in absence and presence, respectively, of hirudin. *P<0.05 vs. wild-type (WT) and PAI-1-transgenic (Tg) groups. (B) Migration of SMC through 3-dimensional collagen matrices. *P<0.05 vs. WT.
PAI-1 regulates fibrin/fibrinogen deposition in VG
PAI-1 regulates fibrinolysis, and deposition of fibrin within the vascular wall has been hypothesized to regulate intimal hyperplasia.16 We used quantitative immunohistochemistry to assess fibrin/fibrinogen concentration in VGs. There was no significant difference in fibrin/fibrinogen concentration in the VG wall of PAI-1-Tggraft/WTrecipient, WTgraft/PAI-1-Tgrecipient, and WTgraft/WTrecipient mice (Supplemental Fig. V). However, fibrin/fibrinogen concentration was significantly elevated in PAI-1-Tggraft/PAI-1-Tgrecipient mice compared to WTgraft/WTrecipient mice (P<0.05). Fibrin/fibrinogen concentration in VGs of Pai1−/−graft/WTrecipient, Pai1−/−graft/Pai1−/−recipient, and WTgraft/WTrecipient mice did not differ significantly, though there was a statistically insignificant trend towards lower fibrin/fibrinogen concentration in the former two groups. Overall, these data supported that hypothesis that PAI-1 regulates fibrin deposition in the VG wall, but did not suggest that enhanced fibrin deposition necessarily promotes intimal hyperplasia.
PAI-1 regulates thrombin activity in the VG wall
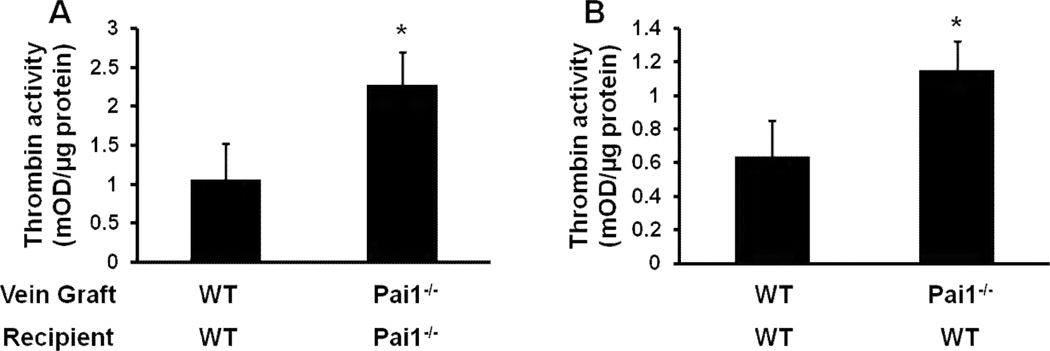
It is hypothesized that thrombin signaling promotes intimal hyperplasia.8, 9 To test the hypothesis that PAI-1 functions in vivo to inhibit thrombin in VGs, we grafted WT vein segments into WT mice and PAI-1-deficient vein segments into PAI-1-deficient mice. After 5 days, VGs were harvested, homogenized, and soluble extracts were prepared. Microscopic examination of longitudinally incised VGs prior to homogenization revealed no evidence of thrombus. Thrombin activity was significantly greater in VG extracts of Pai1−/−graft/Pai1−/−recipient mice than in those of WTgraft/WTrecipient mice (Fig. 5A), suggesting that PAI-1 functions as a physiological thrombin inhibitor in vivo within the VG wall. To examine the role of PAI-1 produced locally in VGs in thrombin inhibition, we performed an experiment in which WT mice received PAI-1-deficient or WT VGs and thrombin activity in VGs was compared 5 days later. Thrombin activity in VG extracts was significantly higher in Pai1−/−graft/WT mice than in WTgraft/WTrecipient mice (Fig. 5B), suggesting that local expression of PAI-1 regulates thrombin activity in VGs.
Figure 5.
PAI-1 deficiency increases thrombin activity retrieved from VGs. (A) Comparison of WTgraft/WTrecipient and Pai1−/−graft/Pai1−/−recipient mice. (B) Comparison of WTgraft/WTrecipient and Pai1−/−graft/WTrecipient mice. Each experimental group consisted of 3–4 VG extracts, each prepared from 2 VGs with identical donor and recipient genotypes. *P<0.05.
Discussion
Several studies have examined fundamental processes by which PAI-1 regulates intimal hyperplasia, including via effects on cell proliferation, migration, and apoptosis.17 These studies have included analyses of the molecular signaling events underlying the effects of PAI-1 on vascular cell function, including cellular receptors, intracellular signaling pathways, and regulation of ECM proteolysis. Together, they have shown that PAI-1 regulates multiple pathways and exerts effects predicted to both increase and decrease intimal hyperplasia. However, no previous single study has examined the in vivo effects of both primary increases and decreases in vascular wall PAI-1 gene expression on intimal hyperplasia, which is essential to understanding the net effect of abnormal PAI-1 expression on vascular remodeling. While previous studies examined the role of PAI-1 in regulating intimal hyperplasia in arteries,9, 13, 18–23 we focused this study on the role of PAI-1 in VG remodeling, as PAI-1 accumulates in diseased VG,11 but it has previously been unknown whether PAI-1 actively regulates VG remodeling or is simply a passive marker of VG disease. The main findings of our study are that 1) PAI-1 regulates VG remodeling, with decreased PAI-1 expression leading to increased intimal hyperplasia; 2) PAI-1’s effects are mediated not only by the plasma pool, but also by a local VG pool that is not discernable by measurement of plasma PAI-1; 3) a primary increase in PAI-1 expression locally in VG or systemically does not increase VG intimal hyperplasia; 4) PAI-1 regulates the function of VG SMC; and 5) PAI-1 regulates thrombin activity in VGs.
Our data suggest that PAI-1 functions in vivo to regulate the cell adhesion function of VN in VGs. VN is synthesized by vascular SMC and other cell types and secreted into the ECM of the vascular wall, where it binds collagen and supports cell migration.24–26 PAI-1 competitively blocks binding of integrins (e.g. αVβ3) and u-PA receptor (uPAR) to VN.5 Therefore, decreased PAI-1 expression could promote VN’s interactions with its receptors and increase cell migration.15 In this study we showed that PAI-1-deficient venous SMC migrate more rapidly through 3-dimensional collagen matrices than WT venous SMC do. We also showed that the proliferative effect of PAI-1 deficiency is blunted in mice also lacking VN, suggesting that this mechanism (i.e. PAI-1 deficiency promoting VN’s cell adhesive function) is active in vivo in VGs. In a recent study we showed that VN-deficient arterial SMC migrate faster through 3-dimensional collagen matrix than WT-SMC, and provided data to suggest that the effect was mediated by an increase in the pool of free, motogenic PAI-1.15 Based on this finding one might hypothesize that VN deficiency would enhance intimal hyperplasia, which was observed in a carotid artery ligation model.9 However, we observed reduced VG intimal hyperplasia in VN-deficient mice, suggesting that during early venous remodeling after bypass grafting, VN supports intimal hyperplasia, most likely by increasing integrin-dependent cell migration.
Over-expression of PAI-1 by vascular SMC, as occurs in diabetes mellitus and other diseases,27 promotes cell proliferation and migration and inhibits apoptosis in vitro.4, 14, 17 We found that over-expression of PAI-1 by venous SMC promotes their migration through 3-dimensional collagen, but did not increase SMC proliferation. However, we did not find that a primary increase of PAI-1 expression, either in VG or systemically, increased VG intimal hyperplasia. These results suggest that while PAI-1 can promote cell migration and proliferation in vitro, the net effect of PAI-1 over-expression on intimal hyperplasia in vivo during the early phase after vein grafting is neutral, perhaps because the proliferative and pro-migratory effects of PAI-1 are counter-balanced by its anti-migratory and antithrombin effects. It is important to realize that our results, observed at a single time point in a murine system, do not disprove that PAI-1 could promote vein graft intimal hyperplasia at other time points, in other pre-clinical models, or in human vein grafts.
An important aspect of our study was the in vivo analysis of the role of PAI-1 as a thrombin inhibitor. While PAI-1 is known to bind and inhibit thrombin in purified, in vitro systems,6 little is known about the significance of this molecular interaction in vivo. Active thrombin can be recovered from the VG wall.28 We have shown that VG thrombin activity is significantly greater in PAI-1-deficient mice, arguing that PAI-1 functions in vivo to regulate vascular wall thrombin activity in VGs. We also showed that PAI-1 produced locally within VGs regulates thrombin, and that PAI-1 produced by SMC regulates their proliferative response to thrombin stimulation. Based on our data, we hypothesize that PAI-1 deficiency promotes VG intimal hyperplasia by effects on thrombin. This hypothesis is supported by reports that thrombin stimulates SMC proliferation,7 and by studies suggesting that sustained thrombin signaling after vascular injury promotes intimal hyperplasia independently of effects on thrombosis.8, 9 While we found that PAI-1-deficient SMC exhibited hyper-responsiveness to thrombin in vitro, we did not observe lessened thrombin responsiveness in PAI-1-Tg SMC, as might be expected. Although our data do not explain the mechanism underlying this observation, we hypothesize that the antithrombin effects of PAI-1 are counterbalanced by the proliferative effects of PAI-1 over-expression.14 In future studies, it will be of interest to examine thrombin activity in VN-deficient VGs to examine the in vivo function of VN as a cofactor for inhibition of vascular wall thrombin by PAI-1.6 In addition to possible effects on thrombin, it is possible that up-regulation of plasmin activity could account for the increased intimal hyperplasia observed in PAI-1-deficient mice, as plasmin has been hypothesized to promote cell migration by degrading ECM.17 However, plasminogen deficiency does not significantly affect VG neointima formation in mice,29 arguing against plasmin as the downstream mediator of PAI-1 deficiency on VG intimal hyperplasia.
PAI-1 regulates vascular fibrin accumulation, which has been proposed as a potential mechanism by which PAI-1 regulates intimal hyperplasia.30 We found increased VG fibrin deposition in PAI-1-Tggraft/PAI-1-Tgrecipient mice, and a non-significant trend towards reduced fibrin deposition in PAI-1-deficient VGs. These results suggested that PAI-1 regulates fibrin deposition in the VG wall. However, we did not observe a correlation between VG fibrin accumulation and intimal hyperplasia in PAI-1-over-expressing mice, suggesting that PAI-1-driven fibrin deposition does not in itself promote intimal hyperplasia. We hypothesize that the lack of a proliferative effect of enhanced fibrin deposition may have been due to the potential anti-migratory effects of enhanced PAI-1 expression.
Our study has some limitations. While we used a published method to study intimal hyperplasia in VG,12 we observed less intimal hyperplasia at 4 weeks after VG implantation than reported previously. The variation between our study and that of Zou et al. may be due to minor differences in surgical technique, differences in the location of cross-sections used to assess intimal hyperplasia, and environmental and dietary differences between studies. Our studies involving implantation of genotype-mismatched VGs into recipient mice provided insights into the function of PAI-1 produced locally within VGs during early venous remodeling. However, some of the PAI-1 in VGs observed in our study could be of systemic origin—i.e. from plasma and/or cells that invade VGs from blood and/or adjacent artery,31, 32 which complicates our functional assessment of VG PAI-1. Nevertheless, our analyses of VG and plasma PAI-1 suggest that cells present in VGs at the time of graft insertion (or subsequently derived from them) are the major source of PAI-1 within VG neointima. These results are consistent with published studies that found that the majority of cells present in VGs are derived from the transplanted graft.33
In summary, we have shown that PAI-1 regulates intimal hyperplasia in a clinically relevant model. Our experiments involving PAI-1-deficient mice and SMC suggest mechanisms by which down-regulation of PAI-1 could promote intimal hyperplasia (i.e. by enhancing VN-dependent SMC migration and thrombin-induced SMC proliferation). These findings are relevant to ongoing development of PAI-1 inhibitors to treat vascular disease—i.e. pharmacological PAI-1 inhibition could potentially up-regulate cell migration and VG wall thrombin activity. Our experiments involving PAI-1-Tg mice suggest that PAI-1 over-expression does not necessarily promote intimal hyperplasia. This finding is significant, given that previous clinical studies, based on associations of PAI-1 concentration and vein graft disease, had hypothesized that increased PAI-1 expression promotes intimal hyperplasia.10, 11 Additional studies involving large animal models of bypass grafting and chemical inhibitors of PAI-1 are warranted to determine the effects of pharmacological targeting of PAI-1 on venous remodeling under clinically relevant conditions.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by a research grant from the Missouri Life Sciences Research Board, NIH grants HL57346 and HL95951, and a Merit Review award from the Department of Veterans Affairs (WPF), and by NIH grants HL55374, HL54710, HL57346, and HL89407 (DAL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Motwani JG, Topol EJ. Aortocoronary Saphenous Vein Graft Disease: Pathogenesis, Predisposition, and Prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 2.Ha H, Oh EY, Lee HB. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nature Reviews Neph. 2009;5:203–211. doi: 10.1038/nrneph.2009.15. [DOI] [PubMed] [Google Scholar]
- 3.Preissner KT, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb Res. 1998;89:1–21. doi: 10.1016/s0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- 4.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The Low Density Lipoprotein Receptor-related Protein Is a Motogenic Receptor for Plasminogen Activator Inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 5.Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin alpha-V beta-3 binding to vitronectin. Nature. 1996;383:441–443. doi: 10.1038/383441a0. [DOI] [PubMed] [Google Scholar]
- 6.Naski MC, Lawrence DA, Mosher DF, Podor TJ, Ginsburg D. Kinetics of inactivation of alpha-thrombin by plasminogen activator inhibitor-1. J Biol Chem. 1993;268:12367–12372. [PubMed] [Google Scholar]
- 7.McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takada M, Tanaka H, Yamada T, Ito O, Kogushi M, Yanagimachi M, Kawamura T, Musha T, Yoshida F, Ito M, Kobayashi H, Yoshitake S, Saito I. Antibody to Thrombin Receptor Inhibits Neointimal Smooth Muscle Cell Accumulation Without Causing Inhibition of Platelet Aggregation or Altering Hemostatic Parameters After Angioplasty in Rat. Circ Res. 1998;82:980–987. doi: 10.1161/01.res.82.9.980. [DOI] [PubMed] [Google Scholar]
- 9.de Waard V, Arkenbout EK, Carmeliet P, Lindner V, Pannekoek H. Plasminogen activator inhibitor 1 and vitronectin protect against stenosis in a murine carotid artery ligation model. Arterioscler Thromb Vasc Biol. 2002;22:1978–1983. doi: 10.1161/01.atv.0000042231.04318.e6. [DOI] [PubMed] [Google Scholar]
- 10.Rifon J, Paramo JA, Panizo C, Montes R, Rocha E. The increase of plasminogen activator inhibitor activity is associated with graft occlusion in patients undergoing aorto-coronary bypass surgery. Brit J Haem. 1997;99:262–267. doi: 10.1046/j.1365-2141.1997.3913205.x. [DOI] [PubMed] [Google Scholar]
- 11.Kauhanen P, Sirén V, Carpén O, Vaheri A, Lepäntalo M, Lassila R. Plasminogen activator inhibitor-1 in neointima of vein grafts - Its role in reduced fibrinolytic potential and graft failure. Circulation. 1997;96:1783–1789. doi: 10.1161/01.cir.96.6.1783. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am J Pathol. 1998;153:1301–1310. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otsuka G, Agah R, Frutkin AD, Wight TN, Dichek DA. Transforming growth factor beta-1 induces neointima formation through plasminogen activator inhibitor-1- dependent pathways. Arterioscler Thromb Vasc Biol. 2006;26:737–743. doi: 10.1161/01.ATV.0000201087.23877.e1. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Budd RC, Kelm RJ, Jr, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- 15.Garg N, Goyal N, Strawn TL, Wu J, Mann KM, Lawrence DA, Fay WP. Plasminogen activator inhibitor-1 and vitronectin expression level and stoichiometry regulate vascular smooth muscle cell migration through physiological collagen matrices. J Thromb Haemost. 2010;8:1847–1854. doi: 10.1111/j.1538-7836.2010.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000;106:1441–1443. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fay WP, Garg N, Sunkar M. Vascular Functions of the Plasminogen Activation System. Arterioscler Thromb Vasc Biol. 2007;27:1231–1237. doi: 10.1161/ATVBAHA.107.140046. [DOI] [PubMed] [Google Scholar]
- 18.Peng L, Bhatia N, Parker AC, Zhu Y, Fay WP. Endogenous vitronectin and plasminogen activator inhibitor-1 promote neointima formation in murine carotid arteries. Arterioscler Thromb Vasc Biol. 2002;22:934–939. doi: 10.1161/01.atv.0000019360.14554.53. [DOI] [PubMed] [Google Scholar]
- 19.Ploplis VA, Cornelissen I, Sandoval-Cooper MJ, Weeks L, Noria FA, Castellino FJ. Remodeling of the vessel wall after copper-induced injury is highly attenuated in mice with a total deficiency of plasminogen activator inhibitor-1. Am J Pathol. 2001;158:107–117. doi: 10.1016/S0002-9440(10)63949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmeliet P, Moons L, Lijnen HR, Janssens S, Lupu F, Collen D, Gerard RD. Inhibitory role of plasminogen activator inhibitor-1 in arterial wound healing and neointima formation--A gene targeting and gene transfer study in mice. Circulation. 1997;96:3180–3191. doi: 10.1161/01.cir.96.9.3180. [DOI] [PubMed] [Google Scholar]
- 21.Sjoland H, Eitzman DT, Gordon D, Westrick R, Nabel EG, Ginsburg D. Atherosclerosis progression in LDL receptor deficient and apolipoprotein E deficient mice is independent of genetic alterations in plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2000;20:846–852. doi: 10.1161/01.atv.20.3.846. [DOI] [PubMed] [Google Scholar]
- 22.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Plasminogen activator inhibitor-1 deficiency protects against atherosclerosis progression in the mouse carotid artery. Blood. 2000;96:4212–4215. [PubMed] [Google Scholar]
- 23.Luttun A, Lupu F, Storkebaum E, Hoylaerts MF, Moons L, Crawley J, Bono F, Poole AR, Tipping P, Herbert J-M, Collen D, Carmeliet P. Lack of plasminogen activator inhibitor-1 promotes growth and abnormal matrix remodeling of advanced atherosclerotic plaques in apolipoprotein-E deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:499–505. doi: 10.1161/hq0302.104529. [DOI] [PubMed] [Google Scholar]
- 24.Dufourcq P, Louis H, Moreau C, Daret D, Boisseau MR, Lamazière JMD, Bonnet J. Vitronectin expression and interaction with receptors in smooth muscle cells from human atheromatous plaque. Arterioscler Thromb Vasc Biol. 1998;18:168–176. doi: 10.1161/01.atv.18.2.168. [DOI] [PubMed] [Google Scholar]
- 25.Izumi M, Shimo-Oka T, Morishita N, Ii I, Hayashi I. Identification of the collagen-binding domain of vitronectin using monoclonal antibodies. Cell Struct Funct. 1988;13:217–225. doi: 10.1247/csf.13.217. [DOI] [PubMed] [Google Scholar]
- 26.Brown SL, Lundgren CH, Nordt T, Fujii S. Stimulation of migration of human aortic smooth muscle cells by vitronectin: Implications for atherosclerosis. Cardiovasc Res. 1994;28:1815–1820. doi: 10.1093/cvr/28.12.1815. [DOI] [PubMed] [Google Scholar]
- 27.Pandolfi A, Cetrullo D, Polishuck R, Alberta MM, Calafiore A, Pellegrini G, Vitacolonna E, Capani F, Consoli A. Plasminogen activator inhibitor type 1 is increased in the arterial wall of type II diabetic subjects. Arterioscler Thromb Vasc Biol. 2001;21:1378–1382. doi: 10.1161/hq0801.093667. [DOI] [PubMed] [Google Scholar]
- 28.Sharony R, Pintucci G, Saunders PC, Grossi EA, Baumann FG, Galloway AC, Mignatti P. Matrix metalloproteinase expression in vein grafts: role of inflammatory mediators and extracellular signal-regulated kinases-1 and -2. AJP - Heart Circ Physiol. 2006;290:H1651–H1659. doi: 10.1152/ajpheart.00530.2005. [DOI] [PubMed] [Google Scholar]
- 29.Shi C, Patel A, Zhang D, Wang H, Carmeliet P, Reed GL, Lee ME, Haber E, Sibinga NES. Plasminogen is not required for neointima formation in a mouse model of vein graft stenosis. Circ Res. 1999;84:883–890. doi: 10.1161/01.res.84.8.883. [DOI] [PubMed] [Google Scholar]
- 30.Fay WP. Plasminogen activator inhibitor 1, fibrin, and the vascular response to injury. Trends Cardiovasc Med. 2004;14:196–202. doi: 10.1016/j.tcm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Cooley BC. Murine model of neointimal formation and stenosis in vein grafts. Arterioscler Thromb Vasc Biol. 2004;24:1180–1185. doi: 10.1161/01.ATV.0000129330.19057.9f. [DOI] [PubMed] [Google Scholar]
- 32.Schafer K, Schroeter MR, Dellas C, Puls M, Nitsche M, Weiss E, Hasenfuss G, Konstantinides SV. Plasminogen activator inhibitor-1 from bone marrow-derived cells suppresses neointimal formation after vascular injury in mice. Arterioscler Thromb Vasc Biol. 2006;26:1254–1259. doi: 10.1161/01.ATV.0000215982.14003.b7. [DOI] [PubMed] [Google Scholar]
- 33.Hu Y, Mayr M, Metzler B, Erdel M, Davison F, Xu Q. Both Donor and Recipient Origins of Smooth Muscle Cells in Vein Graft Atherosclerotic Lesions. Circ Res. 2002;91:e13–e20. doi: 10.1161/01.res.0000037090.34760.ee. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.