Abstract
Backgorund:
Development of biologically inspired experimental processes for the synthesis of nanoparticles is evolving an important branch of nanotechnology.
Methods:
The bioreduction behavior of plant seed extract of Mucuna pruriens in the synthesis of silver nanoparticles was investigated employing UV/visible spectrophotometry, X-ray diffraction (XRD), and transmission electron microscopy (TEM), Fourier transform – infra red (FT- IR).
Result:
M. pruriens was found to exhibit strong potential for rapid reduction of silver ions. The formation of nanoparticles by this method is extremely rapid, requires no toxic chemicals, and the nanoparticles are stable for several months.
Conclusion:
The main conclusion is that the bioreduction method to produce nanoparticles is a good alternative to the electrochemical methods and it is expected to be biocompatible.
Keywords: Biosynthesis, nanomaterials, silver nanoparticle, transmission electron microscopy, Mucuna pruriens
INTRODUCTION
Nanoparticles are being viewed as fundamental building blocks of nanotechnology. The most important and distinct property of nanoparticles is that they exhibit larger surface area to volume ratio. Biological methods for nanoparticle synthesis using microorganisms, enzymes, and plants or plant extracts have been suggested as possible ecofriendly alternatives to chemical and physical methods.[1] Among the above four, silver nanoparticles play a significant role in the field of biology and medicine. Using plants for nanoparticle synthesis can be advantageous over other biological processes because it eliminates the elaborate process of maintaining cell cultures and can also be suitably scaled up for large-scale nanoparticle synthesis.[2]
An important area of research in nanotechnology is the biosynthesis of nanoparticles such as nanosilver. Biologically synthesized silver nanoparticles could have many applications. As a result, researchers in the field of nanoparticle synthesis and assembly have turned to biological systems for inspiration.[3,4] It has been known for a long time that in nature a variety of nanomaterials are synthesized by biological processes; for example, diatoms, which synthesize siliceous materials,[5,6] and S-layer bacteria that produce gypsum and calcium carbonate layers.[7,8] These results showed that microorganisms could indeed be used for the intracellular synthesis of nanoparticles. Biosynthesis of nanoparticles by plant extracts is currently under exploitation. The use of plants Zadirachta indica (Neem),[2] Medicago sativa (Alfalfa),[9] Aloe vera[10] Emblica officinalis (amla, Indian Gooseberry),[11] has already been reported. According to previous reports, the polyol components and the water-soluble heterocyclic components are mainly responsible for the reduction of silver ions and the stabilization of the nanoparticles, respectively. There are also reports on reductases,[12] and polysaccharides,[13] as factors involved in biosynthesis and stabilization of the nanoparticles, respectively. Our hypothesis is that several factors together determine the nanoparticle synthesis, including the plant source, the organic compounds in the crude seed extract, the concentration of silver nitrate, the temperature, and other than these, even the pigments in the leaf extract.
A green method for nanoparticle preparation should be evaluated from three aspects: the solvent, the reducing agent, and the stabilizing agent. In our work, we present a simple and green method for the preparation of silver nanoparticles using naturally occurring plant extract as both the reducing and stabilizing agent. No other chemical-reducing agent is needed. The reaction is carried out in an aqueous solution in a process that is benign to the environment. Parkinson's disease (PD) is the most common disease of motor system degeneration and, after Alzheimer's disease, the second most common neurodegenerative disease.[14] The seed powder has recently been found to show the anti-Parkinsonism effects, which are probably due to the presence of L-DOPA.[15]
In this report, Mucuna pruriens, a medicinal plant used for anti-Parkinsonism has been shown effective to reduce silver ions as well as the formation of silver nanoparticles in the reduction of Ag (III) to Ag (0) and synthesis of nanoparticles. M. pruriens seed extract found to successfully produce silver nanoparticles of different sizes and shapes.
MATERIALS AND METHODS
All the experiments were conducted at room temperature. Material used for the synthesis of silver nanoparticles is silver nitrate (Loba Chemicals), M. pruriens seeds were purchased from a recognized medical shop. They were brought to the laboratory, cleaned thoroughly in fresh water followed by distilled water, and then shade dried for 3-5 days. Dried seeds were ground to powder. The methanolic extract was prepared by Soxhlet apparatus. The extract was kept in deep freezer until use.
Biosynthesis of silver nanoparticles
1 mM solution of 100 ml silver nitrate (0.034 g) at concentration of 10-3 M was prepared by dissolving DDW, kept in a 250 mL Erlenmeyer flask. 100ml of M. pruriens supernatant (0.060g) was added to the silver nitrate solution. The 95% of the bioreduction of AgNO3–ions occurred within 60 min. The colorless solution, which it turned yellow color slowly, indicating the formation of silver nanoparticles.
Characterization
The UV-visible spectra of silver nanoparticles synthesized were measured on a Shimadzu spectrophotometer (model UV-1601), operated at a resolution of 1 nm. X-ray diffraction (XRD) measurement of the M. pruriens reduced silver nanoparticles was carried out on films of the respective solutions drop-coated onto glass substrates on a Rich Seifert P3000 instrument operating at a voltage of 40 kV and a current of 30mA with Cu K α radiations.
The Fourier transform infrared (FTIR) spectroscopy measurements were carried out to identify the biomolecules for the synthesis of silver nanoparticles. Dry powders of the extract and Ag nanoparticles solutions were centrifuged at 5000 rpm for 15 min and resulting suspensions was redispersed in sterile distilled water. The purified pellets were dried and analyzed by Thermo Nicolet Avator 300 instrument in the diffuse reflectance mode at a resolution of 4cm -1 in KBr pellets.
Samples for high-resolution transmission electron microscopic (HR-TEM) analysis were prepared by drop coating Au nanoparticles solutions onto carbon-coated copper TEM grids. The films on the TEM grids were allowed to stand for 2 min following which the extra solution was removed using a blotting paper and the grid is allowed to dry, prior to the measurement. HR-TEM measurements were performed on a JEOL TEMSCAN2000EX instrument operated at an accelerating voltage at 120 kV.
RESULTS AND DISCUSSION
The silver nanoparticle shows brownish red in color. It is due to the excitation of surface plasmon vibrations in the metal nanoparticles. The M. pruriens mixed with deionized water retained its original light green color. The silver nitrate crystals mixed with deionized water show colorless solution. The silver nitrate treated with M. pruriens solution turned dark brownish red after 1 h due to the formation of silver nanoparticles extracellularly. The appearance of a light brown color in solution suggested the formation of silver nanoparticles.[16] This shows that it was a fast process. This color is primarily due to the surface plasmon resonance of silver nanoparticles. But in silver nitrate solution alone, no change in color was observed and the silver nanoparticles analyzed by UV-Vis spectra and SEM were stable for several days.
UV-vis spectrum
Figure 1 shows the UV-visible spectrum of M. pruriens silver nanoparticles. It is quite sensitive to the formation of silver nanoparticles. It is observed that the strong surface plasmon resonance centered at ca. 437 nm clearly increases in intensity with time, stabilizing after ca. 1 h quickly, which is an indication of rapid preparation of silver nanoparticles. It was observed that the solution containing the silver nanoparticles remained stable for more than several weeks, with no signs of aggregation or precipitate. It is known that silver cations are highly reactive and tend to bind strongly to electron donor groups containing sulfur, oxygen, or nitrogen.[17]
Figure 1.
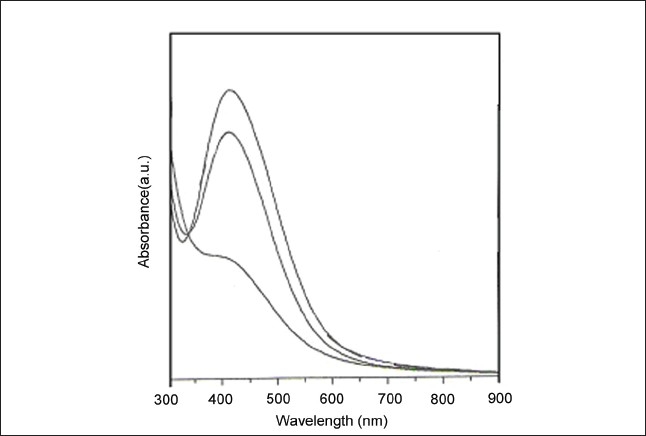
UV-Vis spectrum of silver nanoparticle measured at the time of reaction of Mucuna pruriens seed extract with aqueous solution of 10-3 mol/L AgNO3.
XRD analyses
Figure 2 represents the XRD pattern obtained for the silver nanoparticles. A number of fcc structures of silver Bragg reflections corresponding to (111), (200), (220), and (222) planes were observed. The XRD pattern thus clearly shows that the silver nanoparticles are crystalline in nature.
Figure 2.
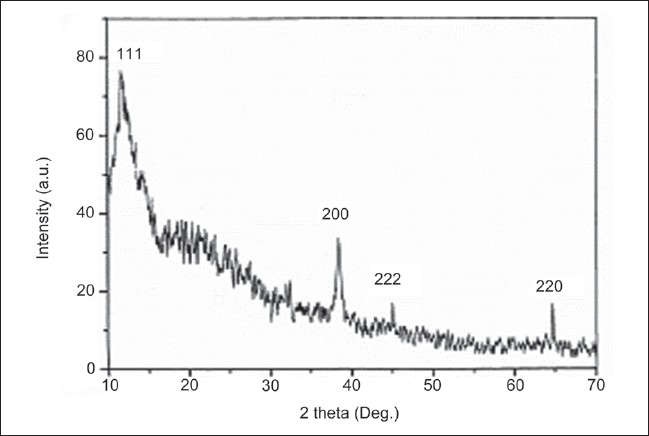
XRD spectrum of silver nanoparticles synthesized by Mucuna pruriens seed extract.
FTIR analyses
A representative FT–IR image of M. pruriens silver nanoparticles. Figure 3 shows the FTIR spectrum recorded from the silver nitrate solution after reaction with M. pruriens seed extract for 1 h. FT-IR analysis revealed the strong bands at 3433, 2924, 1632, and 1072 cm–1. The band at 3433 cm–1 corresponds to NH- amine amino groups, 2924 alkane C-H stretching- lipids, 1632 cm–1 corresponds to amide amino groups. The band at 1384 cm–1 corresponds to C=C stretching of aromatic amine group and the weaker band at 1072 cm–1 is arisen due to carbonyl stretch in proteins. These bands may be assigned to the amide I and II bands of proteins, respectively. It is well known that proteins can bind to silver nanoparticles either through free amine groups or cysteine residues in the proteins and, therefore, stabilization of the silver nanoparticles by surface-bound proteins is a possibility. This is due to that one or more of these proteins may be enzymes that reduce silver nitrate ions and form the silver nanoparticle by reduction technique.
Figure 3.
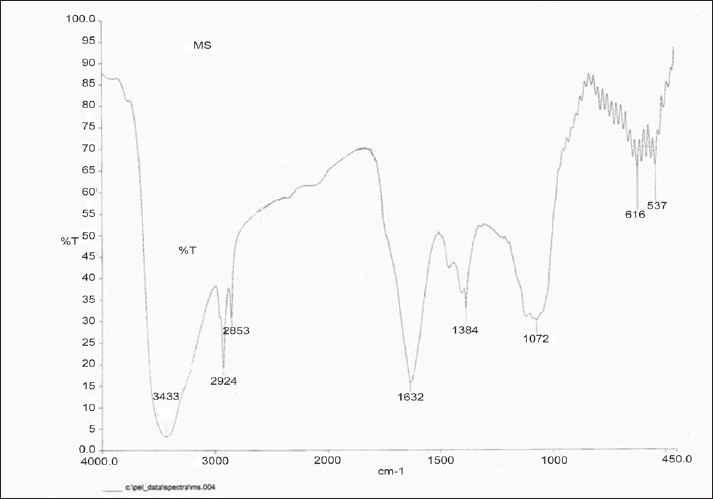
FT-IR spectrum of silver nanoparticles synthesized by reacting AgNO3 with Mucuna pruriens seed extract.
TEM Images
Figure 4a, b shows the TEM image of the Mucuna pruriens silver nanoparticle. The high-resolution study of the nanoparticles using HR-TEM revealed that the nano-Ag predominates with spherical, triangle, oval, and circular morphologies ranging from 10 to 27 nm. TEM analysis revealed that the synthesized nanoparticles are stable in solution over a period of 1 month at room temperature. In this present study, we demonstrate the biosynthesis of M. pruriens silver nanoparticles by green method.
Figure 4.
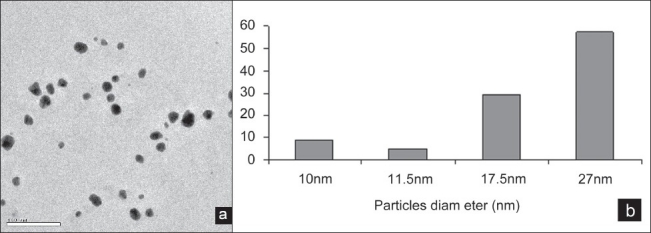
(a) TEM images of Mucuna pruriens silver nanoparticles, (b) TEM images of particle diameter(nm) of Mucuna pruriens silver nanoparticle
CONCLUSION
The reduction of silver ions by M. pruriens seed extract resulted in the formation of stable nanoparticles with spherical, triangle, oval and circular morphologies resulted in 10–27 nm size range of silver nanoparticles. The rate of reaction for the synthesis of nanoparticles by this method (1 h) is rapid. Silver nanoparticles synthesized by the green chemistry approach reported in this study using M. pruriens seed extract could have potent applications in biomedical and biotechnological applications. This simple procedure for the biosynthesis of silver nanoparticles has several advantages such as cost-effectiveness, compatibility for biomedical and pharmaceutical applications, etc.We the authors proudly say that this is the first report of biosynthesis of silver nanoparticles from the anti-Parkinsonian drug M. pruriens.
Acknowledgments
We authors wish to thank Dr.G.Singaravelu, Head, Department of Zoology, Thiruvalluvar University, Vellore and his research Scholars for giving academic support.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: Technological concepts and future applications. J Nanopart Res. 2008;10:507–17. [Google Scholar]
- 2.Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Simkiss K, Wilbur KM. Biomineralization: Cell biology and mineral deposition. New York: Academic Press; 1989. p. 50. [Google Scholar]
- 4.Mann S. Biomimetic Materials Chemistry. New York: VCH; 1996. p. 43. [Google Scholar]
- 5.Mann S. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature. 1993;36:499. [Google Scholar]
- 6.Kroger N, Deutzmann R, Sumper M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science. 1999;286:1129–32. doi: 10.1126/science.286.5442.1129. [DOI] [PubMed] [Google Scholar]
- 7.Pumand D, Sleytr UB. The application of bacterial S-layersinmolecular nanotechnology. Trends Biotechnol. 1999;17:8. [Google Scholar]
- 8.Sleytr UB, Messner P, Pum D, Angew MS. The application of bacterial S-layers in molecular nanotechnology. Chem Int Ed. 1999;38:1034. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1034::AID-ANIE1034>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Gardea-Torresday J, Jose-Yacaman M. Multiple twinned gold nanorods grown by bio- reduction techniques. J Nanoparticle Res. 2001;3:475–81. [Google Scholar]
- 10.Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloe vera Plant Extract. Biotechnol Prog. 2000;22:577–83. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 11.Amkamwar B, Damle C, Ahmad A, Sastry M. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol. 2005;5:1665–71. doi: 10.1166/jnn.2005.184. [DOI] [PubMed] [Google Scholar]
- 12.Anil Kumar S, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmad A, Khan MI. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett. 2007;29:439–44. doi: 10.1007/s10529-006-9256-7. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Yang X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr Res. 2004;339:2627–33. doi: 10.1016/j.carres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Parris M. Parkinson's disease as Multifactorial Oxidative Neurodegeneration Implications for Integrative Management. Altern Med Rev. 2000;5:502–29. [PubMed] [Google Scholar]
- 15.Kulhalli P. Parkinson's disease therapy – an overview. Heritage Heal. 1999:29–30. [Google Scholar]
- 16.Sastry M, Patil V, Sainkar SR. Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine films. Trends Biotechnol. 2002;29:16. [Google Scholar]
- 17.Kowshik M, Ashtaputre SH, Kharazi SH. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14:95–100. [Google Scholar]


