Abstract
Background:
The genus Bersama belongs to the Melianthaceae family and comprises of four species (B. swinnyi, B. yangambiensis, B. abyssinica, and B. engleriana) all of which are very high trees; the latter two detected species are found in Cameroon. Previous phytochemical investigation on B. yangambiensis, B. swinnyi, and B. abyssinica led to the isolation of triterpenes, saponins, flavonoids, and xanthones.
Method:
The stem bark of B. engleriana were collected in the village, Baham near Bafoussam city, Cameroon in August 2003 and identifi ed by Dr. Onana National Herbaruim, Yaoundι, Cameroon. The air dried and powdered stem bark of B. engleriana (1 kg) was extracted at room temperature with CH2Cl2-MeOH (1:1) 5 L for 48 hours. The mixture of the solvent was removed by evaporation to yield 200 g of crude extract. The latter was then dissolved in CH2Cl2 to give the CH2Cl2 soluble fraction of 5 g and a remaining gum of 195 g. Part of the remaining gum (22 g) was dissolved in water and extracted four times with butanol to give 12 g of red oil; which was then separated by paper chromatography, with butanol-acetic acid-water (4:1:5), to give 3 g of orange gum; purification was carried out on HPLC with MeOH (100%) to yield 2 g of mangiferin (1) as red oil. The CH2Cl2 soluble extract was eluted on silica gel n-hexane-CH2Cl2 gradient ratio and Sephadex LH-20 (n-hexane -CH2Cl2 -MeOH, (7:4:0.5) to afford compounds swinniol (2), Δ4-stigmaster-3β-ol (3), 4-methylstigmaster-5,23-dien-3β-ol(4).
Results:
Herein, we carried out a phytochemical study of the stem bark of B. engleriana, and we report herein the isolation and structural elucidation of mangiferin, in addition to three triterpenes, previously reported from other species of the genus.[3,5] The assignment of the signals of mangiferin was determined using 1H, 13C-NMR, and 2D-NMR spectral data (HMQC, COSY, HMBC). The terpenoids were identifi ed by comparison of their 1H and 13C-NMR spectra with the literature data. Fractionation of the CH2Cl2-MeOH (1:1) extract of the stem bark of B. engleriana Guike gave mangiferin (1), in addition to three previously reported triterpenes, swinniol (2), Δ4-stigmaster-3β-ol (3), and 4-methylstigmaster-5,23-dien-3-β-ol (4).
Conclusions:
A chemical investigation of the CH2Cl2-MeOH extract of the stem bark of Bersama engleriana afforded a xanthone C-glucoside (mangiferin) and fi rst isolation of three terpenoids from this species: swinniol (2), Δ4-stigmaster-3β-ol (3), and 4-methylstigmaster-5,23-dien-3-β-ol (4). The complete 1H and 13C chemical shift assignments of mangiferin were determined using 1D and 2D NMR spectroscopic data (COSY, HMQC, HMBC, DEPT). The structures of the terpenoids were determined from their 1H and 13C NMR data and compared with the literature data.
Keywords: Bersama engleriana, melianthaceae, terpenoids, xanthone, mangiferin
INTRODUCTION
The genus Bersama belongs to the Melianthaceae family and comprises of four species (B. swinnyi, B. yangambiensis, B. abyssinica, and B. engleriana) all of which are very high trees; the latter two detected species are found in Cameroon.[1] B. abissinica is used in the form of a decoction for stomach ache, while B. engleriana is used to treat syphilis, injuries, and fever. The pharmacological activity of B. yangambiensis was studied,[2] the antitumor action of B. abyssinica is known[3] and the anti-HIV activity of the Ethiopian species has been studied.[4] Previous phytochemical investigation on B. yangambiensis, B. swinnyi, and B. abyssinica led to the isolation of triterpenes,[3,5] saponins,[6] flavonoids,[3] and xanthones.[3,7] In continuation of our investigation of the Cameroonian medicinal plants, we carried out a phytochemical study of the stem bark of B. engleriana, and we report herein the isolation and structural elucidation of mangiferin, in addition to three triterpenes, previously reported from other species of the genus.[3,5] The assignment of the signals of mangiferin was determined using 1H, 13C-NMR, and 2D-NMR spectral data (HMQC, COSY, HMBC). The terpenoids were identified by comparison of their 1H and 13C-NMR spectra with the literature data.
MATERIALS AND METHODS
General
1H-NMR (500 MHz, CDCl3, and DMSO), 13C-NMR (125 MHz, CDCl3, and DMSO), and the 2D spectra (1H-1H COSY, HMQC, HMBC) were recorded on the JEOL EAC 600 MHz spectrometer, with Trimethylsilane (TMS) as an internal standard. The IR spectrum (KBr) was taken on a HORIBA FT-720 spectrometer. Optical rotation was determined with the help of a HORIBA SEPA-300 spectropolarimeter. Electron impact-mass spectrometry (EI-MS) analyses were recorded on a JEOL SX102A mass spectrometer. Column chromatography was carried out on silica gel 60 (Merck; 230 – 400 mesh) and Sephadex LH-20 (Pharmacia Co. Tokyo, Japan). TLC was performed on silica gel 60 F254 plated (0.25 mm, Merck Co.), Column chromatography (CC) was carried out on Kieslgel 60 (Merck; 230–400 mesh) and spots were detected under UV light and colored by spraying with 10% H2SO4 solution followed by heating.
Plant material
The stem bark of B. engleriana were collected in the village Baham near Bafoussam city, Cameroon in August 2003 and identified by Dr. Onana National Herbaruim, Yaoundé, Cameroon where a voucher specimen (No 3454/SRFK) was also deposited.
Plant identification was performed by Dr. Onana, Cameroon National Herbaruim, Yaoundé, where a voucher specimen (No 3441/SRFK) was deposited.
Extraction and Isolation
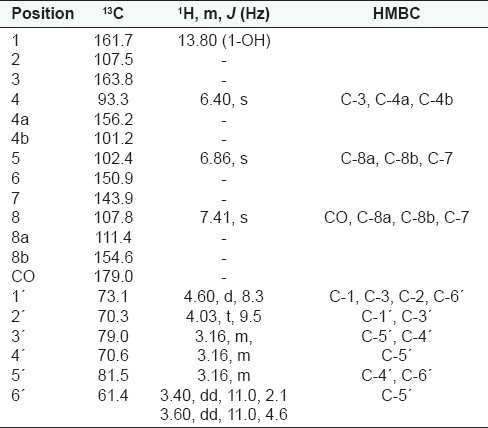
2-C-β-D-glucopyranosyl-1,3,6,7-tetrahydroxyxanthone (mangiferin , 1) — The air dried and powdered stem bark of B. engleriana (1 kg) was extracted at room temperature with CH2Cl2–MeOH (1:1) 5 L for 48 hours. The mixture of the solvent was removed by evaporation to yield 200 g of crude extract. The latter was then dissolved in CH2Cl2 to give the CH2Cl2 soluble fraction of 5 g and a remaining gum of 195 g. Part of the remaining gum (22 g) was dissolved in water and extracted four times with butanol to give 12 g of red oil; which was then separated by paper chromatography, with butanol-acetic acid-water (4:1:5), to give 3 g of orange gum; purification was carried out on HPLC with MeOH (100%) to yield 2 g of mangiferin as red oil; (1H-NMR, 500 MHz, and 13C-NMR, 125 MHz, DMSO) Table 1.
Table 1.
1H, 13C-NMR data of 1 (mangiferin)
Terpenoids – The CH2Cl2 soluble extract was eluted on silica gel n-hexane-CH2Cl2 gradient ratio and Sephadex LH-20 (n-hexane -CH2Cl2 -MeOH, (7:4:0.5) to afford compounds (2-4).
23-Hydroxy betulinaldehyde (swinniol, 2)
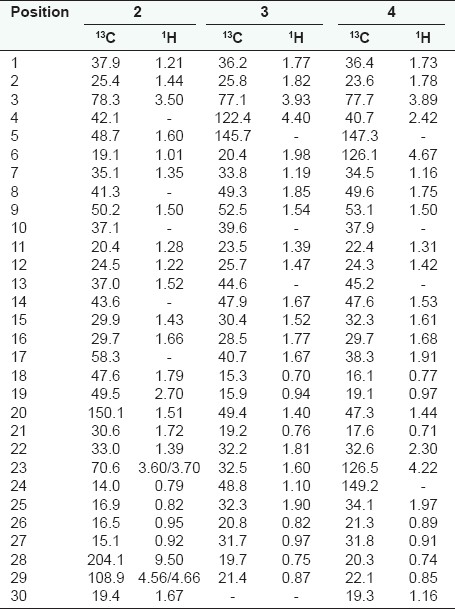
White powder, 12 mg; (1H-NMR, 500 MHz, and 13C-NMR, 125 MHz, CDCl3) Table 2.
Table 2.
1H and 13C-NMR data of compounds 2 – 4
Δ4-stigmaster-3β-ol (3)
White powder, 20 mg; (1H-NMR, 500 MHz, and 13C-NMR, 125 MHz, CDCl3) Table 2.
4-methylstigmaster-5,23-dien-3β-ol (4)
White powder, 24 mg; (1H-NMR, 500 MHz, and 13C-NMR, 125 MHz, CDCl3) Table 2.
RESULTS AND DISCUSSION
Fractionation of the CH2Cl2–MeOH (1:1) extract of the stem bark of B. engleriana Guike gave mangiferin ( 1 ), in addition to three previously reported triterpenes (2 - 4) [Figure 1]. The 1H-NMR of compound 1 revealed the presence of three downfield aromatic singlet signals at δ 6.40 (1H, H-4), 6.86 (1H, H-5), and 7.41 (1H, H-8). Furthermore, the characteristic chemical shifts of the sugar moiety were observed at δ 4.60 (1H, d, J = 8.3 Hz, H-1´), 4.03 (1H, t, J = 9.5 Hz, H-2΄), 3.16 (3H, H-3´, H-4´, H-5´), 3.40 (1H, dd, J = 11.0, 2.1 Hz, H-6´a), and 3.60 (1H, dd, J = 11.0, 4.6 Hz, H-6´b). The 13C-NMR revealed 19 carbons atoms in the molecule. The aglycone, had 13 carbons, including 10 quaternary aromatic carbons at δ 161.7 (C-1), 107.5 (C-2), 163.8 (C-3), 156.2 (C-4a), 101.2 (C-4b), 150.9 (C-6), 143.9 (C-7), 111.4 (C-8a), 154.6 (C-8b), and 179.0 (CO), and three methines at δ 93.3 (C-4), 102.4 (C-5), and 107.8 (C-8). The anomeric carbon at δ 73.1 (C-1´), suggested the C-glucoside moiety (8-9). In addition the other shifts of the sugar carbons appeared at δ 70.3 (C-2´), 79.0 (C-3´), 70.6 (C-4´), 81.5 (C-5´), and 61.4 (C-6´). The location of the OH at C-1 was based on the downfield singlet proton at δ 13.80, hydrogen bonding with the carbonyl. The location of the protons at C-4, C-5, and C-8 was based on their singlet form in 1H-NMR, the COSY and HMBC. The other positions were given according to the HMBC correlations [Table 1]. From the above-mentioned comprehensive NMR data, compound 1 was identified to be mangiferin.[3,7] The 1H-NMR of 2 showed five methyl signals at δ 0.79, 0.82, 0.95, 0.92, and 1.67; accounting for H-24, H-25, H-26, H-27, and H-30, and three protons geminal to the hydroxyl groups at δ 3.50, 3.60, and 3.70 (H-3, H-23a, and H-23b). An aldehyde proton was found at δ 9.50 (H-28), two unsaturated methylenes protons were found at δ 4.56 and 4.66 (H-29a and H-29b). There were a large number of cyclic methylene groups with a chemical shift range (1.00 – 2.50). The 13C-NMR data were in agreement with this information and revealed four carbons in the low field at δ 78.3, 70.6, 108.9, and 204.1, accounting for C-3, C-23, C-29, and C-28, respectively. The 3-OH was assigned a β-configuration from the large value of C-3 (78.3), which could be around 75 ppm in the α-orientation.[5] The β-C-23 stereochemistry was based on the chemical shift of H-23 (3.50/3.60), as this value could be 3.70/4.20 for the β-C-24 configuration.[5] The configuration of C-25, C-26, and C-28 was assigned to be β-oriented from the large multiplets H-5, H-9, and H-18. The stereochemistry of C-20 and C-27 was deduced from H-13 and H-19, which are sharp singlets. Based on this result and comparison with the data of a similar compound,[10] compound 2 was identified as 23-Hydroxy betulinaldehyde, (swinniol), previously isolated from Bersama swinnyi.[5] The stucture of compounds 3 and 4 could be easily determined from the comparison of their 1H and 13C-NMR with literature values.[3,11]
Figure 1.
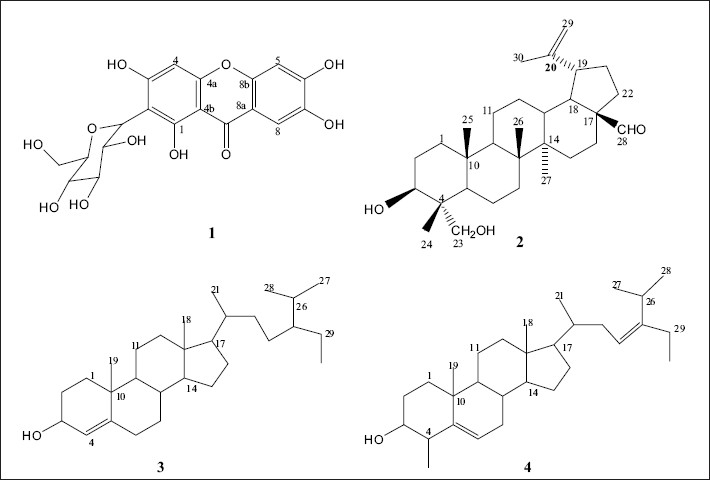
Structures isolated from stem bark extract of Bersama engleriana
Chemotaxonomic Significance
Mangiferin is common in the plant kingdom and has been reported from the genus Hymenophyllum,[9] Arrabidaea[12] and Davallia.[8] Its isolation from B. engleriana is of great interest, as the previous investigation of B. abyssinica, a Kenyan species[3] and B. yangambiensis, a D.R. Congo species[7] reveals the presence of this compound. Thus, this compound may represent a chemotaxonomic marker of this genus, supporting the various uses of these species in traditional medicine.[2]
Acknowledgments
One of the authors, P.C.D, is grateful to the AUF (Agence Universitaire de la Francophonie) and TWAS (Third World Academy of Science) for Fellowships, which enabled him to carry out this study. Dr Onana is acknowledged for plant identification.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Letourze R. Notice sur la carte phytogéographique du Cameroun, imprimerie Jouve, Paris VI, France. 1985 [Google Scholar]
- 2.Vanhaelen M, Indeherberg J, Bauduin J. Further chemical and pharmacological characterisation of Bersama yangambiensis Toussaint (Melianthaceae) J Pharm Sci. 1971;61:1165–7. doi: 10.1002/jps.2600610730. [DOI] [PubMed] [Google Scholar]
- 3.Ian HB, Betty PJ, Hemaia MIM. An investigation of the stem bark of Bersama abyssinica. Planta medica. 1985;51:483–87. doi: 10.1055/s-2007-969569. [DOI] [PubMed] [Google Scholar]
- 4.Asres K, Bucar F, Kartnig T, Witvrouw M, Pannecouque C, De Clercq E. Antiviral activity against human immunodeficiency virus type1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinals plants. Phytother Res. 2001;15:63–9. doi: 10.1002/1099-1573(200102)15:1<62::aid-ptr956>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Thabo M, Mulholland D, Geoff N. Triterpenoids from Bersama swinnyi. Phytochemistry. 1998;49:1819–20. doi: 10.1016/s0031-9422(98)00306-9. [DOI] [PubMed] [Google Scholar]
- 6.Vanhaelen M. New saponoside from Bersama yangambiensis. Phytochemistry. 1969;11:1111–6. [Google Scholar]
- 7.Vanhaelen M. Mangiferin in Bersama yangambiensis. Phytochemistry. 1972;11:854. [Google Scholar]
- 8.Rancon S, Chaboud A, Darbour N, Comte G, Barron D, Raynaud J, et al. A new C-glucosyl xanthone isolated from Davallia solida. Phytochemistry. 1999;52:1677–79. [Google Scholar]
- 9.Kenneth RM, Wallace JW. C-glycosylxanthone and flavonoid variation within the filmy-ferns (Hymenophyllaceae) Phytochemistry. 1980;19:415–20. [Google Scholar]
- 10.Monaco P, Previtera L. Isoprenoids from the leaves of Quercus suber. J Nat Prod. 1984;47:673–6. [Google Scholar]
- 11.Funel C, Berrué F, Roussakis C, Rodriguez RF, Amade P. New cytotoxic steroids from the indian ocean sponge Axinella cf. Bidderi. J Nat Prod. 2004;67:491–4. doi: 10.1021/np034021t. [DOI] [PubMed] [Google Scholar]
- 12.Mendonça PP, Ian Castro-G, Dulce HSS, Young MCM, Tomazela DM, Nogueira ME, et al. New antioxydant C-glucosyl xanthones from the stems of Arrabidaea samydoides. J Nat Prod. 2003;66:1384–7. doi: 10.1021/np030100t. [DOI] [PubMed] [Google Scholar]


