Abstract
Objective
Aldosterone (Aldo) antagonism prevents cardiovascular mortality by unclear mechanisms. Aldo binds to the mineralocorticoid receptor (MR), a ligand-activated transcription factor, which is expressed in human vascular cells. Here we define the early Aldo-regulated vascular transcriptome and investigate the mechanisms of gene regulation by Aldo in the vasculature that may contribute to vascular disease.
Methods and Results
Gene expression profiling of Aldo-treated mouse aortas identified 72 genes regulated by Aldo. These genes are overrepresented in Gene Ontology categories involved in vascular function and disease. QRT-PCR was used to confirm and further explore mechanisms of vascular gene regulation by Aldo. Aldo-regulated vascular gene expression was inhibited by actinomycin-D and MR antagonists supporting a transcriptional MR-dependent mechanism. Aldo regulation of a subset of genes was enhanced in the setting of vascular endothelial denudation and blocked by the free radical scavenger Tempol, supporting synergy between Aldo and vascular injury that is oxidative stress-dependent. In the aortic arch, a region predisposed to atherosclerosis, the injury-enhanced genes also demonstrated enhanced expression compared to the descending aorta, both at baseline and after Aldo exposure. Furthermore, the clinically beneficial MR antagonist spironolactone inhibited expression of the identified genes in aortic tissue from humans with atherosclerosis.
Conclusions
This study defines the Aldo-regulated vascular transcriptome and characterizes a subset of proatherogenic genes with enhanced Aldo-stimulated, oxidative stress-dependent expression in the setting of vascular injury and in areas predisposed to atherosclerosis. Inhibition of MR regulation of these genes may play a role in the protective effects of Aldo antagonists in patients with vascular disease and these pathways may provide novel drug targets to prevent atherosclerosis in humans.
In clinical trials, aldosterone (Aldo) antagonists decrease cardiovascular mortality by unknown mechanisms1,2. Aldo is a steroid hormone that acts by binding to the mineralocorticoid receptor (MR), a member of the nuclear receptor family of ligand-activated transcription factors. Aldo elevates systemic blood pressure by activating renal MR-dependent gene transcription thus, the vascular effects of Aldo have previously been ascribed to renal MR-mediated blood pressure elevation with secondary vascular consequences3. However, the reduction in ischemic events in clinical trials of Renin-Angiotensin-Aldo system (RAAS) antagonism is significantly greater than that expected from the modest decrease in systemic blood pressure in treated patients1,2,4, suggesting a direct role for vascular MR-activation in cardiovascular pathology. The importance of understanding the direct vascular effects of Aldo has recently been highlighted by the failure in clinical trials of the HDL-raising CETP-inhibitor Torcetrapib, despite significant improvements in cholesterol profiles, likely due to off-target increases in serum Aldo levels5. Post Hoc analyses demonstrated a correlation between electrolyte evidence of Aldo excess with increased carotid intimal thickness, coronary atherosclerosis, cardiovascular ischemia and mortality in Torcetrapib-treated patients6,7.
Over the past decade, evidence has accumulated for the presence of functional MR in cardiovascular tissues8. We have demonstrated that human vascular smooth muscle cells (VSMC)9 and endothelial cells (EC)10 express MR capable of regulating vascular specific gene expression and transcriptional programs. We showed further that VSMC MR regulates expression of genes involved in vascular fibrosis and calcification9,11 while in EC, MR regulates genes involved in cell adhesion and oxidative stress10. The MR can be activated by both Aldo and cortisol (the glucocorticoid receptor (GR) ligand); however, hormone specificity is conferred in Aldo-responsive tissues, such as the kidney, by expression of the cortisol-inactivating enzyme 11β-hydroxysteroid dehydrogenase-2 (11βHSD2). Although the in vivo ligand for vascular MR is still debated, multiple groups have demonstrated expression and function of 11βHSD2 in human vascular cells and vessels9,10,12,13. These data support that human vascular cells contain functional MR capable of directly responding to Aldo by regulating gene expression. We hypothesized that Aldo-regulated genes in blood vessels may play a role in the mechanism of Aldo-enhanced vascular disease. Aldo-regulated genes have been identified by gene expression profiling of kidney and heart cells and tissues14–16, however, direct MR target genes in intact blood vessels have not been systematically identified.
In animal models, Aldo promotes vascular inflammation17, vascular remodeling after injury18,19, and atherosclerosis20, although the mechanisms are largely unknown. While several mRNAs and proteins, including monocyte chemoattractant factor 1, osteopontin, and cyclooxygenase 2, have been shown to be increased in vascular tissues after Aldo infusion into animals17,21, whether these expression changes are due to direct effects of Aldo acting on vascular MR or secondary to MR activation in other tissues such as the kidney or heart cannot be determined from these animal studies.
We have recently confirmed in the mouse wire carotid injury model that Aldo enhances vascular remodeling only with the concomitant presence of endothelial damage19. This is consistent with other animal studies demonstrating that Aldo, acting via MR, exacerbates vascular injury responses to a variety of conditions that cause endothelial dysfunction, including mechanical EC injury18, uninephrectomy/high salt-induced hypertension17, or hyperlipidemia-induced vascular damage20. To further explore the molecular mechanisms of Aldo-stimulated vascular disease, we now identify the early Aldo-regulated vascular transcriptome, examine the mechanism of direct regulation of vascular gene expression by Aldo, and explore potential novel mechanisms for the synergy between Aldo and endothelial damage in promoting vascular disease. Understanding the molecular mechanism for the synergistic effects of Aldo on vascular disease in the setting of endothelial damage may help explain the beneficial effects of MR antagonists in patients with vascular diseases and may identify novel therapeutic targets to prevent cardiovascular ischemia.
METHODS
Animal Studies
Animals were handled in accordance with NIH standards and all procedures were approved by the Tufts Medical Center Institutional Animal Care and Use Committee. Surgical procedures used inhaled isoflorane anesthesia. Male C57BL/6J or endothelial nitric oxide synthase (eNOS) knockout (KO) mice22 were pretreated with spironolactone (20 mg/kg/day) for 5 days to suppress basal MR activation. For gene expression profiling, aortas were treated ex vivo with 100 nM Aldo or vehicle for 2, 4, or 8 hours, RNA was isolated, and 3 pooled, biological replicates were performed. For denuded vessel studies, the aorta was opened longitudinally and the EC denuded by gently wiping with a cotton swab. See Supplemental Methods for details and validation of the ex vivo approach.
Human Vessel Studies
Discarded, de-identified, human aorta was collected at the time of coronary artery bypass graft (CABG) surgery with approval from the Tufts Medical Center Institutional Review Board and treated ex vivo with vehicle or spironolactone (1 μM) for 18 hours as described19.
Cell Culture
Primary mouse aortic SMC (MASMC, see Supplemental Methods for culture conditions) pretreated for 24 hours in 10% charcoal stripped serum, were then treated for 8 hours with Aldo or vehicle and total RNA was isolated for QRT-PCR.
RNA Isolation, Microarray Hybridization, and Microarray Data Analysis
Reverse transcribed aortic RNA was hybridized to Mouse Genome 430A 2.0 microarrays (Affymetrix, Santa Clara, CA) at the Dana Farber Cancer Institute Microarray Core Facility (http://chip.dfci.harvard.edu/lab/services.php). The data was normalized and analyzed using the GC content Robust Multichip Analysis (GCRMA) protocol using Benjamini and Horchberg’s adjustment for multiple testing to calculate the adjusted p-values (pAdj) as described (23 and Supplemental Methods). Complete expression data is available at NCBI GEO accession #GSE23566. Genes with highly reproducible (pAdj≤0.05) Aldo-regulated expression changes of at least 1.5-fold were used in all further analyses. The analysis for Gene Ontology enrichment was performed using the Gene Ontology (GO) Tree Machine24.
Quantitative Reverse Transcription Polymerase Chain Reaction (QRT-PCR)
Aortas were treated ex vivo with Aldo (100 nM), cortisol (10 nM), Actinomycin D (ActD, 5 μg/ml), Cyclohexamide (CXMD,40 μg/ml), spironolactone (10 μM), Eplerenone (50 μM), RU485 (Mifepristone, 100 μM), TEMPOL (4-Hydroxy-2,2,6,6-tetramethylpiperidine, 10 mM), Apocynin (4′-hydroxy-3′-methoxyacetophenone, 600 mM), or L-NMMA (NG-methyl-L-arginine acetate, 4 mM) for 8 hours unless otherwise indicated, RNA was isolated, and QRT-PCR performed as described23. See Supplemental methods for details and Supplemental Table V for primer sequences.
QRT-PCR Statistical Analysis
QRT-PCR values are reported as mean +/− standard error of the mean. Within-group differences were assessed with one-factor ANOVA with Student-Newman-Keuls or Dunn’s method posttest when appropriate. P<0.05 was considered significant.
RESULTS
Aldosterone Directly Regulates Aortic Gene Expression
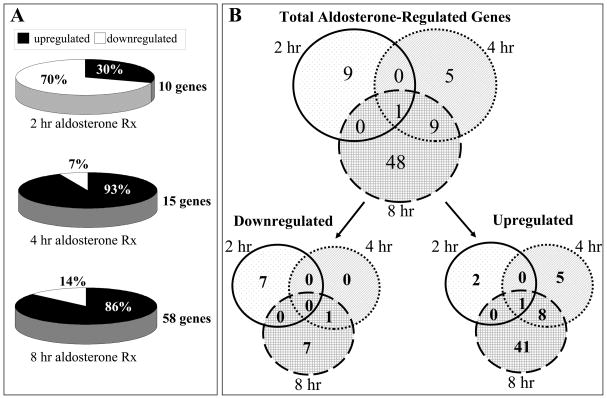
Aldo-responsive vascular genes were identified by gene expression profiling of RNA from mouse aortas treated ex vivo for 2, 4, and 8 hours with Aldo compared to vehicle (NCBI GEO accession #GSE23566). The time points were based on published data demonstrating that after hormone exposure, steroid receptors bind to DNA within 45–60 minutes with detectable changes in gene expression after two hours and protein expression after eight hours19,23,25. Genes with Aldo-responsive expression changes that were significant in magnitude (>1.5 fold compared to vehicle) and highly reproducible (pAdj<0.05) were included in all further analyses. Using these strict criteria, the number of Aldo-regulated genes increased over time with 10, 15, and 58 regulated genes at 2, 4, and 8 hours respectively (Figure 1A and Supplemental Tables I–III). Aldo-mediated gene repression predominated in the first two hours (70% repressed) while gene induction was more prominent at 4 to 8 hours (7% and 14% repressed, respectively, Figure 1A).
Figure 1. Timecourse of Aldo-Regulated Genes in Mouse Aorta.
A: The percentage of up- versus down-regulated genes at each timepoint is indicated. B: Overlap of Aldo-Regulated Gene Sets Over Time. The venn diagrams depict numbers of up- and down-regulated genes in response to Aldo at each time point.
The Aldo-stimulated microarray expression changes were validated by quantitative RT-PCR analysis in independently obtained mouse aorta RNA samples (Supplemental Table IV). Nineteen genes were chosen for confirmation based on large magnitude Aldo-induced changes and/or interesting roles in vascular biology and included both Aldo-stimulated and -repressed genes and genes with changes at each of the three time-points. Sixteen upregulated gene expression changes were tested (28% of Aldo-upregulated genes), 14 of which were reproducible in both the magnitude and direction of the change (88%). Three downregulated genes were tested (20% of Aldo-downregulated genes), and although the downregulation was less consistent, two of the three genes tested decreased at least 25% by Aldo treatment. Overall, 84% of genes tested displayed comparable levels of Aldo regulation by QRT-PCR as in the global gene profile thereby confirming, in new aortic samples, the validity of both the microarray technique and the Aldo-stimulated changes in gene expression.
Temporal Pattern of Aldosterone-Regulated Genes in the Aorta
The temporal pattern of Aldo-regulated genes was explored by analyzing the degree of overlap between gene sets at each time point (Figure 1B). The two-hour Aldo-regulated gene set was distinct from the other time-points as it contained only one gene that was also regulated by Aldo at 4 and 8 hours. In contrast, there was significant overlap between genes regulated by Aldo at 4 hours with ten of the fifteen (67%) genes being persistently Aldo-regulated at 8 hours. A third wave of gene expression begins at 8 hours that is distinct from the earlier regulated genes with 48 of the 58 genes regulated by Aldo at 8 hours being unique. Only one Aldo-regulated vascular gene (baculoviral AIP repeat containing-2 (BIRC2), 1.4%) was persistently significantly induced by Aldo over the entire 8 hour time period.
Functions of Aldosterone-Regulated Vascular Genes
The gene ontology (GO) Tree Machine was used to identify specific functional categories based on GO annotation that are significantly overrepresented in the 72 vascular Aldo-regulated genes. Overall, there are 22 overrepresented GO categories in this gene set. Table 1 lists the 10 categories at the end of each GO tree with their associated genes and the p value for the overrepresentation of genes in the Aldo-regulated vascular gene set compared to randomly generated sets of 72 genes. The overrepresented categories are relevant to the known role of Aldo in regulating oxidative stress (NO-mediated signal transduction), vascular cell proliferation (angiogenesis, negative regulation of progression through cell cycle) and extracellular matrix formation and degradation (integrin-mediated signaling pathway, extracellular matrix (ECM), metaloendopeptidase activity) supporting that Aldo-regulated vascular genes may play a role in mineralocorticoid-mediated vascular dysfunction and disease by altering vascular gene expression.
Table 1. Overrepresented Functional Categories in Aldo-Regulated Vascular Genes.
Gene Ontology (GO) Tree Machine was used to analyze the 72 Aldo-regulated genes. The highest level overrepresented category, the p value for overrepresentation, the associated gene list, and the Aldo-stimulated fold changes at each time-point are listed. Fold changes with Padj<0.05 are indicated in bold. BP=biological process, MF=molecular function, CC=cellular component.
| Category | p value | Gene | 2 hr fold | 4 hr fold | 8 hr fold |
|---|---|---|---|---|---|
| nitric oxide mediated signal transduction (BP) | <0.001 | metallothionein 1 | 1.9 | 2.1 | 2.2 |
| metallothionein 2 | 1.7 | 1.9 | 1.93 | ||
| integrin-mediated signaling pathway (BP) | 0.005 | a disintegrin and metallopeptidase domain 19 (meltrin β) | 0.7 | 0.6 | 0.61 |
| connective tissue growth factor | 1 | 1.4 | 1.82 | ||
| a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 15 | 1.2 | 1.7 | 2.88 | ||
| zinc ion homeostasis (BP) | <0.001 | metallothionein 1 | 1.9 | 2.1 | 2.2 |
| metallothionein 2 | 1.7 | 1.9 | 1.93 | ||
| negative regulation of progression through cell cycle (BP) | 0.009 | sestrin 1 | 1 | 1.9 | 2.34 |
| homeodomain interacting protein kinase 2 | 1 | 1.3 | 1.92 | ||
| large tumor suppressor 2 | 1.2 | 0.9 | 1.57 | ||
| angiogenesis (BP) | 0.002 | connective tissue growth factor | 1 | 1.4 | 1.82 |
| neuropilin 1 | 1.1 | 0.8 | 0.56 | ||
| placental growth factor | 1.7 | 4.6 | 4.59 | ||
| semaphorin 5A | 0.9 | 0.7 | 0.58 | ||
| zinc ion binding (MF) | 0.006 | a disintegrin and metallopeptidase domain 19 (meltrin β) | 0.7 | 0.6 | 0.61 |
| baculoviral IAP repeat-containing 2 | 1.8 | 2.2 | 3.77 | ||
| Kruppel-like factor 9 | 1 | 1.3 | 1.95 | ||
| methyl-CpG binding domain protein 1 | 1.4 | 1.4 | 2.21 | ||
| matrix metallopeptidase 10 | 1 | 1 | 0.63 | ||
| metallothionein 1 | 1.9 | 2.1 | 2.2 | ||
| metallothionein 2 | 1.7 | 1.9 | 1.93 | ||
| src homology three (SH3) and cysteine rich domain | 0.9 | 1 | 0.65 | ||
| zinc finger protein 281 | 1 | 1.4 | 1.79 | ||
| zinc finger protein 52 | 1.5 | 1.3 | 1.49 | ||
| a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 15 | 1.2 | 1.7 | 2.88 | ||
| zinc finger and BTB domain containing 16 | 1 | 3.5 | 7.06 | ||
| nuclear receptor subfamily 1, group D, member 2 | 0.5 | 1 | 0.71 | ||
| metalloendopeptidase activity (MF) | 0.008 | a disintegrin and metallopeptidase domain 19 (meltrin β) | 0.7 | 0.6 | 0.61 |
| matrix metallopeptidase 10 | 1 | 1 | 0.63 | ||
| a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 15 | 1.2 | 1.7 | 2.88 | ||
| polyphosphate-glucose phosphotransferase activity (MF) | <0.001 | phosphorylase kinase alpha 2 | 0.9 | 1.1 | 1.94 |
| homeodomain interacting protein kinase 2 | 1 | 1.3 | 1.92 | ||
| period homolog 2 (Drosophila) | 1.1 | 1.6 | 3.47 | ||
| serum/glucocorticoid regulated kinase | 1 | 1.9 | 1.69 | ||
| mitogen activated protein kinase kinase kinase 8 | 0.7 | 1.2 | 2.19 | ||
| large tumor suppressor 2 | 1.2 | 0.9 | 1.57 | ||
| transcription elongation factor complex (CC) | <0.001 | Max dimerization protein 4 | 1.1 | 1.3 | 2.1 |
| paired related homeobox 1 | 0.9 | 0.7 | 0.58 | ||
| extracellular matrix (CC) | 0.006 | a disintegrin and metallopeptidase domain 19 (meltrin β) | 0.7 | 0.6 | 0.61 |
| connective tissue growth factor | 1 | 1.4 | 1.82 | ||
| matrix metallopeptidase 10 | 1 | 1 | 0.63 | ||
| tissue inhibitor of metalloproteinase 3 | 0.7 | 0.8 | 0.53 | ||
| a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 15 | 1.2 | 1.7 | 2.88 |
Dose Response Relationship for Vascular Aldosterone Gene Regulation
A subset (10%) of highly reproducible Aldo-regulated vascular genes was chosen to further explore the mechanism of Aldo regulation in the vasculature. Six genes were chosen based on their presence in functionally relevant overrepresented GO categories including, Placental Growth Factor (PGF), Metallothionein 1 and 2 (MT1, MT2), Connective Tissue Growth Factor (CTGF), BIRC2, and serum and glucocorticoid-regulated kinase (SGK). The seventh gene, FK506 binding protein 51 (FKBP5), was chosen because it is a well-studied steroid-responsive gene in nonvascular cells26,27. The dose-response relationship of Aldo regulation of these genes was explored revealing, for all seven genes, a dose dependent increase in gene expression with significant increases for individual genes beginning at 10–30 nM (Figure 2A). Two Way ANOVA comparing Aldo-stimulated fold changes for all seven genes at all Aldo doses demonstrates a significant global increase in gene expression even at 1 nM (Figure 2A, p<0.001 for 100 nM, 30 nM, and 10 nM and p<0.05 for 1 nM Aldo, compared to vehicle and to all lower doses tested). These data support that Aldo directly regulates vascular gene expression at physiologic (1 nM) and pathologic (10–30 nM) concentrations.
Figure 2. Dose Dependent Transcriptional Regulation of Vascular Genes by Aldo.
Mouse aortas were treated ex vivo with the Aldo for 8 hours and mRNA was quantified by QRT-PCR. (A) Dose-Response: *p<0.05, **p<0.01, ***p<0.001 versus vehicle. (B) Aldo treatment in the presence or absence of the transcriptional inhibitor actinomycin D (5 μg/ml) or (C) the translational inhibitor cyclohexamide (40 μg/ml). The Aldo-stimulated fold change for each gene in the absence of inhibitor (black bars) is compared to that in the presence of the inhibitor (gray bars). *p<0.05, ***p<0.001 for aldo-stimulated fold change with vehicle compared to inhibitor.
Aldo-Regulated Vascular Gene Expression Requires Transcription but not Translation
To explore the mechanism for the mRNA increase in response to Aldo, we examined Aldo-stimulated gene expression after one hour pretreatment with the transcriptional inhibitor, ActD, or the translation inhibitor, CXMD. ActD inhibited Aldo-stimulated increases in mRNA for all genes tested (Figure 2B, for PGF see19) supporting a transcriptional mechanism rather than mRNA stabilization. The enhanced transcription did not require new protein translation since it was not inhibited by CXMD (Figure 2C, for PGF see19). Thus, the signaling pathway components, transcription factors, and cofactors necessary for Aldo-stimulated transcription must already be present in the vessel.
Receptor-, Hormone-, and Cell Type-Dependence of Aldo-Regulated Vascular Gene Expression
The receptor and hormone-specificity of the vascular Aldo-regulated genes was explored using MR and GR agonists and antagonists. Both spironolactone and the more receptor-selective MR antagonist, eplerenone, inhibited Aldo-stimulated gene transcription in the mouse aorta for all genes tested (Figure 3A, Supplemental Figure I, and Supplemental Table IV) supporting an MR-dependent effect of Aldo. Cortisol (10 nM) treatment resulted in only modest increases in expression of these 7 genes that were not significantly different from vehicle and were not affected by MR inhibition with either spironolactone (Figure 3A) or eplerenone (data not shown). In contrast the same dose of Aldo significantly increased expression of several genes (Figure 2A). It is notable that the modest increase in vascular expression of FKBP5 in the presence of cortisol was significantly inhibited by the GR antagonist, RU486, supporting that cortisol can activate GR in the vessel to regulate FKBP5 expression, as has been previously demonstrated in nonvascular cells27. These data support that the gene expression changes we have identified in the vasculature are Aldo-specific and vascular MR-dependent under these conditions.
Figure 3. Aldosterone- and MR-Dependent Vascular Gene Regulation Predominantly in Smooth Muscle Cells.
(A) Mouse aortae were treated ex vivo with the indicated steroid hormones and inhibitors and (B) Primary Mouse Aortic SMC were treated were treated with vehicle or Aldo. RNA was isolated and QRT-PCR performed with gene specific primers. Aldo (100 nM), cortisol (10 nM), Spirono (10 μM), and RU485 (100 μM). *p<0.05 versus Vehicle and Aldo + Spirono, $ p<0.05 vs Cortisol, #p<0.05 versus vehicle.
Mouse aortas consist predominantly of VSMC with smaller numbers of EC, fibroblasts, and resident immune cells. To explore the cell type in which these genes might be regulated, we treated primary mouse aortic SMC with Aldo for eight hours and quantified expression of the same genes in SMC RNA. Aldo treatment of isolated SMC resulted in significant upregulation of all the genes tested with the exception of PGF (Figure 3B, PGF=1.5 fold, p=0.4). Overall, the pattern of the Aldo-stimulated changes was the same in isolated SMC, although the magnitude was less when compared to whole vessels (i.e. FKBP5 had the greatest magnitude of Aldo regulation in all cases, but it was upregulated 5–10 fold in whole vessels (Figures 2 and 3A) and only 3–4 fold in SMC). We saw no effect of Aldo on expression of these seven genes in isolated endothelial cells under any conditions (data not shown). These data support that SMC are sufficient for Aldo-stimulated regulation of most of these genes however, the structure and/or cellular composition of the whole vessel may modulate the magnitude of the response.
A Subset of Aldo-Mediated Vascular Gene Regulation is Enhanced in the Presence of Endothelial Damage and is Free Radical-Dependent
Animal studies and human clinical trials support that Aldo promotes vascular disease in the setting of endothelial damage or dysfunction. We compared the Aldo-stimulated changes in gene expression in endothelium-denuded vessels (to mimic vascular damage) compared to endothelium intact vessels. For all seven genes tested, Aldo significantly enhanced gene expression in denuded vessels compared to vehicle (Figure 4A, grey bars, p<0.05) further supporting that these gene expression changes do not require the presence of endothelial cells. For a subset of the genes (PGF, MT1, and MT2), the Aldo-stimulated increase in mRNA was significantly greater in denuded vessels compared to intact vessels, supporting that the normal endothelium attenuates Aldo-stimulated activation of this subset of genes. The Aldo effect on these three injury-enhanced genes was inhibited in the presence of the free radical scavenger Tempol (Figure 4B), implicating oxygen free radicals in the mechanism. For two additional genes, CTGF and FKBP5, Tempol treatment partially inhibited Aldo-activated gene transcription, and for BIRC2 and SGK, treatment with Tempol had no effect on Aldo-stimulated gene expression. These data demonstrate that Aldo, acting directly on MR in the blood vessel, regulates vascular gene expression via oxidative stress-dependent and –independent pathways.
Figure 4. Endothelial Damage Enhances Aldo Regulation of a Subset of Vascular Genes and Requires Oxidative Stress.
Mouse aortae were treated with Aldo or Vehicle for 8 hours. The Aldo-stimulated fold change for each gene; (A) in whole vessel, (B, C, D) in the absence of inhibitor, or (E) in wild type aortas is indicated in black bars and is compared to that in the presence of; (A) endothelial denudation, (B) TEMPOL (10 mM), (C) Apocynin (600 mM), (D) L-NMMA (4 mM), or (E) ENOS KO mouse aorta, indicated in the gray bars. *p<0.05, **p<0.01, ***p≤0.001.
Vascular oxidative stress is caused by production of free radicals by vascular oxidases and is mitigated by production of nitric oxide (NO) that inactivates free radicals. To begin to explore the mechanism for the oxidative stress-dependent Aldo-mediated gene expression changes, we examined Aldo regulation of the five genes that were inhibited by Tempol, in the presence of the NADPH-oxidase inhibitor apocynin (Figure 4C), the nitric oxide synthase inhibitor L-NMMA (Figure 4D), and in vessels from mice specifically deficient in the endothelial NOS (Figure 4E). Overall, the genes fell into two patterns, with Aldo regulation of MT1 and MT2 being relatively unaffected by NADPH-oxidase and NOS inhibition (only partial inhibition of MT2 regulation in eNOS-deficient vessels). For PGF, FKBP5, and CTGF, Aldo-stimulated gene expression was significantly attenuated in the presence of apocynin. Inhibition of NOS with L-NMMA resulted in significantly enhanced Aldo regulation of FKBP5 with a trend towards an increase for PGF and CTGF and specific deletion of eNOS in the vessel resulted in enhanced Aldo regulation of both FKBP5 and CTGF with a modest and non-significant trend for PGF. These data support a role for NADPH-oxidase in promoting Aldo upregulation of a specific subset of genes with a contribution of NOS, perhaps from ECs, in attenuating this effect.
Enhanced Expression of Hemodynamically-Regulated Genes in Atherosclerosis-Prone Aortic Segments Exposed to Aldosterone
Although atherosclerosis is a diffuse disease of the vasculature, there is a predisposition for lesion formation at vascular branch points and other areas of turbulent blood flow28. Thus, we compared Aldo-stimulated gene expression in the atherosclerosis-prone aortic arch, an area exposed to non-laminar (pro-atherogenic) blood flow, compared to the descending aorta, an area of laminar flow and decreased atherosclerotic burden28. For all seven genes, Aldo treatment increased mRNA expression in both the arch and descending aorta (Figure 5A). Interestingly, for the three genes with enhanced Aldo-regulation in the setting of endothelial injury (PGF, MT1, MT2), we also found a significant increase in the magnitude of expression in the Aldo-exposed aortic arch compared to the Aldo-exposed descending aorta. A small group of genes (including PGF, MTs, and CTGF) has been previously identified to be upregulated in cultured vascular cells by pro-atherogenic hemodynamic flow29. We compared gene expression levels for this subset of hemodynamically-regulated genes to the genes not induced by atherosclerosis-prone flow (FKBP5, SGK, BIRC2) by three factor ANOVA (comparing flow-dependence, vessel segment, and Aldo treatment). There is a significant increase in expression of the atherosclerosis-prone flow genes in both vehicle- and Aldo-treated aortic arch segments compared to the descending aorta (p<0.05). This is not the case for the genes unaffected by atherosclerosis-prone flow. MR message expression was the same in the arch and descending aorta supporting that differences in Aldo-regulated gene expression in these segments are not mediated by changes in MR message. This finding supports a potential novel role for Aldo in the mechanism underlying the non-uniform distribution of atherosclerotic lesions in the vasculature.
Figure 5. Aldo- and MR-Regulated Gene Expression In Atherosclerosis-Prone Segments and in Diseased Human Vessels.
(A) Mouse aortae were divided into the arch and the descending aorta, treated with Aldo or vehicle for 8 hours, and gene expression was measured in vessel segment RNA by QRT-PCR. Data is presented as the fold change in each gene compared to the level in the vehicle treated, descending aorta. (B) Human aortic samples from coronary artery bypass graft patients were treated ex vivo with vehicle or spironolactone (1 μM) for 18 hours, RNA was isolated, and gene expression measured by QRT-PCR. Data is expressed as the percent of vehicle treated gene expression for each gene. *p<0.05, **p<0.01, ***p<0.001 versus vehicle.
MR-Mediated Gene Regulation in Atherosclerotic Human Vessels
We next explored whether the MR-regulated vascular genes we identified in mouse aortas might also be regulated by MR in human vessels. Aortic punches removed from patients undergoing CABG were treated ex vivo with spironolactone (1 μM) for 18 hours followed by RNA isolation. In human aortic samples, treatment with MR antagonist resulted in a 40–80% reduction in mRNA for all 7 genes tested (Figure 5B, for PGF see19). These data support that the MR-regulated vascular genes identified in mouse vessels using this transcriptional profiling method are also regulated by vascular MR in diseased human vessels and are down-regulated by the clinically beneficial MR antagonist spironolactone and thus could play a role in the mechanisms of vascular protection by MR-antagonist drugs in clinical trials.
DISCUSSION
This study examines the immediate effects of the hormone Aldo on vascular gene expression programs in whole blood vessels and explores the molecular mechanism of Aldo-regulated gene expression for a subset of these genes. The data demonstrate that: 1) Aldo rapidly and directly regulates vascular gene expression; 2) vascular Aldo-regulated genes are overrepresented in GO categories related to vascular function and disease; 3) for those genes tested, vascular gene expression is regulated by Aldo, but not cortisol, at clinically relevant concentrations via a vascular MR-dependent transcriptional mechanism; 4) for a subset of genes tested, Aldo-stimulated expression is enhanced in the presence of endothelial damage and is free-radical dependent with a contribution from NADPH oxidases and protection by NOS; 5) in segments of the vessel prone to atherosclerosis, Aldo further enhances expression of a subset of genes that has been implicated in promoting atherosclerosis under non-laminar flow conditions; and 6) MR antagonists inhibit expression of these genes in human vessels from patients with atherosclerosis. This study identifies direct effects of Aldo-activated vascular MR on vascular gene expression that may contribute to the detrimental vascular effects of this hormone, explores the molecular mechanisms for these vascular gene regulatory effects, and begins to address the mechanism for the vascular protective effects of MR antagonists in human clinical trials.
It has recently become clear that MR in human vascular cells can directly regulate gene transcription9,10 raising the possibility that vascular MR-regulated genes could mediate the effects of Aldo on vascular physiology and disease in humans. Indeed, our further exploration of PGF, one of the vascular MR-regulated genes identified from this profiling study, demonstrated a critical role for PGF in Aldo-mediated vascular injury in vivo19, supporting that this method has identified pathways that are important in the biology of Aldo-induced vascular dysfunction in vivo. Thus, by identifying the early Aldo-regulated transcriptome in whole vessels, this study provides an important resource to further explore mechanisms of mineralocorticoid-mediated vascular dysfunction and may provide novel drug targets to prevent or treat vascular disease in humans.
The trend for very early steroid-mediated gene repression followed by induction over time is consistent with recent RNA expression profiles from hearts of Aldo-injected mice16, estrogen-treated breast cancer cells25, and our recent study in estrogen-treated vessels23. In addition, the pattern of rapid repression of distinct genes after two hours of hormone treatment followed by overlapping gene upregulation cascades at four and eight hours is similar to the pattern of estrogen-regulated genes in the vasculature23, supporting the potential for common mechanisms to mediate this pattern of steroid-mediated vascular gene regulation. Aldo can modulate gene expression by direct, so called “genomic”, effects mediated by MR binding to DNA, and by “non-genomic” mechanisms, mediated by activation of cytoplasmic signaling pathways that regulate other transcription factors. We have found an Aldo-regulated MR binding site in the PGF upstream DNA region19 as well as in intron E of the FKBP5 gene (data not shown), a site that has previously been shown to mediate regulation of FKBP5 by other steroid receptors26. Future computational and experimental analyses are warranted to explore the contributions of genomic and non-genomic signaling mechanisms to the global regulation of vascular genes by Aldo.
Several vascular genes identified in this study were already known to be regulated by Aldo in other tissues including SGK, a known MR target in the kidney, and CTGF, which is regulated by mineralocorticoids in the kidney and heart30. SGK and CTGF play a role in Aldo-mediated renal and cardiac fibrosis30,31, thus, the finding that these genes are regulated by vascular MR supports a potential new mechanism for Aldo-mediated vascular fibrosis that can be further explored in vivo. FKBP5 is known to be regulated by several steroid hormones in non-vascular cells26 but its regulation in the vasculature by Aldo and MR has not been previously reported. A few of the vascular Aldo-regulated genes identified here overlap with Aldo-regulated genes identified by profiling of MR-expressing cardiomyocytes15 including ADAMTS1, FKBP5, SGK, and AKAP12. However, the majority of Aldo-regulated vascular genes identified in this study are distinct from genes regulated by Aldo in the heart16 and in other tissues, supporting tissue-specific effects of Aldo and MR in the vasculature.
In animal models, Aldo promotes vascular inflammation17, vascular remodeling after injury18, and atherosclerosis20. Although, several mRNAs and proteins are increased in vascular tissues in these models, the mechanisms underlying these expression changes are not known. Using an ex vivo Aldo treatment approach, we can conclude that the gene expression changes observed are due to direct effects of Aldo on the vessel rather than to vascular responses that may be secondary to Aldo effects in other tissues, particularly the kidney and heart, which have confounded interpretations of Aldo effects on the vasculature in the past. Inhibition of the Aldo-stimulated effects by ActD and MR antagonists in this study supports a vascular MR-mediated transcriptional mechanism and the lack of regulation by cortisol supports Aldo as the vascular MR ligand under these conditions.
In this study, we identify a number of genes with established roles in vascular function and disease that have not been previously known to be regulated by Aldo in the vasculature. CTGF has been shown by several groups to be upregulated in diseased human vessels and is implicated in vascular remodeling, dissection, atherosclerotic lesion development, and plaque rupture. CTGF regulates SMC proliferation, migration and production of collagen extracellular matrix as well as vascular cell apoptosis, matrix metalloproteinase (MMP) expression, and inflammatory cell recruitment (reviewed in 32). The metallothioneins are metal binding proteins that are upregulated in response to oxidative stress in non-vascular cells where they protect from apoptosis and inflammation33. The in vivo role of MT in protection from oxidative stress is more controversial33. Regulation of this family of genes by Aldo has not been previously reported and the role of this regulation in vascular function deserves further study. PGF is a pro-angiogenic and pro-inflammatory growth factor that promotes vascular cell proliferation and monocyte chemotaxis. We have recently further explored Aldo regulation of PGF and demonstrated that Aldo enhances vascular PGF protein expression and secretion and that PGF plays a role in vivo in Aldo-stimulated vascular remodeling after injury19. By enhancing expression of genes that promote inflammation (PGF, CTGF), increasing expression of matrix proteases that degrade the fibrous cap of atherosclerotic lesions (AdamTS1, CTGF), and decreasing expression of inhibitors of MMPs (TIMP3), Aldo may destabilize atherosclerotic plaques and vessel integrity thereby promoting myocardial infarction, stroke, and aortic dissection. Further in vivo animal and clinical studies will be necessary to test these hypotheses.
The finding that a subset of vascular genes demonstrates enhanced upregulation by Aldo in vessels with endothelial damage is consistent with the hypothesis that the endothelium inhibits Aldo-stimulated expression of these genes in healthy vessels, perhaps by production of NO, and that this protection may be lost in disease states that cause EC dysfunction or injury. This hypothesis could provide a novel explanation for the conundrum that in mouse models, Aldo alone does not promote vascular damage without the concomitant presence of some form of endothelial damage or dysfunction17–20. Indeed, in human trials, the cardiovascular protective effects of drugs that block Aldo production or MR activation, are evident in patients with normal or elevated serum Aldo levels in the setting of cardiovascular risk factors that can produce endothelial dysfunction, including patients with heart failure1, coronary artery disease2, diabetes4, and/or other cardiovascular risk factors4. In this study, treatment of vessels with the superoxide dismutase-mimetic, Tempol (which would be expected to decrease the abundance of oxygen radicals and peroxynitrite), attenuated Aldo activation of this same subset of genes (along with several others) supporting a synergistic role for vascular oxidative stress and vascular MR-activation in regulating a subgroup of vascular Aldo target genes. Aldo is known to increase reactive oxygen species (ROS) production in vascular cells by several mechanisms, including enhanced NADPH oxidase expression and activity10,34,35. Treatment of vessels with the NADPH-oxidase inhibitor apocynin significantly attenuated the Aldo-mediated regulation of only a subgroup of the ROS-dependent MR-regulated genes supporting a role for NADPH oxidase, as well as other oxidases, in this mechanism. Vessel wall shear stress also induces ROS generation36 and hence may play a role in mediating the differential affects of Aldo on gene expression in aortic segments subjected to different flow conditions as in Figure 5A. Synergism between MR activation and oxidative stress has been shown to play a role in other pathologic effects of mineralocorticoids including renal and cardiac fibrosis37, vascular remodeling38 and atherosclerosis20.
The oxidative-stress dependent, Aldo-regulated vascular genes identified in this study overlap significantly with a small group of 11 genes (including PGF, MTs, and CTGF) that have been implicated in atherogenesis due to their induction by blood flow patterns present in atherosclerosis-prone areas of the carotid artery and downregulation by atheroprotective blood flow29. We find increased expression of this subgroup of genes in the atherosclerosis-prone aortic arch in both vehicle and Aldo-treated vessel segments compared to the relatively atherosclerosis-protected descending aorta. These data support the hypothesis that endothelial damage and vascular oxidative stress, mediated by cardiac risk factors and/or hemodynamic forces, synergize with vascular MR-activation by Aldo to enhance expression of a particularly pro-atherogenic group of genes. This mechanism could also play a role in the pro-atherogenic effects of Torcetrapib that correlated with off target increases in serum Aldo5–7 and may play a role in the protective effects of MR antagonists in cardiovascular patients1,2.
Several limitations and areas of further study are worth noting. We chose to profile gene expression in whole vessels (rather than cultured cells) because vascular cell function is known to be regulated by complex paracrine signaling between distinct cell types in the vasculature39 and we believe this strategy would therefore yield more biologically relevant genes. However, the use of whole vessels makes it difficult to precisely determine the cell type in which Aldo regulation is occurring. Overall, since SMC make up the majority of the murine vessel, many of the gene regulatory effects could be recapitulated in isolated SMC, and gene regulation by Aldo was preserved under the denuded vessel conditions, changes in mRNA abundance likely represent predominantly SMC effects. The decreased magnitude of the Aldo effect in cultured SMC could be due to the separation of the SMC from other contributing cell types but could also be due to differences in the experimental techniques. Activation of endothelial MR likely regulates other genes (such as intracellular adhesion molecule 1 (ICAM-1) which we have previously demonstrated to be MR-regulated in human coronary EC10), that would therefore be unlikely to be identified in this study due to the small contribution of EC to total vascular mRNA. However, although the cell number and RNA contribution is small, a paracrine contribution due to MR activation in EC, vascular fibroblasts, and inflammatory cells could contribute to SMC gene expression. Indeed, the enhanced regulation of a subset of genes by Aldo in the absence of the endothelium supports that multiple cell types interact to determine the ultimate magnitude of the transcriptional responses to Aldo in the vessel. In addition, MR in inflammatory cells, including macrophages, has recently been shown to modulate cardiovascular outcomes in animal models40, thus, the role of immune cell MR in regulation of vascular gene expression deserves further study. We also chose an ex vivo hormone treatment approach (rather than in vivo infusion or injection) in order to identify direct vascular MR target genes and to limit the secondary vascular effects of in vivo MR activation in non-vascular tissues. However, removal of the vessel from the animal eliminates the interaction of the vessel with pulsatile and shear hemodynamic forces as well as other circulating factors that may contribute to vascular gene expression and may modulate the response to Aldo. This limitation is controlled for by comparing mRNA expression in Aldo-treated vessels to vessels removed for the same amount of time and treated in an identical fashion with vehicle. The degree to which removal of the vessel modifies basal gene expression over time could be further explored in the vehicle treated expression data over time. However, the finding of increased basal expression of genes previously known to be responsive to hemodynamic shear forces29 in the aortic arch compared to the descending aorta, supports that at these short time-points, there is some “memory” in the vessel for the hemodynamic and shear forces to which it was previously exposed in vivo. Further investigation using in vivo models will be needed to put these direct vascular Aldo target genes into the context of whole animal physiology.
In summary, we have explored the early Aldo-regulated vascular transcriptome and identified vascular genes directly and transcriptionally regulated by Aldo-activated vascular MR. We have further characterized a subset of pro-atherogenic genes with enhanced Aldo-stimulated, oxidative stress-dependent expression in the setting of vascular injury and in areas predisposed to atherosclerosis. We have further established that expression of these genes is inhibited in diseased human vessels by treatment with vascular protective MR antagonist drugs. These data support a novel mechanism for vascular disease in which vascular MR acts synergistically with endothelial injury and oxidative stress to upregulate pro-atherogenic genes in atherosclerosis prone vascular regions. Further exploration of the role of these novel MR-regulated genes in the pathophysiology of vascular disease will help elucidate the mechanism of the protective effects of Aldo antagonists in patients with endothelial dysfunction and provide novel drug targets to prevent cardiovascular ischemic events in humans.
Supplementary Material
Acknowledgments
The authors wish to thank Richard H. Karas for helpful discussions and critical manuscript review.
SOURCES OF FUNDING:
This work was supported by National Institutes of Health grants HL74892 and HL095590 (IZJ) and HL069770 (MEM) and American Heart Association Grant 0855920D (IZJ).
Footnotes
DISCLOSURE:
Michael E. Mendelsohn is employed by Merck and retains an academic appointment at Tufts University School of Medicine.
References
- 1.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. RALES Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective Aldo blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 3.Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids. 2000;65:61–73. doi: 10.1016/s0039-128x(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 4.Dagenais GR, Yusuf S, Bourassa MG, Yi Q, Bosch J, Lonn EM, Kouz S, Grover J, Investigators HOPE. Effects of ramipril on coronary events in high-risk persons: results of the HOPE Study. Circulation. 2001;104:522–526. doi: 10.1161/hc3001.093502. [DOI] [PubMed] [Google Scholar]
- 5.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B ILLUMINATE I. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 6.Nicholls SJ, Tuzcu EM, Brennan DM, Tardif JC, Nissen SE. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE. Circulation. 2008;118:2506–2514. doi: 10.1161/CIRCULATIONAHA.108.790733. [DOI] [PubMed] [Google Scholar]
- 7.Vergeer M, Bots ML, van Leuven SI, Basart DC, Sijbrands EJ, Evans GW, Grobbee DE, Visseren FL, Stalenhoef AF, Stroes ES, Kastelein JJ. Cholesteryl ester transfer protein inhibitor torcetrapib and off-target toxicity: a pooled analysis of the RADIANCE trials. Circulation. 2008;118:2515–2522. doi: 10.1161/CIRCULATIONAHA.108.772665. [DOI] [PubMed] [Google Scholar]
- 8.Lombes M, Farman N, Bonvalet JP, Zennaro MC. Identification and role of Aldo receptors in the cardiovascular system. Annales d’Endocrinologie. 2000;61:41–46. [PubMed] [Google Scholar]
- 9.Jaffe IZ, Mendelsohn ME. Angiotensin II and Aldo regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circulation Research. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 10.Caprio M, Newfell BG, LaSala A, Baur WE, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional Mineralocorticoid Receptors in Human Vascular Endothelial Cells Regulate ICAM-1 Expression and Promote Leukocyte Adhesion. Circulation Research. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thrombs Vasc Bio. 2007;27:799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]
- 12.Alzamora R, Michea L, Marusic ET. Role of 11beta-hydroxysteroid dehydrogenase in nongenomic Aldo effects in human arteries. Hypertension. 2000;35:1099–1104. doi: 10.1161/01.hyp.35.5.1099. [DOI] [PubMed] [Google Scholar]
- 13.Hatakeyama H, Inaba S, Takeda R, Miyamori I. 11beta-hydroxysteroid dehydrogenase in human vascular cells. Kidney International. 2000;57:1352–1357. doi: 10.1046/j.1523-1755.2000.00974.x. [DOI] [PubMed] [Google Scholar]
- 14.Robert-Nicoud M, Flahaut M, Elalouf JM, Nicod M, Salinas M, Bens M, Doucet A, Wincker P, Artiguenave F, Horisberger JD, Vandewalle A, Rossier BC, Firsov D. Transcriptome of a mouse kidney cortical collecting duct cell line: effects of Aldo and vasopressin. Proc Natl Acad Sci USA. 2001;98:2712–2716. doi: 10.1073/pnas.051603198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fejes-Toth G, Naray-Fejes-Toth A. Early Aldo-regulated genes in cardiomyocytes: clues to cardiac remodeling? Endocrinology. 2007;148:1502–1510. doi: 10.1210/en.2006-1438. [DOI] [PubMed] [Google Scholar]
- 16.Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH. Effect of acute Aldo administration on gene expression profile in the heart. Endocrinology. 2006;147:3183–3189. doi: 10.1210/en.2005-1674. [DOI] [PubMed] [Google Scholar]
- 17.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldo induces a vascular inflammatory phenotype in the rat heart. Am Jnl Physio-Heart Circ Physio. 2002;283:H1802–H1810. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 18.Van Belle E, Bauters C, Wernert N, Hamon M, McFadden EP, Racadot A, Dupuis B, Lablanche JM, Bertrand ME. Neointimal thickening after balloon denudation is enhanced by Aldo and inhibited by spironolactone, and Aldo antagonist. Cardiovascular Research. 1995;29:27–32. [PubMed] [Google Scholar]
- 19.Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perreault RE, Ehsan A, Mendelsohn ME. Placental growth factor mediates Aldo-dependent vascular injury in mice. Jnl Clin Invest. 2010;120:3891–3900. doi: 10.1172/JCI40205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldo administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and Aldo. Circulation. 2004;109:2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldo-induced inflammation in the rat heart: role of oxidative stress. Am Jnl Pathy. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 23.Schnoes KK, Jaffe IZ, Iyer L, Dabreo A, Aronovitz M, Newfell BG, Hansen U, Rosano G, Mendelsohn ME. Rapid recruitment of temporally distinct vascular gene sets by estrogen. Molecular Endocrinology. 2008;22:2544–2556. doi: 10.1210/me.2008-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Schmoyer D, Kirov S, Snoddy J. GOTree Machine (GOTM): a web-based platform for interpreting sets of interesting genes using Gene Ontology hierarchies. BMC Bioinformatics. 2004;5:16. doi: 10.1186/1471-2105-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nature Genetics. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 26.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- 27.Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones. 2004;9:243–252. doi: 10.1379/CSC-32R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caro CG. Discovery of the role of wall shear in atherosclerosis. Arterioscler Thrombs Vasc Bio. 2009;29:158–161. doi: 10.1161/ATVBAHA.108.166736. [DOI] [PubMed] [Google Scholar]
- 29.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallon V, Wyatt AW, Klingel K, Huang DY, Hussain A, Berchtold S, Friedrich B, Grahammer F, Belaiba RS, Gorlach A, Wulff P, Daut J, Dalton ND, Ross J, Jr, Flogel U, Schrader J, Osswald H, Kandolf R, Kuhl D, Lang F. SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. Journal Molec Med. 2006;84:396–404. doi: 10.1007/s00109-005-0027-z. [DOI] [PubMed] [Google Scholar]
- 31.Terada Y, Kuwana H, Kobayashi T, Okado T, Suzuki N, Yoshimoto T, Hirata Y, Sasaki S. Aldo-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. Journal Am Soc Nephrol. 2008;19:298–309. doi: 10.1681/ASN.2007050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oemar BS, Luscher TF. Connective tissue growth factor. Friend or foe? Arterioscler Thrombs Vasc Bio. 1997;17:1483–148. doi: 10.1161/01.atv.17.8.1483. [DOI] [PubMed] [Google Scholar]
- 33.Swindell WR. Metallothionein and the biology of aging. Ageing Research Reviews. 2011;10:132–145. doi: 10.1016/j.arr.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldo induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149:1009–1014. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- 35.Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, Leopold JA. Aldo increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. Jnl Biol Chemy. 2009;284:7665–7672. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matlung HL, Bakker EN, VanBavel E. Shear stress, reactive oxygen species, and arterial structure and function. Antioxidants Redox Signaling. 2009;11:1699–1709. doi: 10.1089/ars.2008.2408. [DOI] [PubMed] [Google Scholar]
- 37.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldo mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB Journal. 2006:E846–E854. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 38.Pu Q, Neves MF, Virdis A, Touyz RM, Schiffrin EL. Endothelin antagonism on Aldo-induced oxidative stress and vascular remodeling. Hypertension. 2003;42:49–55. doi: 10.1161/01.HYP.0000078357.92682.EC. [DOI] [PubMed] [Google Scholar]
- 39.Rainger GE, Nash GB. Cellular pathology of atherosclerosis: smooth muscle cells prime cocultured endothelial cells for enhanced leukocyte adhesion. Circulation Research. 2001;88:615–622. doi: 10.1161/01.res.88.6.615. [DOI] [PubMed] [Google Scholar]
- 40.Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.