Abstract
PURPOSE
The aim of this in vitro study was to investigate the adhesion of initial colonizer, Streptococcus sanguis, on resin, titanium and zirconia under the same surface polishing condition.
MATERIALS AND METHODS
Specimens were prepared from Z-250, cp-Ti and 3Y-TZP and polished with 1 µm diamond paste. After coating with saliva, each specimen was incubated with Streptococcus sanguis. Scanning electron microscope, crystal violet staining and measurement of fluorescence intensity resulting from resazurin reduction were performed for quantifying the bacterial adhesion.
RESULTS
Surface of resin composite was significantly rougher than that of titanium and zirconia, although all tested specimens are classified as smooth. The resin specimens showed lower value of contact angle compared with titanium and zirconia specimens, and had hydrophilic surfaces. The result of scanning electron microscopy demonstrated that bound bacteria were more abundant on resin in comparison with titanium and zirconia. When total biofilm mass determined by crystal violet, absorbance value of resin was significantly higher than that of titanium or zirconia. The result of relative fluorescence intensities also demonstrated that the highest fluorescence intensity was found on the surface of resin. Absorbance value and fluorescence intensity on titanium was not significantly different from those on zirconia.
CONCLUSION
Resin specimens showed the roughest surface and have a significantly higher susceptibility to adhere Streptococcus sanguis than titanium and zirconia when surfaces of each specimen were polished under same condition. There was no significant difference in bacteria adhesion between titanium and zirconia in vitro.
Keywords: Biofilm, Resin, Titanium, Zirconia
INTRODUCTION
Oral biofilm is the diverse microbial community found on the tooth surface, embedded in a matrix of polymers of bacterial and salivary origin. Oral biofilm has been known to be closely related with the occurrence of oral disease.1 The formation of oral biofilm may lead to development of dental material surface biodegradation and secondary caries and periodontal inflammation, main reasons for the restoration replacement. In the process of plaque formation on solid substrate surfaces including teeth, implant and restorative materials, initial adhesion of the "early colonizers" to the surface is a critical step.1-3
Oral streptococci have been known to bind to proteins such as alpha-amylase, proline-rich proteins and glycoproteins, and are recognized as early colonizers.4 Streptococcus sanguis (S. sanguis) is thought to be one of the first bacterial species selectively adhere to teeth and colonize on saliva-coated teeth. This species appears in the human oral cavity after tooth eruption, and it becomes a normal inhabitant of the human mouth.4,5
Numerous factors have been identified to influence oral biofilm formation such as surface roughness and surface free energy.6,7 Microscopic examination of early plaque formation on teeth showed the adhesion of the initial colonizing bacteria along cracks and pits in enamel, suggesting the influence of surface structure on bacterial adhesion.3 It is evident that implant and restorative materials have different surface characteristics.
Various affinities of oral bacteria adhesion have been reported for different materials including titanium.7-10 Resin composites have been widely used for operative, esthetic, and prosthodontic treatments.3,8 Dental ceramic materials also applied to broad range of clinical practice. Especially, zirconia has been introduced to improve esthetics for natural teeth and implant prostheses because of its biocompatibility, high resistance to wear and fracture by fatigue loading.10-12 The purpose of this study is to compare and characterize biofilm formation on commonly utilized restorative materials such as composite resin, titanium and zirconia.
MATERIALS AND METHODS
Specimen preparation
Table 1 gives an overview of the materials used in this study. Resin composite was dispensed from a syringe into a mold, and then light-cured for 30 seconds from the top and the bottom side respectively with a light curing unit (Shinwon Dental, Seoul, Korea). Commercially pure titanium (cp-Ti) bars were machined into discs and 3Y-TZP powder was die-pressed into disks and then isostatically pressed at 140 MPa. The green compacts were sintered for 2 hours at 1600℃ in air. Three kinds of 12 mm diameter disc specimens were gradually polished and finished with 1 µm diamond paste to acquire mirror-like surface.
Table 1.
Characteristics of the investigated materials
Determination of surface properties
The average surface roughness (Ra) and topography were measured by the confocal laser microscope (Zeiss, Germany). The static water contact angle of each surface of specimen was measured with a Phoenix 300 contact-angle meter (Surface Electro Optics, Korea) at room temperature.
Bacterial and adhesion assays
S. sanguis 804 (NCTC 10904) was maintained in sterile trypticase soy broth (BD Diagnostics, MD, USA) supplemented with yeast extract (BD Diagnostics). Prior to seeding, sterilized specimens were placed into a 24-well culture plate and were incubated with saliva for 2 hours at 37℃. The saliva was collected from one healthy donor who did not show any active carious lesions or periodontal diseases and sterilized by filtration devices with pore sizes 0.2 µm (Millipore, MA, USA). After washing with PBS, bacteria were seeded onto the specimens at a density of 1×106 bacteria/cm2.
The crystal violet (CV) assay was performed to determine the total amount of biofilm. After 4 hours incubation period, the growth medium was discarded. Then, wells were washed once with PBS and the plates were air-dried. The biofilm was stained using 1% crystal violet solution (Sigma-Aldrich, MD, USA), followed by 10 minutes incubation time at room temperature. Then, the excess of unbound dye was removed by washing the plates with deionized water. The bound CV was extracted with destaining solution (80% ethanol, 20% acetone). The amount of biofilm was measured at optical density of 595 nm using a microplate reader (Bio-Rad Laboratories, CA, USA). The background staining was corrected by subtracting the mean value for CV bound to negative controls.
The fluorescence resazurin (0.75 g/ml, Sigma, MD, USA) was used to determine the quantity of viable adherent bacteria. Bacterial suspension with resazurin was incubated on each specimen at 37℃ for 150 minutes. After washing with PBS, fluorescence intensities were recorded by detection reader (Fluostar optima; MBG Labtech, Offenburg, Germany) at wavelengths of 540 nm excitation and 590 nm emissions.
S. sanguis attachment and morphology were also assessed after 4 hours culture by scanning electron microscopy (JEOL, Tokyo, Japan). Bacteria grown on the different specimen were fixed with sodium phosphate buffered 2.5% glutaraldehyde. After washing twice in the 0.1 M phosphate buffer, bacteria are post fixed with 1% OsO4 in saturated HgCl2. After dehydration in graded ethanol, bacteria are critical point dried and coated with gold by ion sputtering for 2 minutes. Specimens were examined in a SEM at an accelerating voltage of 15 kV.
Statistics
Statistical analysis was performed using one-way analysis of variance (ANOVA) and the Tukey-Kramer multiple comparison test was used for post hoc analysis. Data were expressed as a mean ± standard deviation (SD). Experiments were repeated at least three times. In all analyses, a P-value of <.05 was considered statistically significant.
RESULTS
The surface of resin composite was significantly rougher than that of titanium and zirconia although final polishing condition was the same (Table 1). However, all tested specimens demonstrated mean surface roughness values below 0.2 µm, and were therefore classified as smooth clinically. Table 1 also reports the mean values of water contact angles measurements, ranging between 54.2° and 75.1°. The contact angle of titanium and zirconia were similar, however resin specimen showed significantly lower contact angle.
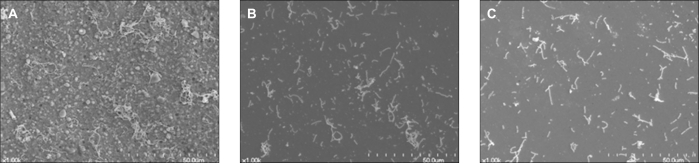
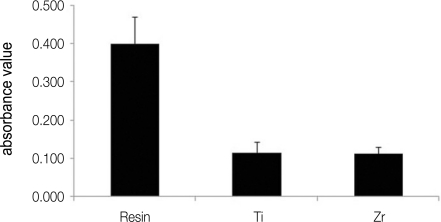
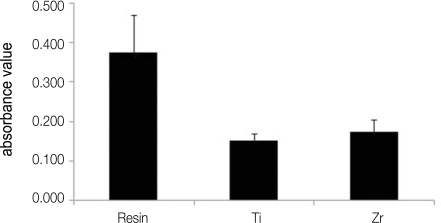
A monolayer of S. sanguis was found on all investigated specimen surface (Fig. 1). It can be seen that there is a difference among the number of bacteria on resin, titanium and zirconia. As observed by SEM, biofilm colonies were more abundant on resin in comparison with those of titanium and zirconia (Fig. 1). A further analysis was performed to investigate the number of attached bacteria on each specimen quantitatively. Figure 2 shows the total biofilm mass determined by crystal violet staining. Absorbance value from resin showed significantly higher than those from titanium and zirconia. There is no difference in bacterial adhesion between titanium and zirconia (Fig. 2). The result of relative fluorescence intensities resulting from resazurin reduction also demonstrated that viable S. sanguis bound on each specimen. The significantly higher fluorescence intensity was found on the surface of resin. The fluorescence intensity on titanium was not significantly different from that on zirconia (Fig. 3).
Fig. 1.
Scanning electron microscope images of the surface of resin (A), titanium (B), zirconia (C) after exposure to a suspension of S. sanguis for 4 hours.
Fig. 2.
Comparison of total biofilm mass determined by crystal violet staining among resin, titanium (Ti) and zirconia (Zr).
Fig. 3.
Relative fluorescence intensities of resin, titanium (Ti) and zirconia (Zr).
DISCUSSION
Oral biofilm formation on the surface of restorative and implant materials leads to secondary caries formation and affects healing process negatively. Therefore, it is important to investigate the initial bacteria adhesion on the surface of biomaterials. The formation of salivary pellicle is considered as the first step in biofilm formation. The initial colonizers, including S. sanguis, S. gordonii and S. oralis, adhere to the pellicles on solid surfaces by several factors, namely, electrostatic and hydrodynamic interactions, thermodynamic parameters and adhesion-receptor interactions.5,11 This event leads to subsequent adhesion of cariogenic microorganisms such as S. mutans and periodontal pathogens, which may induce gingival and periodontal inflammation.13,14
The SEM examination confirmed that S. sanguis can bind directly to the surface of resin, titanium and zirconia. However, adhesion of the total and viable bacteria on resin is significantly higher when evaluated by crystal violet staining and fluorometic quantification, respectively.
Numerous factors influencing oral biofilm formation have been identified. Surface roughness has been regarded as one of the most important factors. With regard to the influence of surface roughness on biofilm formation, rougher surface results to the increased adhesion of bacteria.6,7 In this study, we tried to eliminate the difference of roughness by polishing 1 µm diamond paste. Although, the roughness of resin showed similar level with previous report,3 it is still higher than the roughness of titanium and zirconia. Previous report showed that protein adsorption and bacterial adhesion in vivo are primarily determined by a threshold surface roughness of 0.2 µm, therefore Ra less than threshold level appears to have a negligible effect.15,16 However, correlation of surface roughness and bacterial adhesion could be observed this in vitro study. To confirm this result a further study is needed. When comparing between titanium and zirconia, our results demonstrated that there is no significant difference on S. sanguis adhesion. This contrasts with previous reports9,17 showing that fewer bacteria adhered to zirconia in comparison with titanium of a similar surface roughness in vivo. Discordance may be derived from the difference of roughness. Previous studies compared the bacterial adhesion using titanium and zirconia with rough surface which showed approximately 0.73 µm of Ra. To confirm, a further study will be performed to compare the bacterial adhesion according to the different level of roughness on biomaterials including titanium, zirconia and resin.
Surface hydrophobicity is another crucial element for influencing the bacterial adhesion.6,15 S. sanguis is highly hydrophobic microorganism. It is possible that the difference of bacterial adhesion is derived from the surface hydrophobicity. However, hydrophobicity of resin surface is lower than that of titanium and zirconia and more bacterial adhesion was observed. This observation may be the result from the difference of surface roughness even though all the specimens have surface roughness less than 0.2 µm.
The additional physic-chemical characteristics including crystallinity have been shown to affect biofilm formation in vivo and in vitro.7,18 It is unreasonable to conclude that difference in bacterial adhesion is entirely attributed to different surface roughness in this study. Further studies are needed to investigate the bacterial adhesion on the various biomaterials not only by roughness but also by additional surface characteristics.
CONCLUSION
Within the limitations of this study, composite resin has a significantly higher susceptibility to adhere the initial colonizer, S. sanguis, than titanium and zirconia under the same polishing condition. The amount of both total and viable bacteria on resin was higher than those on titanium and zirconia. There was no significant difference in bacteria adhesion between titanium and zirconia in vitro.
Footnotes
This study was supported by the grant of Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) No.R01-2007-000-10977-0.
References
- 1.Filoche S, Wong L, Sissons CH. Oral biofilms: emerging concepts in microbial ecology. J Dent Res. 2010;89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 2.Scheie AA, Petersen FC. The biofilm concept: consequences for future prophylaxis of oral diseases? Crit Rev Oral Biol Med. 2004;15:4–12. doi: 10.1177/154411130401500102. [DOI] [PubMed] [Google Scholar]
- 3.Ono M, Nikaido T, Ikeda M, Imai S, Hanada N, Tagami J, Matin K. Surface properties of resin composite materials relative to biofilm formation. Dent Mater J. 2007;26:613–622. doi: 10.4012/dmj.26.613. [DOI] [PubMed] [Google Scholar]
- 4.Hojo K, Nagaoka S, Ohshima T, Maeda N. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88:982–990. doi: 10.1177/0022034509346811. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Helmerhorst EJ, Leone CW, Troxler RF, Yaskell T, Haffajee AD, Socransky SS, Oppenheim FG. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol. 2004;97:1311–1318. doi: 10.1111/j.1365-2672.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 6.Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 7.Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. 2009;24:616–626. [PubMed] [Google Scholar]
- 8.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89:657–665. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 9.Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004;75:292–296. doi: 10.1902/jop.2004.75.2.292. [DOI] [PubMed] [Google Scholar]
- 10.Auschill TM, Arweiler NB, Brecx M, Reich E, Sculean A, Netuschil L. The effect of dental restorative materials on dental biofilm. Eur J Oral Sci. 2002;110:48–53. doi: 10.1046/j.0909-8836.2001.101160.x. [DOI] [PubMed] [Google Scholar]
- 11.Hahnel S, Rosentritt M, Handel G, Bürgers R. Surface characterization of dental ceramics and initial streptococcal adhesion in vitro. Dent Mater. 2009;25:969–975. doi: 10.1016/j.dental.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Manicone PF, Rossi Iommetti P, Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. J Dent. 2007;35:819–826. doi: 10.1016/j.jdent.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda M, Matin K, Nikaido T, Foxton RM, Tagami J. Effect of surface characteristics on adherence of S. mutans biofilms to indirect resin composites. Dent Mater J. 2007;26:915–923. doi: 10.4012/dmj.26.915. [DOI] [PubMed] [Google Scholar]
- 14.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 15.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 16.Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 1997;13:258–269. doi: 10.1016/s0109-5641(97)80038-3. [DOI] [PubMed] [Google Scholar]
- 17.Al-Ahmad A, Wiedmann-Al-Ahmad M, Faust J, Bächle M, Follo M, Wolkewitz M, Hannig C, Hellwig E, Carvalho C, Kohal R. Biofilm formation and composition on different implant materials in vivo. J Biomed Mater Res B Appl Biomater. 2010;95:101–109. doi: 10.1002/jbm.b.31688. [DOI] [PubMed] [Google Scholar]
- 18.Puckett SD, Taylor E, Raimondo T, Webster TJ. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31:706–713. doi: 10.1016/j.biomaterials.2009.09.081. [DOI] [PubMed] [Google Scholar]