Abstract
Although the anorexic effects of leptin are lost in obesity, leptin-mediated sympatho-activation is preserved. The cardiovascular consequences of leptin-mediated sympatho-activation in obesity are poorly understood. We tested the hypothesis that 32 weeks of high fat diet (HFD) induces metabolic leptin resistance but preserves leptin-mediated sympatho-activation of the cardiovascular system. HFD in mice significantly increased body weight and plasma leptin concentrations but significantly reduced the anorexic effects of leptin. HFD increased heart rate (HR), stroke volume, cardiac output and plasma aldosterone levels but not blood pressure (BP). As reflected by the contractile response to phenylephrine measured both in vivo and ex vivo, vascular adrenergic reactivity was reduced by HFD suggesting that reductions in sympathetic tone to the periphery vasculature may mitigate sympatho-activation of the heart and the renin angiotensin aldosterone system (RAAS). Tachyphlyaxis was partially restored by symptho-inhibition and not present in ob/ob and db/db mice, despite obesity, arguing for a sympatho-mediated and leptin-specific mechanism. While infusion of leptin in HFD mice had no effect on HR or BP, it further increased aldosterone levels and further reduced vascular adrenergic tone in the absence of weight loss, indicating persistent leptin-mediated stimulation of the cardiovascular system in obesity. In conclusion, these data indicate that despite metabolic leptin resistance, leptin-mediated stimulation of the heart, the vasculature and aldosterone production persists in obesity. BP effects in response to leptin may be limited by a tachyphylactic response in the circulation suggesting that failure of adrenergic desensitization may be a requisite step for hypertension in the context of obesity.
Keywords: blood pressure, adrenergic reactivity, cardiac output, aldosterone, high fat diet
Introduction
Obesity is a metabolic disorder associated with excess body weight affecting 30 to 60 percent of the American population1–3. Although substantial evidence shows that obesity is a major risk factor for the development of hypertension1, the relationship between body weight and blood pressure (BP) is complex and incompletely understood. Indeed while many obese patients develop hypertension 4, 40 to 60 percent of them do not 5–8. Sympatho-activation is thought to be a major component of obesity-induced hypertension 9–11, but its origins and role in the development of hypertension remain to be fully determined.
Leptin is an adipocyte-derived cytokine the concentration of which increases proportionally to the level of adiposity12, 13. Leptin is involved in the control of the metabolism, communicating repletion of body energy stores to the central nervous system in order to suppress food intake and permit energy expenditure 12. Despite high circulating levels, leptin fails to promote weight loss in obesity, a state defined as leptin resistance 14. Besides its metabolic actions, leptin also stimulates the cardiovascular system 15–19, increasing sympathetic outflow via renal, lumbar and adrenal sympathetic nerves 20, 21. Recent studies of leptin action 16, 22 describe a phenomenon of “selective leptin resistance” in which leptin-mediated stimulation of brown adipose tissue innervation is lost with obesity but leptin-mediated activation of renal nerve activity is preserved. While nerve and BP responses to pharmacological infusion of leptin are well-documented22–26, the response of specific target organs in the cardiovascular system to physiological and pathophysiological levels of leptin remains to be clearly defined. Recent studies from our lab have indicated that leptin stimulation results in a sympathetically-mediated stimulation of the vasculature, as indicated by an adaptive down-regulation of adrenergic vasoconstriction and α1D-adrenergic receptors mRNA levels 15. The extent to which selective leptin resistance impacts sympathetic targets in the cardiovascular system is unknown.
A major gap in our knowledge is how leptin-mediated stimulation of the cardiovascular system is affected by obesity and whether there are adaptive responses which parallel “selective leptin resistance”. The goal of the current study was to test the hypothesis that leptin-stimulated activation of the cardiovascular system was preserved in obesity. To test this hypothesis, obesity was generated in C57Bl/6 mice using 32 weeks of a high fat diet. Metabolic function was assessed in terms of metabolic profiling and body weight changes in response to leptin. Cardiovascular responses to obesity were examined in parallel, including cardiac function, vascular reactivity, adrenergic receptor expression, aldosterone production and BP. The goal of these studies was to determine the extent to which leptin-mediated stimulation of cardiovascular target organs contribute to the regulation of BP in obesity.
Methods
Animals
Male C57Bl/6 mice (8 weeks of age, Jackson Laboratory-Bar Harbor, ME, USA) were divided in two groups and fed either a control (CD, 4% of fat calories) or a high-fat diet ad libitum (HFD, 60% of fat calories from lard, Diet F3282, Bioserve, NJ) as previously described 27. Mice were monitored for the following 32 weeks and body weight was measured weekly. After 32 weeks, plasma concentrations of glucose, insulin, triglycerides and leptin, of fasted animals were measured following the procedures previously described 15. In a separate set of experiments, leptin deficient (ob/ob), and leptin receptor deficient mice (db/db) were purchased from the Jackson Laboratory. Mice were housed in an American Association of Laboratory Animal Care–approved animal care facility at Georgia Health Sciences University, and the Institutional Animal Care and Use Committee approved all protocols.
Leptin sensitivity
To determine the effects of 32 weeks HFD on metabolic and cardiovascular sensitivity to leptin, CD and HFD mice were submitted to a 7 day treatment of subcutaneous infusion of leptin at the dose of 10 and 40 μg/day (ALZET, Cupertino, Calif; model 1007D, 0.5 μL/h)15, based on the fact that obesity does not increase blood volume in rodents28.
In vivo vascular adrenergic reactivity
In a separate set of mice, the carotid artery and jugular vein were catheterized under isoflurane anesthesia (1.5%) for the measurement of mean arterial pressure (MAP) and drug delivery, respectively. To eliminate endogenous sympathetic vasomotor tone and baroreceptor-reflex–mediated responses, animals were given the ganglionic blocker mecamylamine(2 mg/kg IV). Effective blockade was confirmed by the absence of reflex bradycardia after vasoconstrictor administration. Changes in MAP were determined after injection of randomized boluses of phenylephrine (0.01 to 1 mg/kg) 15.
Ex vivo aortic reactivity
Thoracic aortas were dissected surgically, cleaned of fat and mounted on a wire myograph (DMT, Aarhus, Denmark)with 1g of basal tension. Briefly, 2 tungsten wires were inserted into the lumen of the arteries and fixed to a force transducer and a micrometer. Arteries were bathed in a physiological salt solution as described previously 29. Arterial viability was determined with a potassium-rich solution (40 mmol/L). Vascular contractility was assessed with cumulative concentration-response curves to phenylephrine (PE, 1 nmol/L to 100 μmol/L), and serotonin (5-hydroxytryptamine[5HT]; 1 nmol/L to 10 μmol/L). Constriction to PE, and 5HT were expressed as a percent of KCl-induced constriction. Endothelial function was assessed with concentration-response curves to acetylcholine (ACh, 1 nmol/L to 10 μmol/L). Endothelium-independent relaxation was analyzed with a concentration-response curve to sodium nitroprusside (SNP, 1 nmol/L to 10 μmol/L). Relaxation curves were performed on preconstricted vessels (5HT 0.1 μmol/L), and relaxation was expressed as a percent of the precontraction.
Relationship between sympathetic activity and vascular adrenergic reactivity
A set of CD and HFD mice was treated with the specific α1-adrenergic receptor inhibitor prazosin (2 mg/kg/d, IP, daily injection )15 or with the combination of leptin (10 μg/day) plus prazosin for 7 days. Cardiovascular consequences of the α1-adrenergic receptor inhibition were determined at the vascular level by assessment of aortic reactivity and the gene expression of the 1D-adrenergic receptor by quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR), as described below.
Real-Time RT-PCR
Total aortic mRNA was extracted (Trizol Plus, Invitrogen, Carlsbad, Calif), purified with RNeasy spin columns, and eluted from the column in 30 μL of DEPC-treated water, and the concentration was established with a NanoDrop 1000 (NanoDrop Technologies, Wilmington, Del). Complementary DNA was generated by RT-PCR with SuperScript III (Invitrogen) from 400 ng of total RNA using random hexamers. Reverse transcription was performed at 50°C for 50 minutes; the enzyme was heat inactivated at 85°C for 5 minutes, and real-time quantitative RT-PCR was performed with the SYBR-Green Supermix (Bio-Rad Laboratories, Hercules, Calif) and the primers previously described 15.
Echocardiographic studies
At the end of the 32 weeks of CD or HFD, mouse heart was imaged in anesthetized animals (isoflurane 2%) in a supine position on a THM100 MousePad with integrated temperature sensor, heater, and ECG electrodes (Indus Instruments, Houston, TX). Ultrasound imaging was performed using the Vevo 770 system (VisualSonics, Toronto, ON, Canada) and the RMV 707B scan head designed for high frame rate and real-time small animal imaging applications with a center frequency of 30 MHz and a frequency band 15–45 MHz. B-mode images of the parasternal long axis view of the heart were used to measure the left ventricular outflow tract (LVOT) and left ventricle (LV) dimensions, and PW-mode was used to determine aortic velocity time integral (Ao VTI). Stroke volume (SV), cardiac output (CO) and fractional shortening were calculated according to the following formulas:
Measurement of plasma aldosterone
To assess the effects of obesity and leptin on the activation of the RAAS, plasma aldosterone levels were measured by radioimmunoassay (Siemens Medical Diagnostic Deerfield, IL) in plasma samples collected from CD and HFD mice submitted to a 7 day treatment with either saline, leptin (10 μg/day), prazosin or leptin (10 μg/day) plus prazosin.
In vivo blood pressure measurement
At the end of the 32 weeks of treatment, C57Bl/6 and ob/ob mice were instrumented with telemetry transmitters to record BP and heart rate (PA-C10, Data Sciences, SaintPaul, Minn) and kept on their specific diet. Transmitters were implanted as described previously 15. After 7 to 12 days of recovery from surgery, necessary for the mice to gain their initial body weight, baseline data were recorded for 7 days before implantation of micro-osmotic pumps (ALZET, Cupertino, Calif; model 1007D, 0.5 μL/h) for subcutaneous infusion of leptin (10 or 40 μg/day 15) or leptin plus prazosin (2 mg/kg/d, IP )15. After one week of leptin treatment, mice were euthanized; tissues and plasma were collected for later analysis.
Statistical Analysis
All data are presented as mean ± sem. Differences in means among groups for non-repeated variables were compared by 1-way ANOVA. Differences in means among groups and treatments, with repeated variables, were compared by 2- or 3-way ANOVA with repeated measures, when appropriate. Bonferroni and Fisher least significant difference tests were used as the post hoc test(SigmaStat).
Results
Baseline phenotypes
The effects of 32 weeks of HFD were determined by measuring body weight and baseline plasma chemistry. As shown in table 1 and figure S1 (Supplemental Figures; available at http://hyper.ahajournals.org) the 32 week HFD induced a much greater increase in body weight compared to CD (HFD: 48±6% vs. CD: 12±3% increase, p<0.0001) and significantly raised circulating leptin levels (HFD: 18.6±3.0 vs. CD: 1.2±0.8 ng/mL, p<0.05, Table 1). As summarized in table 1, HFD was associated with increased plasma cholesterol, triglycerides, glycemia, Hb1Ac and plasma insulin concentration, confirming that our model of diet-induced obesity leads to metabolic dysfunction. As shown in Table 1, diet-induced obesity completely abolished the decrease in body weight induced by low (10 μg/day) and high (40 μg/day) doses of leptin infusion demonstrating that sustained HFD leads to a resistance to the metabolic effects of leptin, as previously described22.
Table 1.
Basic physiological and metabolic parameters of control and high fat diet mice.
| Variable | Control Diet | Control Diet + Leptin 10 μg/day | Control Diet + Leptin 40 μg/day | High Fat Diet | High Fat Diet + Leptin 10 μg/day | High Fat Diet + Leptin 40 μg/day |
|---|---|---|---|---|---|---|
| Body Weight (g) | 34.5±0.5 | *31.2±0.8 | *29.4±0.6 | †47.7±1.5 | †46.4±0.8 | †49.0±2.1 |
| Glycemia (mg/dL) | 141±13 | 121±7 | 123±8 | *203±19 | *166±8 | *197±14 |
| Hb1Ac | 4.8±0.1 | 4.6±0.1 | 4.9±0.1 | *5.7±0.2 | *5.6±0.1 | *5.4±0.1 |
| Insulin (μg/mL) | 61.4±4.0 | 48.6±8.4 | *33.3±7.0 | *603.8±278.5 | *398.8±145.3 | *419.1±52.3 |
| Cholesterol (mg/dL) | 56.5±7.5 | *50.4±7.0 | 87.4±5.9 | †93.3±12 | *84.8±7.35 | 180.9±13.1 |
| Triglycerides (mg/dL) | 37.6±8.2 | 26.1±0.9 | 36.6±3.0 | 38.7±8.9 | 35.9±5.7 | 58.2±8.02 |
Data are presented as mean ± sem,
p<0.05 vs control diet,
p<0.0001 vs. control diet,
p<0.05 vs control condition within the same group, n=10–12 per group.
Effects of HFD on cardiac function and aldosterone secretion
The consequences of obesity and hyperleptinemia on cardiac function s were determined by ultrasonography. As reported in Table 2, HFD promoted an increased CO, notably elevating Ao VTI and LVOT observed in these mice. Obesity has often been associated with an increased activation of the RAAS both in human30 and animals models31. To assess the effects of the HFD on the RAAS, we measured plasma aldosterone levels. As shown in Figure 1, plasma aldosterone levels were increased with HFD and further increased with sustained leptin infusion. Chronic α1-adrenergic receptor inhibition with prazosin completely abolished HFD-induced aldosterone secretions, and also blunted leptin-stimulated aldosterone secretion in obese mice. This suggests a key role for leptin in the control of aldosterone secretion, most likely through the activation of the sympathetic nervous system in HFD mice.
Table 2.
Echocardioagraphic parameters of control and high fat diet mice.
| Variable | Control Diet | High Fat Diet |
|---|---|---|
| Ao VTI (cm) | 1.39±0.13 | *1.91±0.16 |
| LVOT (mm) | 1.40±0.05 | *1.54±0.03 |
| HR (bpm) | 436±63 | 467±14 |
| SV (μL) | 21.1±1.0 | *35.9±3.7 |
| CO (mL/min) | 9.2±1.5 | *16.8±1.9 |
| FS (%) | 17.9±1.1 | 18.6±0.9 |
Aortic velocity time integral (Ao VTI), left ventricular outflow tract (LVOT), heart rate (HR), stroke volume (SV), cardiac output (CO), and fractional shortening obtained from the ultrasound analysis of mouse’s heart. Data are presented as mean ± sem,
p<0.05 vs control, n=8 per group.
Figure 1. Diet-induced obesity and sustained leptin infusion increase plasma aldosterone levels.
Plasma aldosterone levels measured in control and high fat diet mice submitted to 7 days of saline, leptin (10 μg/day), prazosin or leptin (10 μg/day) plus prazosin (2 mg/kg/d, IP.) infusion. Data are presented as mean ± sem, # p<0.05 vs. saline, $ p<0.05 vs. leptin, * p<0.05 vs. control diet. n=6–12 per group.
Effects of obesity and leptin on blood pressure and heart rate
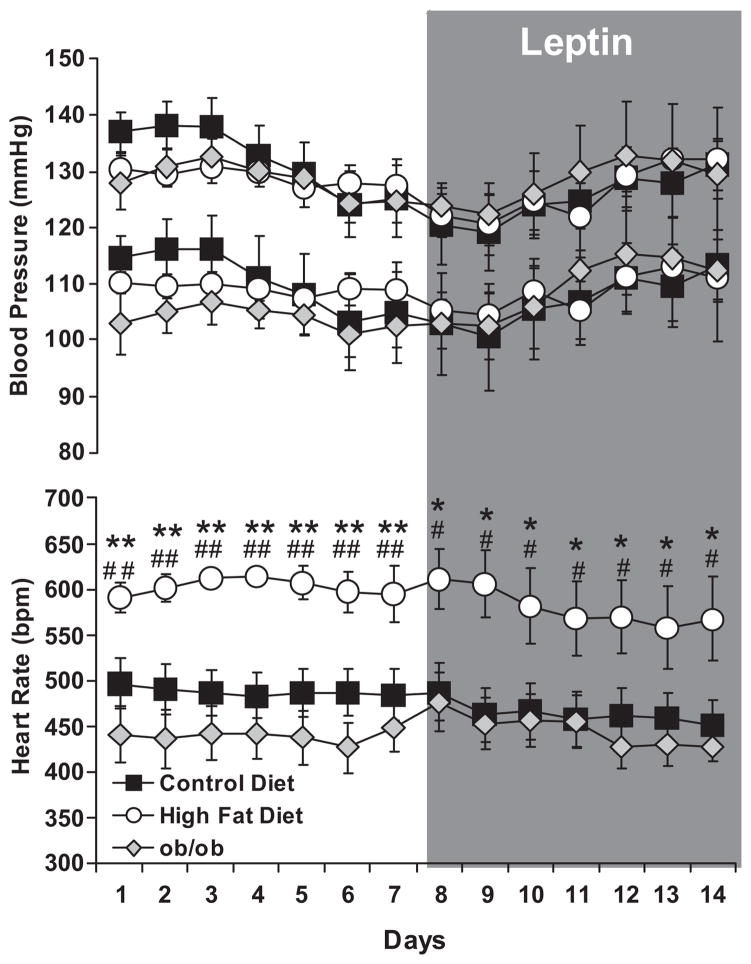
The effects of obesity and hyperleptinemia on BP and heart rate are shown in Figure 2. 32 weeks of HFD in mice, increased neither systolic nor diastolic BP compared to controls. Sustained leptin infusion at low (Figure 2) or high dose (Figure S2, please see http://hyper.ahajournals.org), did not affect the BP of the CD and HFD mice. Prazosin did not alter responses in either group (Figure S3, Supplemental Figures, please see http://hyper.ahajournals.org). As further shown in Figure 2, leptin deficient mice (ob/ob) presented systolic and diastolic BP values similar to those of the CD mice, despite obesity. BP data from the HFD and ob/ob mice suggest that leptin does not directly affect BP.
Figure 2. High fat diet or leptin infusion does not increase blood pressure.
Systolic, diastolic blood pressure and heart rate measured via radio telemetry, in baseline condition and during 7 days leptin infusion at the dose of 10 μg/day in mice submitted to 32 weeks of control or high fat diet, as well as in leptin deficient mice (ob/ob). Data are presented as mean ± sem, # p<0.05 vs. control diet, ## p<0.0001 vs. control diet, * p<0.05 vs. ob/ob, ** p<0.0001 vs. ob/ob. n=6–8 per group.
In contrast to the lack of effect on BP, HFD induced a 23% increase in heart rate. Obesity without leptin in ob/ob mice had no significant effect on HR, suggesting the leptin is required for obesity-related tachycardia. Further increases in plasma leptin via leptin infusion, did not affect heart rate in CD and HFD mice. Despite obesity, sympatho-activation of the heart, and an increased RAAS activation, HFD mice did not present an elevated BP. These data indicate that HFD mice may evidence parallel counter-regulatory mechanisms to offset the sympatho-excitatory effects of leptin and maintain their BP in a physiological range.
Effects of HFD on adrenergic tone
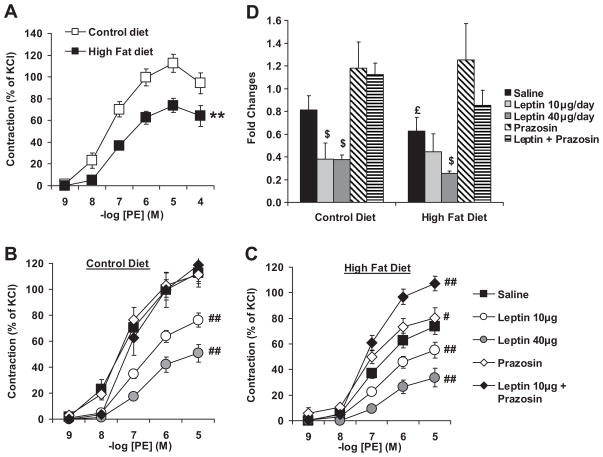
Total peripheral resistance (TPR) is determined in large part by sympathetic innervation of the vasculature. Because sympathetic innervation is the most likely aspects of peripheral control to be influence by obesity-related hyperleptinemia, we assessed changes in MAP induced by α1-adrenergic stimulation (phenyleprhine) in anesthetized mice under ganglionic blockade. Under these conditions, the rise in MAP reflects the constriction of the resistance vasculature. As shown in Figure 3, HFD significantly blunted PE-mediated rises in MAP (CD: baseline 72.2±5.8, peak 129.6±4.1; HFD: baseline 96.6±3.4 peak 140.8±1.07 mmHg) suggesting that obesity decreases vascular adrenergic tone and likely TPR. To localize this effect directly to the vasculature, we assessed aortic ring reactivity on a wire myograph. As observed in Figure 4A, diet-induced obesity blunted vascular adrenergic constriction in vitro. To determine the effect of leptin on adrenergic reactivity, PE-induced constriction was assessed in CD and HFD mice treated with low and high doses of leptin for 7 days. Sustained leptin treatment blunted aortic reactivity to PE in CD mice (Fig. 4B) and further reduced it in HFD mice (Fig 4C). In CD mice, sustained leptin infusion reduced vascular adrenergic reactivity to the level of the HFD mice. To determine the role of the sympathetic activity in the development of these defects we measured the vascular consequences of a chronic peripheral α1-adrenergic receptor inhibition with prazosin. As reported in figure 4B-C, α1-adrenergic receptor inhibition alone or in combination with leptin did not affect adrenergic reactivity in CD mice, while it restored it in HFD mice treated with saline and leptin. As previously reported by our group leptin-induced vascular adrenergic desensitization is linked to decreased expression of α1D-adrenergic receptor15. As reported in figure 4D, sustained leptin infusion as well as diet-induced obesity significantly reduced α1D-adrenergic receptor gene expression as measured by quantitative real-time RT-PCR. Chronic α1-adrenergic receptor inhibition restored α1D-adrenergic receptor gene expression in obese mice and blunted the leptin effects.
Figure 3. 32 weeks of high fat diet reduces vascular adrenergic tone in resistance arteries.
Changes in MAP induced by phenylephrine injection in mice under mecamylamine blockade. Data are presented as mean ± sem. Effects of the diet were assessed with ANOVA. *p<0.05 vs. control diet, n=5 per group.
Figure 4. 32 weeks of high fat diet lead to a leptin-mediated adrenergic desensitization.
Cumulative concentration response curves to phenylephrine (PE) performed on aortic rings taken from control and high fat diet mice (A), submitted or not, either to a 7 day subcutaneous leptin infusion (10 or 40 μg/day), to a 7 day prazosin treatment (2 mg/kg/d IP) or to a 7 day treatment with leptin (10 μg/day) plus prazosin (B and C). Data are expressed as percent of KCl-induced constriction. (D) Quantification of α1D-adrenergic receptor subtype gene expression by quantitative real time RT-PCR performed on aortas taken from control and high fat diet mice. Effects of the group and the treatment were determined with a two-way ANOVA. Data are presented as mean ± sem, **p<0.0001 vs. control diet, # p<0.05 vs. saline within group, ## p<0.001 vs. saline within group, $ p<0.05 vs. saline within group, £ p<0.05 vs. control diet (n=8–13 per group).
To confirm that leptin specifically affect vascular adrenergic reactivity we analyzed endothelium-dependent relaxation and assessed the properties of the aorta to constrict in response to 5HT infusion and to relax to SNP. As shown in figure S4 to S6 (Supplemental Figures, please see http://hyper.ahajournals.org), obesity-induced high levels of leptin as well as sustained leptin infusion did not affect the endothelium dependent relaxation nor the vascular smooth muscle cell function as reflected by the reactivity to KCl, 5HT and SNP. These results argue for a specific effect of leptin on vascular adrenergic reactivity. Moreover, 7 days of leptin treatment also did not alter non-adrenergic vascular reactivity (Figure S6, Supplemental Figures, please see http://hyper.ahajournals.org)
Role of obesity and leptin receptor in vascular adrenergic desensitization
To determine the role of leptin in obesity-related vascular adrenergic tachyphylaxis we measured adrenergic reactivity in leptin receptor- (db/db) and leptin-deficient (ob/ob) mice. As reported in figures 5A and 5B, neither db/db nor ob/ob mice, both obese, presented a reduced vascular adrenergic reactivity nor a decreased in α1D-adrenergic receptor gene expression (Figures 5E–F) compared to their respective control, suggesting that intact leptin signaling is required for adrenergic desensitization with obesity-related sympathoactivation. This was further supported by the lack of effect of leptin on adrenergic tone in db/db mice (Fig 5C) and the reduced adrenergic reactivity in ob/ob treated with leptin (fig 5D, 5G).
Figure 5. Leptin-induced vascular adrenergic desensitization requires functional leptin receptor.
Cumulative concentration response curves to phenylephrine (PE) performed on aortic rings taken from leptin receptor (db/db, A) or leptin deficient (ob/ob, B) mice and in mice submitted or not to a 7 day subcutaneous leptin infusion (C, D). Effects of leptin receptor deletion and of leptin treatment were determined with ANOVA for repeated measurements. Quantification of α1D-adrenergic receptor subtype gene expression by quantitative real time RT-PCR performed on aortas taken from db/db (E), ob/ob (F) and ob/ob mice treated with leptin (G). Data are presented as mean ± sem, **p<0.0001 vs. db/+, ## p<0.0001 vs. ob/ob, n=5–8 per group.
All together these data suggest that obesity-induced high leptin secretion triggers a sympatho-mediated vascular adrenergic tachyphylaxis likely decreasing TPR which may offset the prohypertensive effects of the high CO and RAAS activation.
Discussion
The goal of the present study was to determine the effects of leptin on sympatho-activation of the cardiovascular system in the context of obesity. The key observations of the current study are 1) obesity induces resistance to the metabolic action of leptin, 2) sympatho-activation of the cardiovascular system as indicated by increases in heart rate and stroke volume persist in obesity, despite metabolic leptin resistance, 3) HFD- and leptin-mediated sympatho-activation stimulates the renin angiotensin aldosterone system (RAAS) as reflected by the plasma aldosterone levels, 4) vascular adrenergic tachyphylaxis caused by leptin-mediated sympatho-activation persists in obesity, indicating a lack of resistance to the effects of leptin in the vasculature, 5) obesity in the absence of leptin signaling does not result in vascular adrenergic tachyphylaxis and 6) the decreased adrenergic tone in the vasculature correlates with an increase in heart rate, CO and RAAS activation but not with changes in pressure. Relevant to these observations are the concepts of selective leptin resistance, the mechanisms of sympatho-activation of the vasculature in obesity and the balance of hemodynamic forces that regulate blood pressure in obesity.
Selective Leptin Resistance and the Cardiovascular System
Although many studies have reported that obesity leads to a resistance to the metabolic effects of leptin14, whether the vasculature becomes resistant to the high circulating leptin levels remained to be determined. As demonstrated in our previous study, a “footprint” of the leptin-mediated innervation of the vasculature can be detected by a compensatory vascular adrenergic tachyphylaxis and a reduction in the gene expression of the vascular adrenergic receptor15. Whether this chronic effect of leptin in obese animals is preserved was unknown. In the current study, we observed that sustained exogenous leptin infusion caused a sympatho-mediated vascular adrenergic tachyphylaxis in lean leptin sensitive as well as in obese mice with metabolic leptin resistance suggesting that the ability of leptin to increase sympathetic nerve activity to the vasculatture is preserved in obese animals and is in support of the theory of selective resistance to the metabolic effects of leptin 16, 22, 32.
Another major observation in the current study is that obesity increases aldosterone secretion through a leptin-dependent mechanism requiring sympatho-activation. Indeed, sustained leptin infusion increased aldosterone secretion in CD mice and further enhanced it in HFD mice. Furthermore, sympatho-inhibition restored aldosterone levels in HFD and blunted the increased induced by leptin infusion. One potential mechanism of this is an increase in renal nerve traffic in response to leptin 21, 22 leading to an increase in renin release 33, 34, though this has historically thought to be β-adrenergic in character. While detailed mechanisms will require further study, the prazosin data further support the concept that neural components related to the metabolic function develop a resistance to high leptin levels while components relevant to cardiovascular control do not.16, 22, 32.
Hemodynamic impact of leptin in obesity
While leptin’s role in sympatho-activation is well-documented, the impact on the regulation of BP is incompletely understood. Indeed, the role of leptin as a pressor agent in normal and obese states remains to be fully determined. While several studies reported an increased BP with acute or sustained leptin infusion 19, 22–24 several others did not report any changes 15,21, 24, 35, 36. Some of these differences between these studies comes from the techniques used to administrate leptin (intravenous vs. intracerebrovascular or intraperitoneal), the duration of the treatment (acute vs. chronic), the dose (physiologic vs. pharmacologic) and the technique used to measured blood pressure (tail cuff plethysmography, intra arterial catheter vs. telemetry). As leptin is mainly secreted from the subcutaneous adipose tissue37 into the blood stream, we infused leptin subcutaneously with osmotic mini-pumps. Blood pressure was recorded in conscious animals via radiotelemetry in order to avoid the manipulation of animals with exaggerated cardiovascular response to stress38. Thus, we believe that the experimental approach used in this study provides the best experimental reflection of physiologic control of leptin.
Another deficit in our understanding of leptin’s effects stems from a lack of information of how the complex mechanisms that control BP are impacted by obesity and increases in leptin. In the current study, we use gold-standard measurements of arterial pressure combined with examination of indices of each major compartment of BP control. In obese mice characterized either by a high (HFD) or low (ob/ob) circulating levels of leptin, we did not observed any difference in BP between lean and obese animals. However, high leptin levels were associated with an increased heart rate while increased heart rate was absent in mice which lack leptin (ob/ob), consistent with leptin mediated sympatho-activation of the heart, a relationship between leptin and heart rate previously documented both in humans39 and animal models19. This increase in heart rate was paralleled by an increase in cardiac output, further supporting the concept of sympathoactivation of the heart in obesity. When the effects of obesity and leptin on aldosterone levels are added to these cardiac indices, it is clear that obesity and leptin have direct sympathetically mediated actions on the cardiovasvular system. The observation that leptin does not increase BP despite increasing heart rate and sympathetic activity suggest that counter-regulatory mechanisms oppose the prohypertensive influence of the sympathetic activation.
The absence of an increased in BP despite these increases in cardiac and RAAS mechanism suggests that parallel changes in the vasculature must occur that limit the expression of hypertension in these animals. The results of the present study and several others36, 40 do not support the vasodilator effects of leptin as the primary mechanism moderating the blood pressure response to leptin, and instead propose that this is mediated by rather a vascular adrenergic tachyphylaxis. Interestingly, human studies also support the role of altered adrenergic tone in the modulation of the BP response to obesity. While studying obese normotensive patients, Agapitov et al., observed a dissociation between the increased forearm muscle sympathetic nerve activity and reduced brachial adrenergic tone41, suggesting that the reduced vascular adrenergic tone could compensate or counterbalance increased sympathetic activity. Moreover, Egan et al., 42 noted that obese patients with increased forearm vascular adrenergic tone were hypertensive, suggesting that loss of compensatory reductions in adrenergic tone favors the actions of prohypertensive factors. All together these data suggest that the failure to induce a vascular adrenergic tachyphylaxis under conditions of sympatho-activation, such as obesity, may indeed be a major factor in determining whether frank hypertension develops.
Mechanisms of adrenergic desensitization in obesity
Although a vascular adrenergic tachyphylaxis, reflected by a reduced vascular adrenergic tone has often been reported in human obese patients41 as well as in animals models of obesity28, 43, the underlying mechanisms of theses vascular changes remains undetermined. After 32 weeks of HFD, mice of the present study presented a significant reduction in their vascular adrenergic tone as reflected, in vivo, by the reduced PE-mediated increased in blood pressure as well as in vitro with the blunted aortic constriction to PE. Without effects in control mice, sustained peripheral inhibition of the α1-adrenergic receptor with daily prazosin injection partially restored vascular constriction to PE in HFD mice and completely restored α1D-adrenergic receptor gene expression. These results confirmed our previous study15 and present vascular adrenergic tachyphylaxis as a compensatory mechanism to obesity induced sympatho-activation.. Moreover sustained leptin infusion further decreased aortic adrenergic sensitivity in HFD mice and reduced the gene expression of the α1D-adrenergic receptor both at the level of the conduit arteries (aorta) and resistance arteries (mesenteric and tail caudal arteries, Figure S7, online supplement) an effect that was absent in mice lacking functional leptin receptors (db/db). Leptin-deficient ob/ob mice likewise demonstrated no tachyphylaxis to phenylephrine despite obesity but when leptin was restored by s.c. infusion, desensitization was evident.. These data indicate that sympathetic link between obesity and vascular adrenergic tone is mediated by leptin. The mechanism of this tachyphylaxis and whether it is defective in obese hypertensives is worthy of further study.
In summary, by studying the cardiovascular response to leptin in obese mice resistant to the metabolic effects of leptin, we have been able to demonstrate that leptin, despite increasing heart rate, CO and RAAS, is also responsible for a vascular adrenergic tachyphylaxis most likely protecting against the development of hypertension (Figure 6).
Figure 6.
Schematic representation of the dissociation between the metabolic and cardiovascular effects of leptin-induced sympathoactivation.
Perspectives
Known for its ability to increase the sympathetic tone, leptin has been suggested to be the link between the metabolic and cardiovascular dysfunctions associated with obesity and to be a potential player in the development of hypertension related to obesity. In the present study we observed that diet-induced obesity leads to a resistance to the metabolic effects of leptin. The cardiovascular consequences of leptin stimulation in obesity are complex. Pro-hypertensive effects such an increased cardiac output and aldosterone secretion are parallel by sympathetically driven tachyphylxis of adrenergic tone. These results suggest that the reduction in the vascular adrenergic tone could be a compensatory mechanism reducing the total peripheral resistances and limiting the development of frank hypertension. These data obtained in a mouse model of obesity are supported by two clinical studies reporting that obese normotensive patients presented a decrease adrenergic tone while obese hypertensive patients developed an increased response to adrenergic stimulation. All together these data suggest that the failure to reduce adrenergic tone in the face of chronic sympathoactivation may be important to the development of hypertension in obesity.
Supplementary Material
Acknowledgments
The authors wish to thank Jennifer Rowland for assistance with the aldosterone assays and Christina Salet for assistance in animal husbandry.
Funding sources
The authors acknowledge research support from NIH R01 HL092446 to D.W.S. and an AHA Post-Doctoral Fellowship to EJBC.
Footnotes
Disclosures
None
References
- 1.Kannel W, Brand M, Skinner J, Dawber T, McNamara P. The relation of adiposity to blood pressure and development of hypertension: The framingham study. Ann Intern Med. 1967;67:48–59. doi: 10.7326/0003-4819-67-1-48. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the united states. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure: Findings in hypertension screening of 1 million americans. JAMA. 1978;240:1607–1610. doi: 10.1001/jama.240.15.1607. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JK, Dennis EW, Smith WG, Amad KH, Duncan WC, Austin RC. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull. 1962;1:39–44. [PubMed] [Google Scholar]
- 6.Bramlage P, Pittrow D, Wittchen H-U, Kirch W, Boehler S, Lehnert H, Hoefler M, Unger T, Sharma AM. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled[ast] American journal of hypertension. 2004;17:904–910. doi: 10.1016/j.amjhyper.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Prevalence of uncomplicated obesity in an italian obese population[ast][ast] Obesity. 2005;13:1116–1122. doi: 10.1038/oby.2005.130. [DOI] [PubMed] [Google Scholar]
- 8.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 9.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 10.Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- 11.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 12.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 13.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: Measurement of plasma leptin and ob rna in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 14.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 15.Belin de Chantemele EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW. Protein tyrosine phosphatase 1b, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120:753–763. doi: 10.1161/CIRCULATIONAHA.109.853077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correia MLG, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 18.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: New insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 19.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–414. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 20.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 21.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 23.Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–1252. doi: 10.1172/JCI8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correia MLG, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension. 2001;37:936–942. doi: 10.1161/01.hyp.37.3.936. [DOI] [PubMed] [Google Scholar]
- 25.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: Role of leptin and sympathetic nervous system. American journal of hypertension. 2001;14:103S–115S. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- 26.Young JB, Landsberg L. Diminished sympathetic nervous system activity in genetically obese (ob/ob) mouse. The American journal of physiology. 1983;245:E148–154. doi: 10.1152/ajpendo.1983.245.2.E148. [DOI] [PubMed] [Google Scholar]
- 27.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1b gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 28.Schreihofer AM, Hair CD, Stepp DW. Reduced plasma volume and mesenteric vascular reactivity in obese zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R253–261. doi: 10.1152/ajpregu.00498.2004. [DOI] [PubMed] [Google Scholar]
- 29.Romanko OP, Stepp DW. Reduced constrictor reactivity balances impaired vasodilation in the mesenteric circulation of the obese zucker rat. Am J Physiol Heart Circ Physiol. 2005;289:H2097–2102. doi: 10.1152/ajpheart.00213.2005. [DOI] [PubMed] [Google Scholar]
- 30.Saiki A, Ohira M, Endo K, Koide N, Oyama T, Murano T, Watanabe H, Miyashita Y, Shirai K. Circulating angiotensin ii is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Metabolism. 2009;58:708–713. doi: 10.1016/j.metabol.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 31.de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41–47. doi: 10.1161/01.HYP.0000105624.68174.00. [DOI] [PubMed] [Google Scholar]
- 32.Mark AL, Sivitz WI. Uncoupling metabolism and coupling leptin to cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:881–883. doi: 10.1161/01.atv.0000023181.54279.fe. [DOI] [PubMed] [Google Scholar]
- 33.Fray JC. Stimulus-secretion coupling of renin. Role of hemodynamic and other factors. Circ Res. 1980;47:485–492. doi: 10.1161/01.res.47.4.485. [DOI] [PubMed] [Google Scholar]
- 34.Osborn JL, Kopp UC, Thames MD, DiBona GF. Interactions among renal nerves, prostaglandins, and renal arterial pressure in the regulation of renin release. Am J Physiol Renal Physiol. 1984;247:F706–713. doi: 10.1152/ajprenal.1984.247.5.F706. [DOI] [PubMed] [Google Scholar]
- 35.Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: Obesity-induced hypertension: New concepts from the emerging biology of obesity. Hypertension. 1999;33:537–541. doi: 10.1161/01.hyp.33.1.537. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JL, Morgan DA, Correia MLG, Mark AL, Sivitz WI, Haynes WG. Does leptin stimulate nitric oxide to oppose the effects of sympathetic activation? Hypertension. 2001;38:1081–1086. doi: 10.1161/hy1101.096053. [DOI] [PubMed] [Google Scholar]
- 37.Van Harmelen V, Reynisdottir S, Eriksson P, Thörne A, Hoffstedt J, Lönnqvist F, Arner P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 38.D’Angelo G, Mintz JD, Tidwell JE, Schreihofer AM, Pollock DM, Stepp DW. Exaggerated cardiovascular stress responses and impaired {beta}-adrenergic-mediated pressor recovery in obese zucker rats. Hypertension. 2006;48:1109–1115. doi: 10.1161/01.HYP.0000247306.53547.d4. [DOI] [PubMed] [Google Scholar]
- 39.Narkiewicz K, Somers VK, Mos L, Kato M, Accurso V, Palatini P. An independent relationship between plasma leptin and heart rate in untreated patients with essential hypertension. J Hypertens. 1999;17:245–249. doi: 10.1097/00004872-199917020-00009. [DOI] [PubMed] [Google Scholar]
- 40.Gardiner SM, Kemp PA, March JE, Bennett T. Regional haemodynamic effects of recombinant murine or human leptin in conscious rats. British journal of pharmacology. 2000;130:805–810. doi: 10.1038/sj.bjp.0703381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agapitov AV, Correia MLdG, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension. 2008;52:687–695. doi: 10.1161/HYPERTENSIONAHA.107.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egan BM, Schork NJ, Weder AB. Regional hemodynamic abnormalities in overweight men. Focus on alpha-adrenergic vascular responses. American journal of hypertension. 1989;2:428–434. doi: 10.1093/ajh/2.6.428. [DOI] [PubMed] [Google Scholar]
- 43.Mingorance C, Alvarez de Sotomayor M, Jimenez-Palacios FJ, Callejon Mochon M, Casto C, Marhuenda E, Herrera MD. Effects of chronic treatment with the cb1 antagonist, rimonabant on the blood pressure, and vascular reactivity of obese zucker rats. Obesity (Silver Spring, Md. 2009;17:1340–1347. doi: 10.1038/oby.2009.20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.