Abstract
The consistent association between adolescent sexual initiation (ASI) and risky adult sexual behavior (RASB) has sometimes been interpreted as causal, with the resulting assumption that delaying ASI will reduce RASB. Yet the ASI-RASB association might be better accounted by some third variable. We evaluated the causal role of ASI (initiation of oral, anal, or vaginal sex at or before age 16) on RASB in a longitudinal sample of 2173 twins (followed from age 11 to 24 or from 17 to 29) using two methods: discordant twin and propensity score design. The former controlled for unmeasured genetic and shared environmental factors while the latter controlled for measured non-shared environmental factors. We replicated the link between ASI and RASB reported previously, but results from the discordant twin and propensity score analyses suggested that this association is better explained by common genetic and/or environmental risk factors. These findings suggest that preventing ASI is unlikely to reduce RASB.
Initiation of sexual behavior in early and middle adolescence, defined as initiation of oral, anal, or vaginal sex at or before 16 years (Cavazos-Rehg et al., 2010) is associated with contraction of sexually transmitted diseases, increased number of lifetime sexual partners, and frequency of sex under the influence of drugs or alcohol (Seidman et al, 1994, Dickson et al., 1998; Sandfort et al., 2008). Further, adolescent sexual initiation is associated with having or contributing to an adolescent pregnancy (Wellings et al, 2001).
The consistent link between adolescent sexual initiation (ASI) and risky adult sexual behavior (RASB) has had a strong influence in shaping public policy regarding sex education. Twenty-two states mandate that abstinence be stressed in school curricula, another 12 states require thorough coverage, while only 15 states require mention of contraceptive methods (Alan Guttmacher Institute, 2009). The aim of abstinence only education programs is to delay sexual initiation until marriage, with one of the major goals being to reduce the rates of RASB and early pregnancy. Notably, proponents for abstinence-only education draw arguments based on the crucial assumption that ASI causes later RASB. Stated otherwise, a causal assumption suggests that delaying sexual initiation will decrease RASB.
However, the association between ASI and later RASB may be better accounted for by some third variable. Indeed, environmental risk factors such as family SES (Caminis et al., 2007) and negative peer influences (Svenson & Hanson, 1996) can give rise to both ASI and RASB. Alternatively, both ASI and RASB may be manifestations of a genetic predisposition to engage in disinhibited behavior (Donohew et al., 2000; Caspi et al., 1997; McGue & Iacono, 2005). If either of these competing explanations is true, then delaying ASI might be ineffective in reducing RASB.
Unfortunately, few studies have tested the causal assumption empirically. Here, we use two approaches to test the causal assumption of ASI: discordant twin and the propensity score method. The discordant twin design involves an analysis of members of monozygotic (MZ) and dizygotic (DZ) twin pairs that differ on ASI. The logic of this design is that twin similarity is due to additive genetic effects—which are shared completely by MZ twins but only 50% by DZ twins—and shared environmental effects that are shared completely by both MZ and DZ twins. Consequently, an association between ASI and RASB might reflect causality, or it might represent common genetic or shared environmental effects. Associations within MZ and DZ twin pairs discordant for ASI control for shared environmental effects (i.e., environmental effects that contribute to twin similarity), while associations within discordant MZ twin pairs control for genetic effects (discordant DZ twin pairs provide a partial genetic control) (McGue et al., 2010).
However, twins could differ on factors present prior to ASI, and these pre-existing differences might then account for discordance in ASI and the link between ASI and RASB. For instance, factors such as early puberty, deviant peer affiliation, prior drug/alcohol use, or previous romantic relationships might contribute to ASI discordance and later RASB. In this case, the propensity score method adds additional rigor to testing the causal effect of ASI on RASB (Rosenbaum & Rubin, 1983). A propensity score is a single variable obtained by modeling the probability of being in the ASI vs. non-ASI group as a function of covariates that existed prior to the “ASI event” (Rosenbaum & Rubin, 1983; 1984). Review of the ASI literature indicates these covariates include SES, parental psychopathology, parental sexual history, parent-child relationships, externalizing and internalizing psychopathology, peers, level of psychosexual maturity, and stressful life events (Paul et al, 2000; Zimmer-Gembeck & Helfand, 2008). The propensity score mathematically mimics randomized group assignment based on measured covariates when true randomization is not possible. As the discordant design controls for unobserved genetic and shared environmental contributions to RASB, the propensity score provides partial control of measured non-shared environmental effects (i.e., environmental effects that contribute to differences between members of a twin pair). The use of a combined discordant twin and propensity score design thus provides an especially rigorous test of causality.
The current study employed this design to evaluate the causal relationship between ASI and RASB. We expected that, similar to what has been reported previously, ASI would predict RASB. In testing the causal assumption, however, we stipulated several specific hypotheses. First, if ASI has a direct and causal influence on RASB, then within twin pairs, only the twin with ASI (hereinafter referred to as the “affected twin”) would be expected to develop RASB. If shared genetic and environmental risk accounts for the ASI-RASB association, then both the affected and unaffected twin would develop RASB. If genetic risk per se contributed, then MZ twins would be more similar on RASB than DZ twins. If shared environmental risk alone contributed, then the similarity in RASB would be comparable for MZ and DZ twins discordant for ASI. Finally, if pre-existing twin differences that predict ASI account for the ASI-RASB association, then the propensity score a) would be significantly different in twins discordant on ASI, b) would be related to RASB, and c) reduce any effect of ASI on the outcomes in both MZ and DZ twins.
Method
Sample
Participants were same-sex twin pairs taking part in the ongoing, longitudinal Minnesota Twin Family Study (MTFS). Families were identified using public birth records of twins born in Minnesota between 1972 to 1984, and recruited into the study at age 11 or 17 (see Iacono et al., 1999 for a full description of the MTFS sample and study design).
The current study primarily utilized data from two assessments administered the year the twins turned 11 and 24 years old. Data used to calculate the propensity score was collected at age 11 as no participants reported engaging in sexual intercourse at or prior to this age. Importantly, we aimed to keep the timeline of predictors, ASI, and the outcomes clearly separated. Thus, the covariates for the current study were measured at age 11 so that it can be certain that the predictor variables were not a consequence of ASI.
To increase our power, we augmented our sample by utilizing data from the older cohort whose intake occurred at age 17. Although a propensity score could not be calculated for participants from this older cohort, everyone had data for ASI and early pregnancy; only the female twins from this older cohort completed the full assessment of RASB1. As a result, the number of discordant pairs was increased by 85% for analyses based on early pregnancy, and by 54% for the other RASB data.
The final sample included 1044 twins from the 11-year old and 1129 twins from the 17-year old cohort that completed their respective adult assessments. Only participants who were sexually active at the follow-up (i.e., had at least one lifetime sexual experience of oral, anal, or vaginal sex) were included in the study as including abstinent participants might inflate the differences between affected and unaffected groups2. In some cases, one of the members of the twin pair had not completed the age 24 or 29 assessment (n=154) so that concordance could not be determined. Of the remaining 1082 pairs, 691 were MZ (51.8% female) and 391 DZ (54.5% female). One hundred fifty-eight MZ pairs and 128 DZ pairs were ASI-discordant. ASI probandwise concordance was 64% and 52% for MZ and DZ pairs, respectively. The mean age of sexual initiation was 15.27 years (SD=.97) for affected twins, and 19.24 years (SD = 2.36) for unaffected twins. All twins were included in the regression analysis regardless of concordance status to estimate both between-pair effects (for which the concordant pairs are informative) as well as within-pair effects (for which only the discordant pairs are informative).
Over 95% of the twins were Caucasian, reflecting the ethnic composition of Minnesota for the birth years sampled. The mean age of participants at the age 24 and 29 assessments was 25.26 (SD = .72) and 29.62 (SD = .62) years. As sexual risk-taking is correlated with age (Dariostis et al., 2008), the age of assessment and cohort were included as covariates in all analyses.
Measures
Sexual Behavior Inventory (SBI)
ASI and RASB were measured via the SBI, a self-report instrument administered at the age 24 assessment. The SBI assesses the age of onset and frequency of oral sex and sexual intercourse (vaginal or anal) with romantic and casual partners as well as recent sexual risk behavior. ASI was coded “0” if first oral sex or sexual intercourse occurred after age 16, and coded “1” if first it occurred at or before age 16 (35% of the sample)3. We used age 16 as the ASI cutoff because it has been used previously (Cavazos-Rehg et al., 2010), and based on our sample characteristics age 16 provide a good balance between being “risky enough” to be considered a risk factor while also providing sufficient power (number of discordant pairs) for within-pair effects4.
RASB included six variables derived from SBI items:1) lifetime number of regular partners; 2) lifetime number of casual partners; 3) past year number of regular partners; 4) past-year number of casual partners; 5) past year sexual behavior under the influence of drugs/alcohol, and 6) early pregnancy. The number of regular and casual partners (lifetime and past year) was reported using a 6-point scale ranging from 1 (0 partners) to 6 (>20 partners). The scale reflecting sexual behavior under the influence of drugs/alcohol includes 4 items (range 0=never to 4=more than 10 times) and assesses the frequency that alcohol and drug use: a) increased the participant’s decision to do something sexual; b) helped the participant feel more comfortable with his/her sexual partner; c) lead to more sexual activity than the participant was comfortable with; and d) lead to unprotected sex over the past year. All variables were standardized to ease interpretation, and natural log transformations were made on all but the early pregnancy variable. Notably, the male and female twins from the younger cohort and female twins from the older cohort completed this measure.
Early pregnancy was assessed by asking “How old were you when you first become pregnant?/How old were you when you got your partner pregnant?”, and coded 0 (no pregnancy before age 20) or 1 (pregnancy reported at or before age 20). We made efforts to increase honest and accurate responding on the SBI by having participants complete the form in private using only a numeric code rather than their names. All participants across cohorts provided early pregnancy data. Probandwise concordance for early pregnancy was 30% for MZs and 26% for DZs.
ASI Risk Factors at Age 11
Table 1 lists the MTFS measures used to tap the content domains of risk factors for ASI identified in prior research (Zimmer-Gembeck & Helfand, 2008). These domains include parent-child relationships, externalizing and internalizing psychopathology, peer factors, psychosexual development, and stressful life events. Because the discordant twin design already controls for shared environment effects, there are no family-level variables in the propensity score (i.e., variables than cannot differ for twins such as SES). We took advantage of the extensive MTFS assessment battery to create composite variables where possible.
Table 1.
Measures, descriptions, and reliability of ASI Risk Factors
| Assessment | Measure | Description | Reliability |
|---|---|---|---|
| Familial Variables | |||
| Parent-child conflict | Parental Environment Questionnaire (Elkins et al, 1997) | Composite of mother and child ratings of parent-child conflict | α= 78 to .87 |
| Stressful Life Events | Life Events Interview (Billlig et al, 1996) | Stressful life occurrences (e.g., being bullied by other kids) | ICC = .89 |
| Childhood Trauma | Assessment of Childhood Maltreatment Derringer et al (2010). | Childhood experiences of sexual, physical, emotional abuse | α= .91 |
| Academic and Religious History | |||
| Estimated Full-Scale IQ | Wechsler Intelligence Scale for Children-Revised (Wechsler, 1974) | Verbal (Vocabulary and Information) and Performance (Block Design and Object Assembly) scales | Inter-item r = .36 |
| Puberty | Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988) | Level of development of secondary sex characteristics | N/A |
| Academic history | Academic History Questionnaire (Johnson et al, 2006) | Composite of mother, teacher, and child ratings for measures of academic motivation, GPA, expected academic attainment, and school behavior problems. | ICC = .77 |
| Self esteem | Piers-Harris Children’s Self-Concept Scale (Piers, 1984; Neiss, et al, 2009) | Self report assessment of one’s own behavior and attributes | α= .73 |
| Religiosity | Religious Background and Practices Questionnaire (Koenig et al, 2005) | Self report of the frequency and importance of religious practices | α= .88 |
| Psychopathology/Drug Use | |||
| Externalizing Factor | Diagnostic Interview for Children and Adolescents-Revised (DICA-R; Reich & Welner, 1998) Teacher Rating Form (Walden et al, 2004) Delinquent Behavior Inventory (Blazei, Iacono, & McGue, 2008). | First principal component of mother, teacher, and child report of ADHD, CD, and ODD symptoms, and mother and child reports on the Delinquent Behavior Inventory | Kappas= .71–.81 α= .80–.94 |
| Negative Affect Factor | DICA-R Teacher Rating Form | First principal component of mother, teacher, and child reports of depression and anxiety symptoms | Kappas = .72–.82 |
| Substance Use Initiation before Age 11 | Computerized Substance Use Interview (Malone, Iacono, & McGue, 2002) | Has the participant ever used drugs or alcohol at or before intake? | N/A |
| Social/Environmental Factors | |||
| Prosocial Peers | Friends Inventory (Walden et al, 2004) Teacher Rating Form | Composite of child and teacher ratings of prosocial peers | α= .79–.86 |
| Deviant Peers | Friends Inventory Teacher Rating Form | Composite of child and teacher ratings of deviant peers | α= .78–.84 |
| Number of Friends | Social Adjustment Interview (Johnson et al, 2007; Elkins et al, 1997) | Self-report of total number of friends | N/A |
| Interest in Opposite Sex | Social Adjustment Interview | Self-report of interest in opposite sex | α=.71 |
| Friendliness | Social Adjustment Interview | Self-report ratings of extraversion or friendliness (e.g., Is it easy or hard for you do make new friends?) | N/A |
| Dating Initiation before Age 11 | Life Events Inventory | Has the participant ever dated at or before intake? | N/A |
Note: Diagnostic reliability was calculated from the kappa coefficient, scale internal consistency was evaluated using Cronbach’s α, the reliability of a composite measure composed of multiple scales was determined via mean inter-scale correlations, and reliability of composite measures that involve the ratings across multiple informants was calculated via the inter-class correlation coefficient (ICC). Finally, mean inter-item correlations were used to index correlations among categorical items (e.g., experience of some type of negative life event such as rape) composing a scale. When reliability = N/A, then α or inter-item correlations could not be computed due to the small number of items (3 or less).
Data Analyses
Calculation of Propensity Score
To determine the variables to use to calculate the propensity score, a series of univariate ANOVAs examined the differences between ASI and non-ASI 11-year old participants on the putative risk factors. Next, we entered these predictors into a logistic regression predicting ASI status. The predicted probability of ASI served as a propensity score, and was used as a covariate to adjust for pre-existing differences that may have contributed to ASI discordance.
Regression Analyses of Individual-level and Twin-Level Data
A mixed-level regression framework (Begg & Parides, 2003) predicting our key outcome variables was used to investigate the individual-level, within twin pair, and between twin pair effects of ASI on RASB, before and after controlling for the ASI propensity score. An individual-level effect [ASIIND] is estimated by regressing RASB on ASI, regardless of the status of a co-twin, and simply tests for the positive association between ASI and RASB.
In the discordant-twin analysis, the regression of the outcome on ASI is decomposed into a within [ASIW] and a between [ASIB] pair effect (Begg & Parides, 2003). The ASIB effect is similar to the ASIIND effect, as it is a reasonable approximation for the association of exposure with outcome uncorrected for genetic and shared environmental confounding. The ASIW effect gives the difference in outcome for the members of a twin pair who are discordant for ASI. The ASIW effect is the main interest of this study, as it measures the similarity between ASI discordant twins. Finally, each model provides a zygosity x ASIW interaction term. This interaction indicates whether this effect is significantly different across MZ and DZ twins. It also indicates whether the effect of ASI and RASB stems from genetic confounding (nonsignificant ASIW in MZ pairs, significant in DZ pairs, and significant zygosity x ASIW interaction), shared environmental factors (nonsignificant ASIW for both MZ or DZ pairs, and nonsignificant zygosity x ASIW), or causal influences (ASIW is significant for both MZ or DZ pairs and nonsignificant zygosity x ASIW). As a supplement to the latter analysis, we also examined twin correlations as an additional index of genetic and shared environmental influences on RASB.
For each outcome, we fit three mixed-level regression models using PROC MIXED in SAS. First, we determined whether ASIIND was associated with each of the six RASB measures. In this step, we also tested if the effect of ASI on RASB variables differs by gender. If the interaction term was significant in this analysis, subsequent models were computed separately for males and females5. Next, we examined whether the ASI twin is more likely to have RASB than the non-ASI twin in a discordant twin regression model.
The final model included the ASI propensity score as a covariate. Here, the aim was to determine whether any within-pair effects could be accounted for by twin differences in the propensity score. As appropriate, the clustered nature of the twin data was accounted for using either mixed-level analyses for quantitative outcomes or generalized estimating equations for early pregnancy, the only categorical outcome.
Results
Calculating Propensity Scores
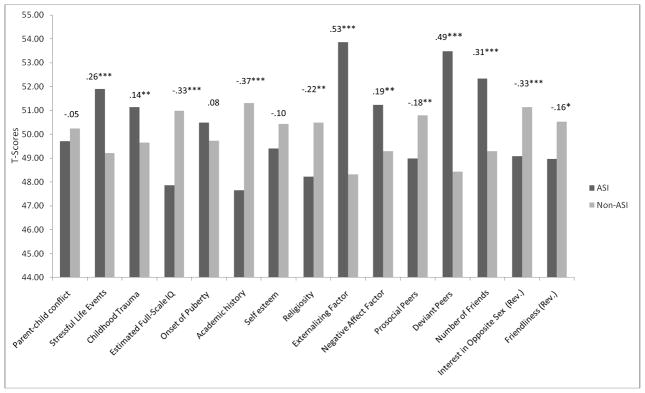
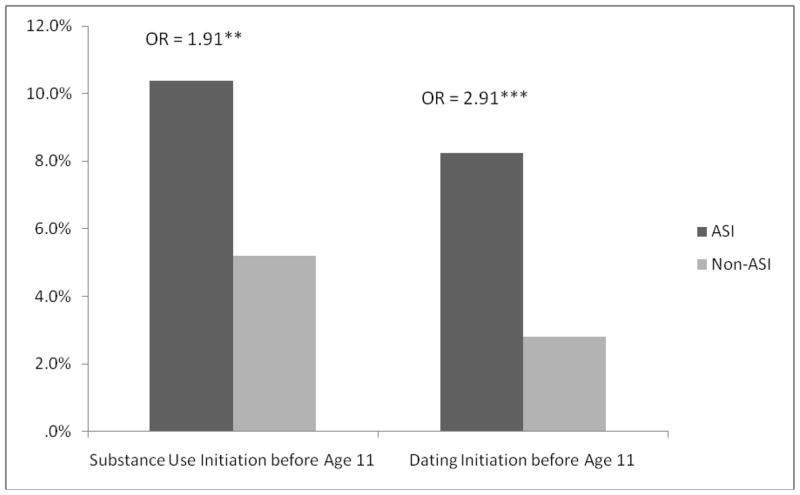
Fourteen of 17 predictors significantly differentiated the ASI and non-ASI participants (Figures 1 and 2). In all but one case (parent-child conflict), the ASI participants scored in the more deviant direction than non-ASI participants. Propensity scores were estimated in a multivariate logistic regression in which ASI status was predicted by the 14 significant predictors. The regression correctly classified 86.8% of adolescents in the non-ASI group, and 36.2% of adolescents in the ASI group, with an overall accurate classification rate of 68.6% (omnibus χ2(14) =156.04, p<.001), accounting for 19% of the variance in ASI (Nagelkerke R2=.19). The predicted probability score was used as the propensity score in further analyses. Affected twins exhibited higher propensity scores than unaffected twins [F(1, 133)=8.56, p<.004], indicating that the propensity score captures important but otherwise unaccounted for pre-existing differences between twins associated with ASI. Additionally, the propensity score was significantly correlated with each outcome variable except past year regular partners (rs = .08–.24, ps < .05 for continuous outcomes; F(1, 1042) = 32.56, p < .001 for early pregnancy).
Figure 1.
Differences between ASI and non-ASI participants on contunuous risk factors.
Note: All variables are standardized (T-scored). Numbers above each set of bars represent effect size (Cohen’s d) and significance level. * <.05; ** < .01; ***< .001. (R) indicates reverse scoring (higher score = lower on construct).
Figure 2.
Differences between ASI and non-ASI participants on categorical risk factors.
Note: Bars represent the percentage of individuals endorsing “yes” on a given variable. Numbers above the bars represent effect size (odds ratios) and significance level. * <.05; ** < .01; ***< .001.
Does ASI predict RASB?
There was a significant effect of ASIIND on each measure of RASB except lifetim e number of regular partners (Table 2). Gender was also a significant predictor for these outcome variables, with men reporting higher levels of sexual risk taking than women. Additionally, the gender x ASIIND interaction was significant for past year and lifetime casual partners and sexual behavior under the influence of drugs/alcohol. Specifically, the association between ASI and these three outcomes was stronger for men. For these three outcome variables, the models were fit separately across gender. The effect of ASIIND on past year number of casual partners was significant among men but not women. The ASIIND effect on sexual behavior under the influence of drugs/alcohol was stronger for men than women, although in both cases it reached statistical significance. Finally, for the lifetime partner variables there was a reasonable concern that individuals who have been having sex longer are more likely to acquire more lifetime partners. Therefore, for these variables, we also controlled for “sexual opportunity” (current age – age of sexual initiation). The ASIIND effect remained significant for lifetime number of casual partners but only for men.
Table 2.
Relationships between ASI and RASB outcomes at an individual level, gender effects, and gender X ASI interactions
| Outcome Variable | Past year number of regular partners | Past year number of casual partners | Past year sex behavior under influence of drugs/alcohol | Lifetime number of casual partners | Lifetime number of regular partners | Early pregnancy |
|---|---|---|---|---|---|---|
| ASIIND | .12 (.05)** | .24 (.05)*** | .25 (.05)*** | .02 (.07) | .03 (.06) | 1.18 (.15)*** |
| Gender | −.11 (.06)* | .45 (.06)*** | .30 (.07)*** | .25 (.07)*** | .02 (.05) | −.73 (.15)*** |
| ASIIND X Gender | −.15 (.10) | .58 (.11)*** | .30 (.11)** | .54 (.10)*** | .18 (.09) | .37 (.31) |
| Sexual opportunity | N/A | N/A | N/A | .32 (.04)*** | .45 (.04)*** | N/A |
Note: ASI, adolescent sexual initiation; early pregnancy: ASIIND refers to the fact that this is an individual-level regression (using all participants); early pregnancy, having been pregnant or having made someone pregnant before age 20; sexual opportunity: current age - time at initial sexual experience. All variables but early pregnancy (which was categorical) were z-scored. Unstandardized regression weights [B(SEs)] are presented. The gender effect in early pregnancy is in the same direction as all other gender effects (higher RASB in men), despite the opposite sign.
<.05;
< .01;
< .001.
How similar in RASB outcome are twins who were discordant for ASI?
Discordant twin models were fit to compare ASI effects within MZ and DZ pairs. As noted above, discordant twin models for past year number of casual partners was limited to men as the ASI effect was not significant for women.
The results for the first set of discordant twin models are reported in Table 3, and twin correlations for each RASB variable are provided as well. For nearly every RASB outcome, the effect of ASIW was not significant for both MZ and DZ twins. Moreover, the standard error for most effects was larger than the estimate, and the effects were close to zero in six out of twelve cases. These results indicate that the affected and nonaffected twins within a pair did not generally differ in RASB, especially for women. The two exceptions were for men on past year number of casual partners (MZ twins only) and past year sexual behavior under the influence of drugs/alcohol (DZ twins only). Additionally, despite a few instances where the MZ twin correlations were small to moderate and the DZ correlations close to zero, the nonsignificant ASIW x zygosity interactions indicated no difference in MZ and DZ twin similarity for RASB
Table 3.
Twin Correlations and relationships between ASI and sexual risk outcomes within twin pairs before and after controlling for propensity score.
| RASB Outcome Variable | rfMZ | rDZ | MZ Twins | DZ Twins | ||
|---|---|---|---|---|---|---|
| Model (B, SE) | Model (B, SE) | |||||
| Without Propensity Score | With Propensity Score | Without Propensity Score | With Propensity Score | |||
| n of pairs | 158 | 128 | ||||
| Past year number of regular partners | ||||||
| Twin Correlation | .17 | .13 | ||||
| ASIB | 19 (.07)** | .18 (.10) | .12 (.10) | .13 (.13) | ||
| ASIW | −.03 (.10) | −.14 (.14) | .08 (.12) | −.04 (.15) | ||
| Zygosity* ASIW | .10 (.16) | −.10 (.21) | ||||
| Propensity Score | −.01 (.03) | |||||
| Past year number of casual partners among men | ||||||
| Twin Correlation | .32* | .01 | ||||
| ASIB | .68 (.17)*** | .57 (.18)** | .49 (.25) | .36 (.26) | ||
| ASIW | .78 (.30)** | .79 (.30)** | .46 (.30) | .40 (.30) | ||
| Zygosity* ASIW | −.33 (.42) | −.39 (.42) | ||||
| Propensity Score | .10 (.06) | |||||
| Past year sexual behavior under influence of drugs/alcohol among men | ||||||
| Twin Correlation | .10 | −.11 | ||||
| ASIB | .38 (.16)* | .38 (.18)* | .30 (.24) | .30 (.25) | ||
| ASIW | .50 (.26) | .50 (.26) | .76 (.26)** | .76(.26)** | ||
| Zygosity* ASIW | .26 (.37) | .26 (.37) | ||||
| Propensity Score | .00 (.05) | |||||
| Past year sexual behavior under influence of drugs/alcohol among women | ||||||
| Twin Correlation | .44** | .21 | ||||
| ASIB | .25 (.09)** | .01 (.15) | .16 (.12) | −.02 (.19) | ||
| ASIW | .03 (.11) | −.01 (.18) | .14 (.13) | .34 (.22) | ||
| Zygosity* ASIW | .11 (.17) | .34 (.28) | ||||
| Propensity Score | .04 (.05) | |||||
| Lifetime casual partners among men | ||||||
| Twin Correlation | .26* | .14 | ||||
| ASIB | .70 (.17)*** | .64 (.17)** | .25 (.23) | .19 (.24) | ||
| ASIW | .40 (.23) | .41 (.23) | .12 (.24) | .10 (.24) | ||
| Zygosity* ASIW | −.28 (.31) | −.32 (.31) | ||||
| Sexual opportunity | .49 (.10)*** | .46 (.10)*** | ||||
| Propensity Score | .07 (.05) | |||||
| Early Pregnancy | ||||||
| Twin Correlation | .14 | .04 | ||||
| ASIB | 1.82(.22)*** | .94 (.37)** | 1.59(.31)*** | 1.17 (.64) | ||
| ASIW | −.11 (.34) | −.52 (.54) | .34 (.41) | −.08 (.61) | ||
| Zygosity* ASIW | .45 (.53) | .44 (.81) | ||||
| Gender | −.74(.15)*** | −.97(.26)*** | ||||
| Propensity Score | .50 (.14)*** | |||||
Abbreviations: MZ, monozygotic; DZ, dizygotic; ASI, adolescent sexual initiation; early pregnancy: having been pregnant or having made someone pregnant before age 20 (coded yes/no); sexual opportunity: current age - time at initial sexual experience. All variables but early pregnancy (which was categorical) were z-scored. Unstandardized regression weights are presented. If ASI failed to predict an outcome in the individual-level model, we did not use that variable as an outcome measure in the discordant twin models (number of lifetime partners). If the ASI x Gender interaction was significant and ASI predicted the outcome for both genders, we report the results of the discordant twin model separately for each gender. If the ASI x Gender was significant and ASI predicted the outcome for only one gender, we report the results of the discordant twin model for that gender only. The first model termed “without propensity score” is a discordant twin model, testing the within-pair (ASIW) and between-pair (ASIB) effects on the outcomes among MZ and DZ twins. In this model, zygosity*ASIW tests whether the within-pair effect is significantly different across zygosity. The second model, termed “with propensity score” is identical to the first one, except for the addition of the propensity score. The former model included both the younger and older cohort, whereas the latter included only the younger cohort, as propensity score variables were available only for that cohort. Propensity score is identical for MZ and DZ pairs; thus, it is presented only once per model (in MZs).
<.05;
< .01;
< .001.
Can the association between ASI and ASB be accounted for by pre-existing risk factors associated with ASI?
Finally, we included the ASI propensity score in the twin models to adjust for pre-existing differences between members of a twin pair. After controlling for the within and between pair differences in ASI, the propensity score was significant only for early pregnancy, indicating that some of the effect of ASI on this outcome can be accounted for by pre-existing differences.
Discussion
In the current study, we tested whether the ASI-RASB association is consistent with the causal assumption inherent in the logic of abstinence-only programs using a longitudinal twin study that follows the twin pairs from ages 11 to 24. Moreover, we used a novel approach combining the discordant twin design and propensity score method. This allowed us to control for common genetic and environmental factors between ASI and RASB, as well as twin differences in risk prior to ASI that might account for the association between ASI and RASB.
We replicated the general association between ASI and most RASB variables (Sandfort et al., 2008; Seidman et al., 1994; Wellings et al., 2001). Results from our discordant twin regression models, however, indicated that some combination of genetic and environmental factors accounted for the association between ASI and RASB as twins discordant on ASI were similar in RASB, with the zygosity interaction analyses indicating no significant difference between MZ and DZ twins. Additionally, the propensity score for ASI was significantly associated with early pregnancy, indicating that differences between members of twin pairs prior to ASI are also important for this particular outcome. These findings are consistent with the interpretation that ASI per se is unlikely to play a causal role in most types of RASB; rather their association is largely a consequence of common risk factors that influence both ASI and RASB.
The one exception was for past-year casual partners, for which there was evidence of a causal effect of ASI, but only for men. Given the failure to detect a causal effect for the other outcome variables, we are hesitant to interpret these findings as strong evidence of a causal effect of ASI on RASB in men. Although we may have detected this effect simply by chance, we cannot rule out the possibility that ASI may be a specific environmental risk exposure for a particular type of sexual risk behavior in men.
Nevertheless, the failure to find causal associations for most of the RASB outcomes has several implications for public health programs that aim to reduce early pregnancy and other RASB. From our results, it follows that attempting to delay sexual initiation per se might not affect many types of sexual risk-taking in early adulthood. These findings are consistent with a comprehensive review of sex education programs that found abstinence based programs failed to influence adolescent sexual behavior (Kirby, 2007). Furthermore, research suggests “virginity pledges” may be successful in delaying ASI up to 18 months, however pledgers and non-pledgers have similar rates of sexually transmitted infections in early adulthood (Brückner & Bearman, 2005).
More broadly, our results speak to the need to submit the assumptions underlying public policies designed to reduce harmful outcomes to rigorous empirical testing. Our failure to detect a consistent pattern of causal effects of ASI on RASB should be considered in the context of what is known about adolescent problem behaviors and adult psychopathology more generally. Specifically, McGue and Iacono (2005) found that different adolescent problem behaviors (precocious sexual intercourse, substance use, police involvement) all predict a diverse set of adult disorders (substance use disorders, antisocial behavior, major depression), suggesting there is a group of youth who are at an especially high and generalized risk for poor outcomes in adulthood. These findings suggest that even if successful, prevention and intervention for specific behaviors may not forestall deleterious adult outcomes. Rather, resources would be best allocated to identify those at highest risk and then provide comprehensive, individualized interventions (e.g., multi-systemic therapy, Curtis et al., 2004; Henggeler & Schaeffer, 2010; Klietz et al., 2010) instead of universal prevention and intervention efforts.
Despite strong results, it is important to be cautious in interpreting the current findings. First, regardless of its strengths, no research design can ever “prove” the null hypothesis. Second, the current study focuses on a specific definition of ASI: namely, any type of sexual initiation (oral sex or sexual intercourse) before the age of 16. Results may be different if ASI is defined differently or when using an earlier age. Third, though we failed to detect many causal effects of ASI on RASB, we are not suggesting that ASI has no negative consequences. Indeed, ASI may increase risk for major depression and substance use. Thus, future studies might utilize the current method for testing whether the results differ by ASI definition and examining the causal role of ASI on other negative outcomes.
Moreover, although we took care to maximize the power by including the older cohort when possible, we lacked the power needed to detect small effects in ASIW in MZ twins. Additionally, we were powered to detect only large effects for the zygosity X ASIW interactions (a common problem in discordant twin designs). As such, though MZ twins did not differ significantly from DZ twins, we cannot disentangle genetic from shared environmental effects.
Despite these caveats, the current research has utilized a powerful test of causality, and the results are compelling. Future research can extend these findings by providing insight into the factors that do contribute to such behavior, which can then be used to inform the design of interventions that ultimately reduce RASB.
Acknowledgments
This work was supported by National Institute on Drug Abuse Grant DA05147, National Institute on Alcohol Abuse and Alcoholism grants AA09367 and AA015621, and National Institute on Mental Health grant T32 MH017069.
Footnotes
The female twins from the older cohort completed the full assessment at age 29. Age and cohort were used as covariates in all analyses.
We re-analyzed the data with and without the abstinent participants, and the results were essentially unchanged.
We repeated the analyses, this time with age 15 and age 17 as our cutoff ages for ASI. At age 15, 16.3% of the sample was ASI-exposed. At age 17, 50% of the sample was ASI-exposed. The outcomes and interpretations were unchanged. That is, in no cases did a non-significant within-pair effect become significant.
With any variable that is measured via retrospective reporting, there is a concern of response distortion. Thus, we examined the correlation of our ASI measure with another, longitudinal ASI index. This second index was an interview question on a separate measure: “How old were you when you started having sex?” asked at ages 14, 17, 24, and 29. We chose not to use the interview-based question because of the very sensitive nature of the topic and social desirability concerns. Nevertheless, the correlation of our retrospective ASI measure with the mean of the responses to the interview question across the four time points was .78.
We also tested the interactions by examining whether the betas are significantly different between males and females. In every case where we detected a significant gender interaction, the male and female betas differed significantly from each other.
References
- Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Statistics in Medicine. 2003;22:2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- Blazei RW, Iacono WG, McGue M. Father-child transmission of antisocial behavior: The moderating role of father’s presence in the home. Child and Adolescent Psychiatry. 2008;47:406–415. doi: 10.1097/CHI.0b013e3181642979. [DOI] [PubMed] [Google Scholar]
- Billig JP, Hershberger SL, Iacono WG, McGue M. Life events and personality in late adolescence: Genetic and environmental relations. Behavior Genetics. 1996;26:543–554. doi: 10.1007/BF02361227. [DOI] [PubMed] [Google Scholar]
- Brückner H, Bearman PS. After the promise: The STD consequences of adolescent virginity pledges. Journal of Adolescent Health. 2005;36:271–278. doi: 10.1016/j.jadohealth.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Caminis A, Henrich C, Ruchkin V, Schwab-Stone M, Martin A. Psychosocial predictors of sexual initiation and high-risk sexual behaviors in early adolescence. Child and Adolescent Psychiatry and Mental Health. 2007;1:1–14. doi: 10.1186/1753-2000-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Moffitt TE, Begg D, Dickson N, Langley J, Silvia PA. Personality differences predict health-risk behaviors in young adulthood: Evidence from a longitudinal study. Journal of Personality and Social Psychology. 1997;73:1052–1063. doi: 10.1037//0022-3514.73.5.1052. [DOI] [PubMed] [Google Scholar]
- Cavazos-Rehg PA, Spitznagel EL, Bucholz KK. Predictors of Sexual Debut at Age 16 or Younger. Archives of Sexual Behavior. 2010;39:664–673. doi: 10.1007/s10508-008-9397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis NM, Ronan KR, Borduin CM. Multisystemic treatment: A meta-analysis of outcome studies. Journal of Family Psychology. 2004;18:411–419. doi: 10.1037/0893-3200.18.3.411. [DOI] [PubMed] [Google Scholar]
- Dariotis JK, Sonenstein FL, Gates GJ, Capps R, Astone NM, Pleck JH, Sifakis F, Zeger S. Changes in sexual risk behavior as young men transition to adulthood. Perspectives on Sexual and Reproductive Health. 2008;40:218–225. doi: 10.1363/4021808. [DOI] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Irons DE, Iacono WG. Harsh discipline, childhood sexual assault, and MAOA genotype: An investigation of main and interactive effects on diverse clinical externalizing outcomes. Behavior Genetics. 2010;40:639–48. doi: 10.1007/s10519-010-9358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson N, Paul C, Herbison P, Silva P. First sexual intercourse: age, coercion, and later regrets reported by a birth cohort. British Medical Journal. 1998;316(7124):29–33. doi: 10.1136/bmj.316.7124.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohew L, Zimmerman R, Cupp PS, Novak S, Colon S, Abell R. Sensation seeking, impulsive decision making, and risky sex: Implication for risk-taking and design of interventions. Personality & Individual Differences. 2000;28:1079–1091. [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Genetic and environmental influences on parent-son relationships: Evidence for increasing genetic influence during adolescence. Developmental Psychology. 1997;33:351–363. doi: 10.1037//0012-1649.33.2.351. [DOI] [PubMed] [Google Scholar]
- Guttmacher Institute. Sex and STI/HIV education. State Policies in Brief. 2009 Retrieved from http://guttmacher.org/statecenter/spibs/spib_SE.pdf.
- Henggeler SW, Schaeffer C. Treating serious antisocial behavior using multisystemic therapy. In: Weisz JR, Kazdin AE, editors. Evidence-based psychotherapies for children and adolescents. 2. New York: Guilford Press; 2010. pp. 259–276. [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-case disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Johnson W, McGue M, Iacono WG. Genetic and environmental influences on academic achievement trajectories during adolescence. Developmental Psychology. 2006;42:514–532. doi: 10.1037/0012-1649.42.3.514. [DOI] [PubMed] [Google Scholar]
- Johnson W, Hicks BM, McGue M, Iacono WG. Most of the girls are alright but some aren’t: Personality trajectory groups from ages 14 to 24 and some associations with outcomes. Journal of Personality and Social Psychology: Personality Processes and Individual Differences. 2007;93:266–284. doi: 10.1037/0022-3514.93.2.266. [DOI] [PubMed] [Google Scholar]
- Kirby D. Abstinence, sex, and STD/ HIV education programs for teens: Their impact on sexual behavior, pregnancy, and sexually transmitted diseases. Annual Review of Sex Research. 2007;18:143–117. [Google Scholar]
- Klietz SJ, Borduin CM, Schaeffer CM. Cost-benefit analysis of multisystemic therapy with serious violent juvenile offenders. Journal of Family Psychology. 2010;24:657–66. doi: 10.1037/a0020838. [DOI] [PubMed] [Google Scholar]
- Koenig LB, McGue M, Krueger RF, Bouchard TJ., Jr Genetic and Environmental Influences on Religiousness: Findings for Retrospective and Current Religiousness Ratings. Journal of Personality. 2005;73:471–88. doi: 10.1111/j.1467-6494.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- Malone SM, Iacono WG, McGue M. Drinks of the father: Father’s maximum number of drinks consumed predicts externalizing disorders, substance use, and substance use disorders in preadolescent and adolescent offspring. Alcoholism: Clinical and Experimental Research. 2002;26:1823–1832. doi: 10.1097/01.ALC.0000042222.59908.F9. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. American Journal of Psychiatry. 2005;162:1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal inference and observational aging research: the utility of twins. Perspectives in Psychological Science. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiss MB, Stevenson J, Legrand LN, Iacono WG, Sedikides C. Self-esteem, negative emotionality, and depression as a common temperamental core: a study of mid-adolescent twin girls. Journal of Personality. 2009;77:327–346. doi: 10.1111/j.1467-6494.2008.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Fitzjohn J, Herbison M, Dickson N. The determinants of sexual intercourse before age 16. Journal of Adolescent Health. 2000;27:136–147. doi: 10.1016/s1054-139x(99)00095-6. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards MH, Boxer AM. Measuring pubertal status: Reliability and validity of a self-report measure. Journal of Youth and Adolescence. 1988;7:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Piers E, Harris D. Nashville Tennessee: Counselor Recording and Tests. 1969. The Piers-Harris Children’s Self-Concept Scale. [Google Scholar]
- Reich W, Weiner Z. Revised version of the Diagnostic Interview for Children and Adolescents (DICA-R) St. Louis, MO: Department of Psychiatry, Washington University School of Medicine; 1998. [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Rosenbaum P, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. Journal of American Statistical Association. 1984;79:516–24. [Google Scholar]
- Sandfort TGM, Orr M, Hirsch JS, Santelli J. Long term health correlates of timing of sexual debut: Results from a national US study. American Journal of Public Health. 2008;98:155–161. doi: 10.2105/AJPH.2006.097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman SN, Mosher WD, Aral SO. Predictors of high-risk behavior in unmarried American women: Adolescent environment as risk factor. Journal of Adolescent Health. 1994;15:126–132. doi: 10.1016/1054-139x(94)90539-8. [DOI] [PubMed] [Google Scholar]
- Svenson GR, Hanson BS. Are peer and social influences important components to include in HIV-STD prevention models? Results of a survey on young people at Lund University, Sweden. European Journal of Public Health. 1996;6:203–211. [Google Scholar]
- Walden B, McGue M, Iacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: The respective roles of peers and parents. Journal of Abnormal Psychology. 2004;113:440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- Wellings F, Nanchahal K, Macdowall W, McManus S, Erens B, Mercer CH, et al. Sexual behaviour in Britain: early heterosexual experience. Lancet. 2001;358(9296):1843–1850. doi: 10.1016/S0140-6736(01)06885-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children—Revised. New York: Psychological Corporation; 1974. [Google Scholar]
- Zimmer-Gembeck MJ, Helfand M. Ten years of longitudinal research on U.S. adolescent sexual behavior: Developmental correlates of sexual intercourse, and the importance of age, gender, and ethnic background. Developmental Review. 2008;28:153–224. [Google Scholar]