Abstract
Background:
Stevia rebaudiana regulates blood sugar, prevents hypertension and tooth decay. Other studies have shown that it has antibacterial as well as antiviral property.
Methods:
Preliminary phytochemical screening of aqueous, ether and methanolic extracts of S. rebaudiana was done. Acute and sub-acute toxicity were conducted on twenty four Albino rats, divided into one control (Group I) and three treatment groups viz. aqueous extract (Group II), ether extract (Group III) and methanolic extract (Group IV). For the study of antidiabetic effect of S. rebaudiana rats were divided into seven groups (n=6). Diabetes was induced by a single dose of 5% alloxan monohydrate (125 mg/kg, i.p.) after 24 hour fasting.Blood samples were analysed on day 0, 1, 5, 7, 14 and 28.
Results:
Phytochemical tests showed presence of different kinds of phyto-constituents in aqueous, ether and methanol extract of Stevia rebaudiana leaves. Daily single dose (2.0 g/kg) administration of aqueous extract (A.E.) , ether extract (E.E.) and methanol extract (M.E.) for 28 days of S. rebaudiana could not show any significant change in ALT and AST levels in rats. Blood sugar level was found to be decreased on day 28 in groups of rats treated with A.E., E.E. and M.E. of S. rebaudiana.
Conclusion:
The extracts of Stevioside rebaudiana could decrease the blood glucose level in diabetic rats in time dependent manner.
Keywords: Aqueous extract, diabetes, ether extract, methanol extract, rats, Stevia rebaudiana, sub-acute toxicity
INTRODUCTION
Stevia rebaudiana Bertoni commonly known as sweet leaf is a perennial shrub and is a member of Asteraceae family. It is native to the valley of the Rio Monday in highlands of Paraguay, between 25 and 26 degrees south latitude where it grows in sandy soils near streams. Its medicinal use includes regulating blood sugar, preventing hypertension, treatment of skin disorder, and prevention of tooth decay. It also possesses antibacterial and antiviral properties. Standard extracts of S. rebaudiana are used as natural sweetener or dietary supplements in different countries for their content of stevioside or rebaudioside A. These compounds possess upto 250 times the sweetness intensity of sucrose and they are noncalorigenic.
The principles of S. rebaudiana are due to natural sweet active components present in the leaves that is stevioside and rebaudiosides A, B, C, D, and E; dulcoside A; and steviolbioside. Stevioside has a slight bitter aftertaste and provides 250–300 times the sweetness of sugar.[1] The sweet diterpenoid glycoside, rebaudioside F has been isolated from leaves and its structure was established by chemical and spectral studies.[2,3]
In Japan, cultivation of stevia is done as an alternative to artificial sweeteners such as cyclamates saccharine, which are suspected carcinogens. The plants leaves, the aqueous extract of leaves, and purified steviosides are used as sweeteners. Japan currently consumes more stevia than any other country, with stevia accounting for 40% of the sweetener market. Today stevia is cultivated and used in food elsewhere in East Asia, including in China, Korea, Thailand, and Malaysia. China is the world's largest exporter of stevioside.
In US, stevia is mostly employed as sugar substitute. About one-fourth teaspoon of the natural ground leaves is equivalent to one teaspoon of sugar. In South America, a standard infusion is sometimes used as a natural aid for diabetes and hypertension. The difference between stevia and sugar is that stevia does not cause tooth decay. It has been reported that stevia kills the bacteria Streptococcus mutans, which is the prime factor in teeth plaque.
Active principles of many plant species are isolated for direct use as drugs, lead compounds, or pharmacological agents. Different species of medicinal plants are used in the treatment of diabetes mellitus. For diabetes treatment, before the discovery of insulin, the only options were those based on traditional practices.[4] Till today, metformin is the only ethical drug approved for the treatment of noninsulin-dependent diabetes mellitus patients, which is derived from a medicinal plant Galega officinalis. Among those plants used traditionally for the treatment of diabetic complications is S. rebaudiana Bertoni.[5] Hence, the present experiment was undertaken to study the antidiabetic effect of S. rebaudiana in rats.
MATERIALS AND METHODS
Experimental animals
Rats of Wister strain (180–200 g) of both sex and guinea pigs were used in this experiment after approval of the protocol by Institutional Animal Ethics Committee. Rats were kept in cages (2–3 rats per cage) under standard laboratory conditions (light period of 12 h per day and temperature 27 ± 2°C). They were fed standard pelleted feed and access to water ad lib. The rats were acclimatized to the animal house conditions. Prior to each study, the animals were made to fast for 12–14 h but had free access to water.[6]
Plant materials
Fresh mature leaves of authenticated S. rebaudiana leaves were obtained from Directorate of Research, BAU, Kanke, Ranchi. It was air-dried under shed at room temperature and finely powdered with the help of grinder. Leaves powders were always prepared fresh for aqueous, ether, and methanolic extraction.
Preparation of extracts
Aqueous extract – 50 g of powdered leaves were kept in a beaker to which 250 ml of distilled water was added. The mixture was shaken properly and kept at room temperature for 24 h. It was stirred 2–3 times a day. After 24 h, mixture was filtered through ordinary filter paper and the filtrate was evaporated using rotary vacuum evaporator at 40–45°C. The extractability percentage was determined as per the method suggested by Rosenthaler.[7]
Ether extract – 50 g dried powdered leaves of S. rebaudiana was taken into thimble and 750 ml of petroleum ether was added into the soxhlet and boiled on the water bath. After 10–15 cycles, it was decanted into the beaker and was evaporated using rotary vacuum evaporator at 40–45°C.
Methanol extract (ME) – 50 g dried powdered leaves of S. rebaudiana was taken into thimble and 750 ml of methanol was taken into the flask of soxhlet apparatus and cycled 10–15 times. After that it was decanted into the beaker and was left open, so that the methanol evaporated using rotary vacuum evaporator at 40–45°C.
Preliminary phytochemical screening
Standard screening tests of three extracts were carried out for various plant constituents. The crude extracts were screened for the presence or absence of secondary metabolites such as alkaloids, steroidal compounds, phenolic compounds, flavonoids, saponins, tannins, and anthraquinones using standard procedures.[8]
Test for alkaloids
Preliminary test. A 100 mg of an extract was dissolved in dilute hydrochloric acid. Solution was clarified by filtration. Filtrate was tested with Dragendroff's and Mayer's reagents. The treated solutions were observed for any precipitation.
Confirmatory test. Five grams of the extract was treated with 40% calcium hydroxide solution until the extract was distinctly alkaline to litmus paper, and then extracted twice with 10 ml portions of chloroform. Chloroform extracts were combined and concentrated in vacuo to about 5 ml. Chloroform extract was then spotted on thin layer plates. Solvent system (n-hexane-ethyl acetate, 4:1) was used to develop chromatograms and detected by spraying the chromatograms with freshly prepared Dragendroff's spray reagent. An orange or dark colored spots against a pale yellow background was confirmatory evidence for the presence of alkaloids.
Test for steroidal compounds
Salkowski's test – 0.5 g extracts were dissolved in 2 ml chloroform in a test tube. Concentrated sulfuric acid was carefully added on the wall of the test tube to form a lower layer. A reddish brown color at the interface indicated the presence of a steroid ring (i.e., the aglycone portion of the glycoside).
Lieberman's test – 0.5 g extracts were dissolved in 2 ml of acetic anhydride and cooled well in an ice-bath. Concentrated sulfuric acid was then carefully added. A color change from purple to blue to green indicated the presence of a steroid nucleus, i.e., aglycone portion of the cardiac glycosides.
Test for phenolic compounds
To 2 ml of filtered solution of the aqueous macerate of the plant material, three drops of a freshly prepared mixture of 1 ml of 1% ferric chloride and 1 ml of potassium ferrocyanide was added to detect phenolic compounds. Formation of bluish-green color was taken as positive.
The dried EE and ME extracts (100 mg) were dissolved in water. Few crystals of ferric sulfate were added to the mixture. Formation of dark-violet color indicated the presence of phenolic compounds.
Flavonoids
Test for free flavonoids. Five milliliters of ethyl acetate was added to a solution of 0.5 g of the extract in water. The mixture was shaken, allowed to settle, and inspected for the production of yellow color in the organic layer, which is taken as positive for free flavonoids.
Lead acetate test. To a solution of 0.5 g extract in water about 1 ml of 10% lead acetate solution was added. Production of yellow precipitate is considered as positive for flavonoids.
Reaction with sodium hydroxide. Dilute sodium hydroxide solution was added to a solution of 0.5 g of the extract in water. The mixture was inspected for the production of yellow color which considered as positive test for flavonoids.
Test for saponins
Froth test – 0.5 g extracts were dissolved in 10 ml of distilled water in a test tube. The test tube was stoppered and shaken vigorously for about 30 sec. The test tube was allowed to stand in a vertical position and observed over a 30-min period of time. If a “honey comb” froth above the surface of liquid persists after 30 min. the sample is suspected to contain saponins.
Test for tannins
Ferric chloride test. A portion of the extracts were dissolved in water. The solution was clarified by filtration; 10% ferric chloride solution was added to the clear filtrate. This was observed for a change in color to bluish black.
Formaldehyde test. To a solution of about 0.5 g extract in 5 ml water, three drops of formaldehyde and six drops of dilute hydrochloric acid were added. The resulting mixture was heated to boiling for 1 min and cooled. The precipitate formed (if any) was washed with hot water, warm alcohol, and warm 5% potassium hydroxide successively. A bulky precipitate, which leaves a colored residue after washing, indicated the presence of phlobatannins.
Test for phlobatannins. Deposition of a red precipitate when an aqueous extract of the plant part was boiled with 1% aqueous hydrochloric acid was taken as an evidence for the presence of phlobatannins.
Modified iron complex test. To a solution of 0.5 g of the plant extract in 5 milliliter of water a drop of 33% acetic acid and 1 g sodium potassium tartarate was added. The mixture was warmed and filtered to remove any precipitate. A 0.25% solution of ferric ammonium citrate was added to the filtrate until no further intensification of color is obtained and then boiled. Purple or blackish precipitates, which are insoluble in hot water, alcohol, or dilute ammonia, denote the presence of pyrogallol tannin.
Test for anthraquinones
Test for free anthraquinones (Borntrager's test). The hydro-extracts of the plant material (equivalent to 100 mg) was shaken vigorously with 10 ml of benzene, filtered, and 5 ml of 10% ammonia solution added to the filtrate. Shake the mixture and the presence of a pink, red, or violet color in the ammonia (lower) phase indicated the presence of free anthraquinones.
Test for o-anthraquinone glycosides (Modified Borntrager's test). For combined anthraquinones, 5 g plant extracts were boiled with 10 ml 5% sulfuric acid for 1 h and filtered while hot. The filtrate was shaken with 5 ml benzene; the benzene layer separated and half its own volume of 10% ammonia solution added. The formation of a pink, red, or violet color in the ammonia phase (lower layer) indicated the presence of anthraquinone derivatives in the extract.
Toxicity study
Acute oral toxicity
The acute oral toxicity studies of all the three extracts were undertaken as per the Organization for Economic Co-operation and Development (OECD) guidelines for testing of chemicals by up-and-down procedure. The rats were fasted overnight and the weight of each rat used was recorded just before use. Animals were divided randomly into a control and three treatment groups for each extract, each group consisting of four mice (two males and two females). Control group received only the vehicle and each treatment group received orally the EE , ME, and AE of the studied plant in the limit test at a rate of 2000 mg/kg body weight was conducted and terminated after four survivals out of four animals.
Again a higher dose of 5000mg/kg of all extracts were given to three groups of rats. Animals were kept under close observation for 4 h after administering the extracts,[9] and then they were observed daily for 3 days for any change in general behavior and other physical activities
Subacute toxicity
Subacute toxicity of AE, EE, and ME of S. rebaudiana leaves was studied in albino rats of either sex (n = 24). Rats were divided randomly into four groups. Group I (n = 6) served as control and the other three groups were used as experimental groups. Group II, AE (n = 6) III, EE (n = 6), and IV, ME (n = 6) were given 2 g/kg, i.p. of S. rebaudiana leaves per day for 4 weeks. The blood samples were collected on day 0, 14th, and 28th by heart puncture after anesthetizing the rats by ethyl alcohol. The biochemical parameters (ALT and AST) were measured by kit supplied by ERBA chemicals on semiautoanalyzer.
Antidiabetic effect
Diabetes in rats was induced by a single dose of 5% alloxan monohydrate (125 mg/kg, i.p.) after 24 h fasting. Induction of diabetes was confirmed after a week of alloxan treatment by estimation of fasting blood glucose level. Only those rats with blood glucose level between 200–300 mg/dl were included in the study. These rats were further divided into seven groups (I-nondiabetic control; II-diabetic control; III-Aqueous extract, IV-Ether extract, V-Methanolic extract, VI-Glibenclamide, VII-Glibenclamide + Aqueous extract) of six rats each. Groups III–V were subgrouped (IIIA, IIIB, IVA, IVB, VA, VB). Groups I and II (control) received comparable volume of NSS. Groups III–V received lower and higher daily doses of AE, EE, and ME at a rate of 50 and 100 mg/kg p.o., respectively, once daily for 4 weeks. VIth group was administered hypoglycemic drug glibenclamide (5 mg/kg, p.o.) once daily for 4 weeks and group VII was administered daily dose of glibenclamide (50 mg/kg) and 100 mg/kg AE, p.o., respectively. The blood glucose levels were measured by glucometer on day 0, 1, 5, 7, 14, and 28. The blood samples were collected from tail vein puncture and blood glucose levels were analyzed.
RESULTS AND DISCUSSION
Phytochemical studies
In order to determine the presence of chemical constituents, phytochemical tests were performed, which revealed the presence of phytoconstituents in aqueous, ether, and methanol extracts [Table 1], which is in consonance with the report ofRef.[10]
Table 1.
Results of phytochemical screening of the extracts of S. rebaudian leaves
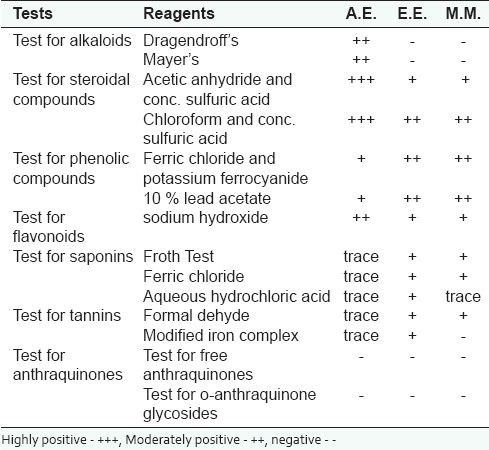
Acute toxicity (determination of ALD50)
A preliminary toxicity study was designed to demonstrate the appropriate safe dose range that could be used for subsequent experiments rather than to provide complete toxicity data on the extract. Acute toxicity studies conducted revealed that the administration of graded doses of three crude aqueous, ether, and methanol extracts (up to a dose of 5000 mg/kg) of S. rebaudiana did not produce significant changes in behaviors such as alertness, motor activity, breathing, restlessness, diarrhea, convulsions, coma, and appearance of the animals. No death was observed up to the dose of 5 g/kg body weight. The mice were physically active. These effects were observed during the experimental period (72 h). The result showed that in single dose, the plant extracts had no adverse effect, indicating that the medium lethal dose (LD50) could be greater than 5 g/kg body weight in mice. Search for the available literature revealed the nontoxic effect of the leaves of S. rebaudiana in mice.[10]
[Table 2] shows the mean concentration of ALT and AST. ALT was estimated to be 40.00 ± 1.40, 40.33 ± 1.39, 40.21 ±1.23 unit/ml in AE-, EE-, and ME-treated groups, respectively. On 28th day, it was found to be 40.75 ± 1.42, 39.75 ± 0.97, and 40.15 ± 1.00 unit/ml in all the three groups. No significant difference in the mean concentration was found.
Table 2.
Effect of A.E, E.E., and M.E. (2.0 g/kg, oral) of S. rebaudiana on serum enzyme activity (Mean±S.E.) of albino rats after once daily administration for 28 days
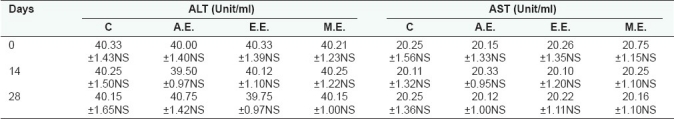
The AST level was recorded as 20.15 ± 1.33, 20.26 ± 1.35, 20.75 ± 1.15 in AE, EE, and ME, respectively. On day 28, the level of AST was recorded as 20.12 ± 1.00, 20.22 ± 1.11, and 20.16 ± 1.10 units/ml in AE, EE, and ME, respectively. Mean values did not show significant difference on day 0 and 28.
Antidiabetic effect
The blood glucose levels were 220.16 ± 8.63, 220.00 ± 11.20 mg/dl in AE-, 209.66 ± 4.15, 220.83 ±09.24 mg/dl in EE-, 218.66 ±4.93, 232.00 ± 11.81 mg/dl in ME-treated rats on day 0 [Table 3]. In the glibenclamide-treated group, the blood glucose was 211.00 ± 5.10 mg/dl on day 0, whereas in glibenclamide + AE treated rats the blood glucose level on day 0 was found to be 208.16 ± 9.23 mg/dl. It may be noted in the above table that a significant decrease in the mean blood glucose levels was found on day 28 in AE-, EE-, and ME-treated rats, both after 50 and 100 mg/kg daily dose administration. The results obtained in this study for extracts of S. rebaudiana showed decrease in the mean blood glucose levels which were in agreement with the observations of Abdula et al.[11]
Table 3.
Effect of treatment (mean±S.E.) of S. rebaudiana extracts on blood glucose level in alloxaninduced diabetic rats
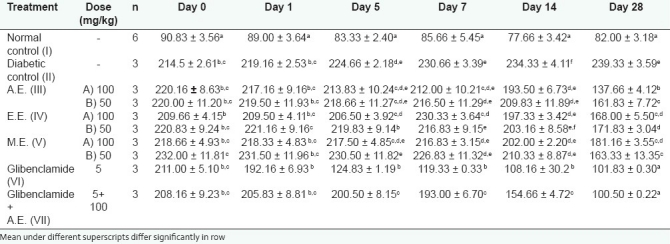
The study also showed that the rats which had been given the extracts of AE and EE at higher dose (100 mg/kg) exhibited greater decrease in mean blood glucose level as compared to those given at a rate of 50 mg/kg b.w. on day 28. Therefore, it is obvious from the results obtained in this study that antihyperglycemic activity of AE and EE were dose-dependent. The findings obtained in this investigation are similar to that of Jeppesen et al.,[12] who pointed that stevioside and steviol dose dependently enhanced insulin secretion. The data showed that there was significant decrease in the mean blood glucose level (100.50 ± 0.22) in the group VII where AE was given with glibenclamide. However, it did not differ with the blood glucose level on day 28 (101.83±0.30) as compared to that group which received only glibenclamide. Therefore, it is obvious that glibenclamide and AE both are working differently in rats.
The stevia leaves powder has also been reported to reduce the blood glucose concentration of diabetic rats. The findings of this experiment are similar to the reports of Chang et al.[13] However, it was observed that as hypoglycemic drug, glimepiride, was better, though powdered form of stevia (S. rebaudiana Bertoni) leaves at a rate of 250 mg/kg body weight showed very potent hypoglycemic efficacy, but comparatively less effective than glimepiride. It is known that sulfonylureas like glimepiride produce hypoglycemia by increasing the secretion of insulin from the pancreas and these compounds are active in mild streptozotocin-induced diabetes, whereas they are inactive in intense streptozotocin diabetes (nearly all β-cells have been destroyed).[14,15] Since our results showed that glimepiride reduces the blood glucose levels in hyperglycemic animals, so it can be postulated that the state of diabetes was not severe. It may be mentioned that stevioside regulates blood glucose level by enhancing insulin secretion and also enhances glucose utilization in peripheral tissues and muscles in diabetic rats.[16]
It was concluded that the extracts of S. rebaudiana could decrease the blood glucose level in diabetic rats in time-dependent manner. The antidiabetic effect might be due to steviosides counteracting the glucotoxicity in β-cells or also by suppressing the glucagon secretion by α-cell of pancreas; both the mechanisms have been depicted by Shibata et al.[17] and Chen et al.[18]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kolb N, Herrera JL, Ferreyra DJ, Uliana RF. Analysis of sweet diterpene glycosides from Stevia rebaudiana: Improved HPLC method. J Agric Food Chem. 2001;49:4538–41. doi: 10.1021/jf010475p. [DOI] [PubMed] [Google Scholar]
- 2.Brandle J. Nature's Natural Low Calorie Sweetener. Canada: Agriculture and Agri-Food; 2004. FAQ- Stevia. [Google Scholar]
- 3.Starratt AN, Kirby CW, Pocs R, Brandle JE, Rebaudioside F. A diterpene glycoside from Stevia rebaudiana. Phytochemistry. 2002;59:367–70. doi: 10.1016/s0031-9422(01)00416-2. [DOI] [PubMed] [Google Scholar]
- 4.Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. Antihyperglycemic activity of Tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13:550–7. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. Antihyperglycemic effects of Stevioside in Type 2 diabetic subjects. Metab. 2004;53:73–6. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Ragavan B, Krishnakumari S. Antidiabetic effect of T. arjuna bark extract in alloxan induced diabetic rats. Indian J Clin Biochem. 2006;21:123–8. doi: 10.1007/BF02912926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthaler L. The chemical investigation of plant. 1st ed. London: Bell and Sons; 1930. p. 36. [Google Scholar]
- 8.Hymete A. MSc thesis, ILE-IFE. Nigeria: University of IFE; 1986. Phytochemical investigation of the fruit of Lagenaria breviflora Robert; pp. 54–67. [Google Scholar]
- 9.Burger C, Fischer DR, Cordenunzzi DA, Batschauer AP, Filho VC, Soares AR. Acute and subacute toxicity of the hydroalcoholic extract from Wedelia paludosa (Acmela brasiliensis) (Asteraceae) in mice. J Pharm Sci. 2005;8:370–3. [PubMed] [Google Scholar]
- 10.Geuns MJ. Stevioside. Phytochemistry. 2003;64:913–21. doi: 10.1016/s0031-9422(03)00426-6. [DOI] [PubMed] [Google Scholar]
- 11.Abdula R, Jeppesen PB, Rolfsen SE, Xiao J, Hermansen K. Rebaudioside A potently stimulates insulin secretion from isolated mouse islets: Studies on the dose, glucose and calcium dependency. Metab. 2004;53:1378–81. doi: 10.1016/j.metabol.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Jeppesen PB, Gregersen S, Poulsen CR, Hermansen K. Stevioside acts directly on pancreatic beta cells to secrete insulin: Actions independent of cylcic adenosine monophosphate and adenosine triphosphate sensitive potassium ion channel activity. Metab. 2000;49:208–14. doi: 10.1016/s0026-0495(00)91325-8. [DOI] [PubMed] [Google Scholar]
- 13.Chang JC, Wu MC, Liu IM, Cheng JT. Increase of insulin sensitivity by stevioside in fructose-rich chow-fed rats. Hormone and Metabolic Geuns M J. Stevioside. Phytochemistry. 2003;64:913–21. doi: 10.1055/s-2005-870528. [DOI] [PubMed] [Google Scholar]
- 14.Grodsky GM, Epstein GH, Fanska R, Karam JH. Pancreatic action of sulphonylureas. Fed Proc. 1971;36:2719–28. [PubMed] [Google Scholar]
- 15.Yallow RS, Black H, Villazan M, Berson SA. Comparison of plasma insulin levels following administration of tolbutamide and glucose. Diabetes J. 1960;9:356–62. doi: 10.2337/diab.9.5.356. [DOI] [PubMed] [Google Scholar]
- 16.Chen TH, Chen SC, Chan P, Chu Y L, Yang HY, Cheng JT. Mechanism of the polyglycemic effect of stevioside, a glycoside of Stevia rebaudiana. Planta Med. 2005;71:108–13. doi: 10.1055/s-2005-837775. [DOI] [PubMed] [Google Scholar]
- 17.Shibata H, Sawa Y, Oka T, Sonoke S, Kim KK, Yoshioka M. Steviol and steviol glycoside. Glucosyl transferase activities in S. rebaudiana Bertoni. Purification and partial characterization. Arch Biochem Biophys. 1995;321:390–6. doi: 10.1006/abbi.1995.1409. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Jeppesen PB, Nordentoft I, Hermansen K. Stevioside improves pancreatic? cell function during glucotoxicity via regulation of acetyl-CoA carboxylase. Am J Physiol Endocrinol Metab. 2007;292:1906–16. doi: 10.1152/ajpendo.00356.2006. [DOI] [PubMed] [Google Scholar]


