Summary
Background
Concurrent sexual partnerships are widely believed to be one of the main drivers of the HIV epidemic in sub-Saharan Africa. This view is supported by theoretical models predicting that increases in prevalence of concurrent partnerships could substantially increase the rate of spread of the disease. However, the effect of concurrent partnerships on HIV incidence has not been appropriately tested in a sub-Saharan African setting.
Methods
For this population-based cohort study, we used data from the Africa Centre demographic surveillance site in KwaZulu-Natal, South Africa, to try to find support for the concurrency hypothesis. We used a moving-window approach to construct estimates of the geographical variation in reported concurrent and lifetime partners in sexually active men aged 15–55 years (n=2153) across the study area. We then followed up 7284 HIV-negative women (≥15 years of age) in the population and quantified the effect of the sexual behaviour profiles of men in the surrounding local community on a woman's hazard of HIV acquisition.
Findings
During 5 years' follow-up, 693 new female HIV infections occurred (incidence 3·60 cases per 100 person-years). We identified substantial intercommunity heterogeneity in the estimated point-prevalence of partnership concurrency (range 4·0–76·3%; mean 31·5%) and mean number of lifetime sexual partners (3·4–12·9; mean 6·3) in sexually active men in this population. After adjustment for individual-level sexual behaviour and demographic, socioeconomic, and environmental factors associated with HIV acquisition, mean lifetime number of partners of men in the immediate local community was predictive of hazard of HIV acquisition in women (adjusted hazard ratio [HR] 1·08, 95% CI 1·03–1·14, p=0·004), whereas a high prevalence of partnership concurrency in the same local community was not associated with any increase in risk of HIV acquisition (adjusted HR 1·02, 95% CI 0·95–1·09, p=0·556).
Interpretation
We find no evidence to suggest that concurrent partnerships are an important driver of HIV incidence in this typical high-prevalence rural African population. Our findings suggest that in similar hyperendemic sub-Saharan African settings, there is a need for straightforward, unambiguous messages aimed at the reduction of multiple partnerships, irrespective of whether those partnerships overlap in time.
Funding
US National Institute of Child Health and Human Development; Wellcome Trust.
Introduction
Concurrent sexual partnerships are widely held to be one of the primary drivers of the HIV epidemic, especially in sub-Saharan Africa,1–3 where about two-thirds of the world's HIV-positive population live and more than 70% of all new HIV infections occurred in 2008.4 This view is supported by theoretical mathematical models predicting that under specific conditions, small increases in the prevalence of concurrent partnerships could substantially increase the rate of spread of HIV.5–7 Consequently, researchers have argued that reduction of concurrent partnerships should be a major focus of global HIV prevention strategies.8–11 In contrast to serially monogamous sexual partnerships, concurrent partnerships are thought to allow an increased rate of spread of HIV by linking up what would otherwise be discrete sexual networks in time and space. Additionally, being in a concurrent relationship could increase the probability of an infected individual having sex with a susceptible partner during the acute HIV infection stage, when there is higher potential for onward transmission of the virus. However, to date there is no clear empirical evidence to show the effect of concurrent partnerships on the rate of new HIV infections.12,13
Measurement of any effect of concurrency on risk of new infection poses a challenge for standard individual-focused epidemiological methods, in which populations are usually viewed as collections of individuals, rather than as meaningful entities with inherent properties related to the likelihood that individuals within them acquire disease.14 Having concurrent partners is not expected to change an individual's risk of acquiring HIV, provided that partner concurrency does not increase an individual's cumulative number of unprotected sex acts. However, for the two reasons described above, concurrency could increase the rate of HIV spread through a population.2,6 Consequently, studies that test the association between partnership concurrency and infection status at an individual level have been labelled as “theoretically misguided and empirically irrelevant” by proponents of the theory.15 Hence, to test the concurrency hypothesis appropriately, the focus has to be shifted away from an individual's own sexual behaviour patterns and onto the behaviours of the individuals in the network of people from whom an HIV-negative person is likely to choose a sexual partner.
To test this transmission dimension of the concurrency theory and try to find empirical support for the hypothesis, we followed up a large, population-based cohort of HIV-negative women in a rural South African population over 5 years and quantified the effect of the sexual behaviour profiles of men in the surrounding local community (from which an HIV-negative individual most often chooses a sexual partner) on their individual risk of acquiring an infection. We used detailed geographical data and a novel spatial statistical method to create unique virtual communities around each woman to derive sensitive and realistic community-level sexual behaviour estimates of men in the surrounding community. We then used a multivariable statistical model to contrast the increased risk of infection in an HIV-negative woman living in a community with high numbers of reported lifetime sexual partners in men (a widely accepted and robust index of risky sexual behaviour) against that of a woman living in a community with a high prevalence of male concurrent sexual partnerships.
Methods
Setting
This population-based cohort study used data from one of the most comprehensive demographic surveillance sites in Africa—the Africa Centre Demographic Information System.16 The site has collected sociodemographic information on a population of 87 000 individuals within a circumscribed geographic area (434 km2) in rural KwaZulu-Natal, South Africa, for more than a decade. All individuals under surveillance are geolocated to their respective homesteads (accuracy <2 m).17 With the exception of the township, the homesteads in the study area are scattered across the landscape and are not concentrated into villages or compounds as they are in other parts of Africa.
Nested within the demographic information system are the population-based HIV surveillance and sexual behaviour surveys, which take place annually in all consenting resident individuals aged 15 years or older. After written informed consent was given, fieldworkers obtained blood by finger prick and prepared dried blood spots for HIV testing according to Joint UN Programme on HIV/AIDS and WHO guidelines.18 The Africa Centre's HIV cohort is open—ie, individuals continually enter the cohort when they reach the age of 15 years or migrate into the area and leave it because of death or out-migration. 60% of individuals contacted agreed to be tested at least once. More than 24% of the adult population are infected with HIV and infection peaks at more than 50% in women aged 25–29 years and 44% in men aged 30–34 years.19 The rate of new infections remains high and fairly constant over time at around 3·2 new infections per 100 person-years20 and peaks in women at 7·5 per 100 person-years (at age 24 years) and in men at 5·0 per 100 person-years (at age 29 years). These longitudinal HIV incidence estimates have been independently confirmed using a locally calibrated test of recent HIV infection in a cross-sectional sample of individuals.21 The population is characterised by low rates of marriage with only 31% of women and 23% of men ever having been married and 14% of those marriages being polygamous for men.22
Sexual behaviour survey
We used data from a questionnaire about sexual partnership patterns undertaken in 2004 from 2699 men (of whom 2153 were sexually active) who were aged 15–55 years and were resident in the surveillance area.16 63% of men contacted agreed to participate in the survey. Men's sexual behaviour data was used because of the well documented greater reluctance of women compared with men to report on multiple sexual partnerships.23 Participants were asked how many sexual relationships they were currently in and how many lifetime sexual partners they had had. Men were coded as having concurrent partners if they reported currently being in more than one sexual relationship.
Construction of community-level variables
To produce robust estimates of the sexual behaviour variables that vary across continuous geographical space, we used a Gaussian kernel method.17,24 The method does not impose any static geographical boundaries on the data and resulted in community-level estimates that were both sensitive to local variations and robust. Data from 2153 sexually active men were plotted on a map of the study area consisting of a grid of 30 m × 30 m cells in Idrisi Taiga (Clark University, Worcester, MA, USA). We then passed a standard Gaussian kernel of search radius 3 km (total area 28 km2) across the map to derive community-specific measures of the point-prevalence of partnership concurrency (a robust population-level indicator of concurrency25,26) and mean numbers of lifetime partners. The kernel moved systematically across the map and for each cell calculated a Gaussian-weighted estimate of mean lifetime partners and concurrency prevalence within a search radius of 3 km (figure 1). A median of 285 (IQR 104–491) men were included in each community-level calculation. The resulting estimate was then placed onto a new map at the same location as the central cell. The kernel was moved one cell to the right (and then down one row at the end of the row) and the process was repeated. We then standardised the resulting community measures of sexual behaviour against the eligible population of the sexual behaviour survey using 10-year age-bands to adjust for differences in age distribution across the surveillance area. Additionally, we derived male HIV prevalence (n=4982) across the study area for the 2004 HIV survey using the same method.
Figure 1.
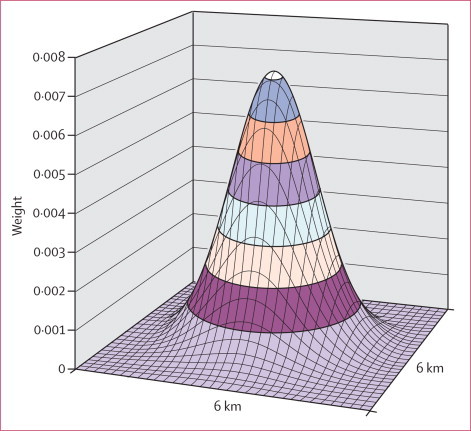
Two-dimensional standard Gaussian kernel of search radius 3 km used to map geographical variations in mean lifetime partners and point-prevalence of concurrency in sexually active men across the surveillance area
The Z axis shows the weights given to each cell. The greater the distance from the centre of the kernel, the lower the weight assigned to that cell in the community-level calculation.
In this population, partner choice is strongly affected by geography and 61% of women reported at least one partnership with a man in the same immediate isigodi (a Zulu term for a small community headed by a local chief, which have a median area of 16·9 km2) during the observation period. However, in view of the scattered distribution of the population and because the optimum size of the individual's community was not precisely known, we investigated the potential effect of the size and shape of the kernel, producing estimates with kernels of 2·5 km, 3·0 km, and 3·8 km radii, which evaluated an area of between 19·6 km2 and 45·4 km2. We also checked the potential effect of the weighting in the kernel by deriving estimates from kernels of SD 1 and 3. The smaller the search radius used in the kernel, the greater the range in community-level estimates obtained and the greater the sensitivity to local variation in the resulting estimates. The use of a large kernel will result in smoothing towards the mean and important geographical variation in the outcome variable might be lost.
Statistical analysis
We followed up 7284 women who were HIV negative at baseline, were tested at least twice during the study period (2004–09), and were resident in the surveillance area at least 50% of the time (only 273 did not meet the latter criterion). Each woman was geolocated to her homestead of residence and the estimated mean number of lifetime partners and point-prevalence of concurrency of men in the surrounding unique local community (as calculated with the Gaussian kernel of search radius 3 km) was extracted. We then used an interval-censored parametric survival analysis of time to HIV seroconversion to investigate the effect of mean number of lifetime partners and point-prevalence of concurrency of men in each woman's virtual community on her hazard of HIV acquisition. The survival model assumed that time to seroconversion followed a Weibull distribution and accounted for the fact that few individuals were tested every year (on average 1·8 years) and therefore precise seroconversion times were not known27 (a detailed description of statistical methods is shown in the webappendix pp 1–2). The resulting estimates from the survival analysis were adjusted for other important individual-level determinants of HIV incidence in the study population (age, number of years in education, wealth tertile, urban locale, marital status, and reporting more than one partner in the previous 12 months over the duration of the study28) and male community-level HIV prevalence. Survey participants were not required to answer all questions, and thus data were missing for the covariates age, marital status, wealth, years of education, and sexual behaviour. A complete case analysis of these covariates would include 5364 of the 7284 eligible women in the cohort. Coverage was lowest for women's sexual behaviour data (75%). To account for missing covariate information in the incidence cohort, we used a multiple imputation procedure with five imputed datasets.29 Hazard ratios, standard errors, and test statistics were adjusted appropriately to account for the imputation procedure. All analyses were done with Stata (version 11.0).
To assess the potential role of the size and shape of the community on the results of the statistical analysis, we did a full set of parallel statistical analyses for kernels of different sizes and shapes. Additionally, we ran the analysis using an alternative community-level index of partnership concurrency—mean numbers of concurrent partners. Furthermore, we ran a secondary analysis to assess the effect of combined community-level male and female sexual behaviour (derived from 2153 men and 4815 women) on the hazard of HIV acquisition in 11 861 participants of both sexes who had undergone repeat testing.
Role of the funding source
The funders had no role in the design and conduct of the study, interpretation of the data, or approval of the report. The corresponding author had access to all study data and made the final decision to submit for publication.
Results
Over the duration of the study (2004–09), we measured an incidence of 3·60 cases (95% CI 3·33–3·87) per 100 person-years (693 new female infections in 19 275·58 person-years of follow-up). The overall point-prevalence of men reporting concurrent partners in the 2004 sexual behaviour survey was 23·2% (95% CI 21·8–24·7). Among men who reported being sexually active, 28·9% (95% CI 27·0–30·8) reported having two or more concurrent partners and the median number of reported lifetime partners was five (IQR three to eight).
The geographical analysis revealed substantial variation in men's sexual behaviour across the different communities of the study area (figure 2). The estimated age-standardised point-prevalence of sexually active men in concurrent relationships varied between 4·0% and 76·3% (mean 31·5%) and standardised mean number of reported lifetime sexual partners varied between 3·4 and 12·9 (mean 6·3) in communities across the surveillance area. There were clear disparities between the two indices in many areas. For example, on the eastern side of the study area around the township (characterised by the highest HIV prevalence17) communities are characterised by high numbers of reported lifetime partners, but low prevalence of partnership concurrency. Substantial differences also occurred along the western boundary of the study area, where partnership concurrency was highly prevalent, but where numbers of lifetime partners were generally low.
Figure 2.
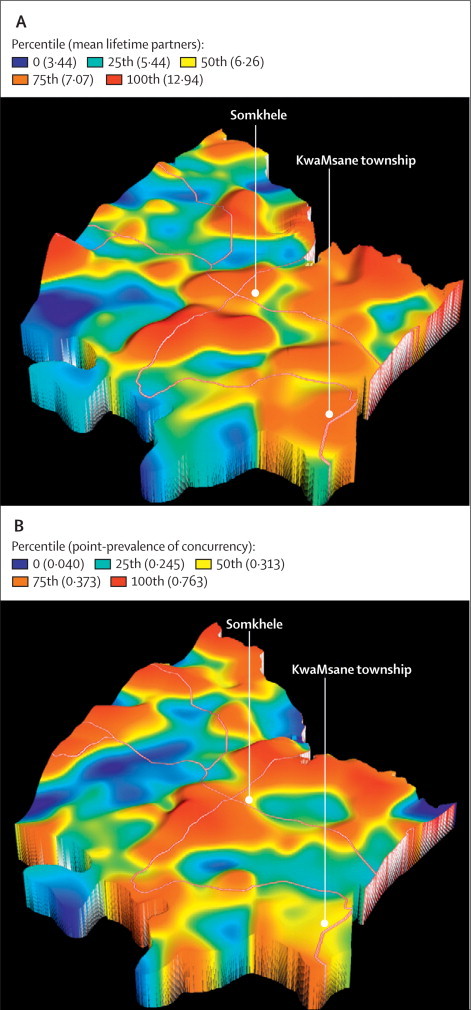
Age-standardised geographical variations in mean lifetime partners (A) and point-prevalence of concurrency (B) in sexually active men across the surveillance area
Obtained by a standard Gaussian kernel of radius 3 km (main roads are superimposed). The Z axis is proportional to the value of the community-level sexual behaviour covariate for any given geographical location.
At an ecological level, female HIV incidence did not differ by prevalence of male concurrency in the surrounding local community (figure 3). With respect to reported lifetime partners, a clear exposure-response relation was evident. Communities with the highest number of lifetime partners in men had the highest HIV incidence in women.
Figure 3.
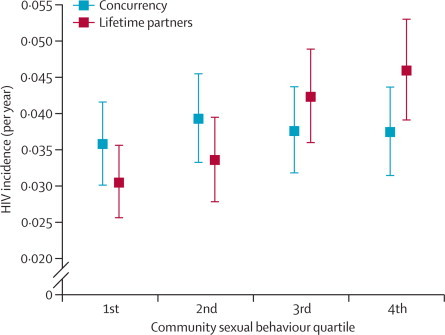
A comparison of female age-standardised HIV incidence by mean lifetime partners and point-prevalence of concurrency among sexually active men in the surrounding local community (as shown in figure 2)
Error bars show 95% CIs.
Table 1 shows the characteristics of women in the cohort and number of HIV seroconversions reported. At an individual level, age, place of residence (urban or rural), marital status, years of education, and reporting of more than one partner in the previous 12 months (at least once during follow-up) were all significantly associated with a woman's hazard of HIV acquisition (table 2). Being in a polygamous marriage was not associated with hazard of HIV acquisition in our analyses, but only 242 women (3%) in the cohort reported being married to a polygamous man. For every 10% increase in community-level HIV prevalence in men, the hazard of HIV acquisition in women increased by 26% (p<0·0001). After adjustment for individual risk factors and sexual behaviour profiles of men in the surrounding community, the hazard ratios decreased to 1·17–1·20 in the three models shown in table 2.
Table 1.
Descriptive characteristics of women in the HIV incidence cohort (N=7284)
| Person-years* | Events | Crude HIV incidence rate (per 100 person-years of observation) | ||
|---|---|---|---|---|
| Community level (men)† | ||||
| Concurrency, quartiles | ||||
| 1st (4·0–24·3%) | 4737·19 | 161 | 3·40 (2·89–3·97) | |
| 2nd (24·4–31·2%) | 4917·73 | 194 | 3·94 (3·41–4·54) | |
| 3rd (31·3–37·3%) | 4824·77 | 170 | 3·52 (3·01–4·09) | |
| 4th (37·4–76·3%) | 4795·89 | 168 | 3·50 (2·99–4·07) | |
| Lifetime partners, quartiles | ||||
| 1st (3·4–5·4) | 4919·32 | 154 | 3·13 (2·66–3·67) | |
| 2nd (5·5–6·2) | 4816·34 | 151 | 3·14 (2·66–3·68) | |
| 3rd (6·2–7·1) | 4746·17 | 188 | 3·96 (3·42–4·57) | |
| 4th (7·1–12·9) | 4793·75 | 200 | 4·17 (3·61–4·79) | |
| HIV prevalence, quartiles | ||||
| 1st (0·0–8·8%) | 4898·92 | 155 | 3·16 (2·67–3·70) | |
| 2nd (8·9–13·1%) | 4918·61 | 168 | 3·42 (2·92–3·97) | |
| 3rd (13·2–17·6%) | 4788·25 | 166 | 3·47 (2·96–4·04) | |
| 4th (17·6–31·1%) | 4669·80 | 204 | 4·37 (3·79–5·01) | |
| Individual level | ||||
| Age (years) | ||||
| 15–19 | 6701·51 | 342 | 5·10 (4·58–5·67) | |
| 20–24 | 2033·72 | 152 | 7·47 (6·33–8·76) | |
| 25–29 | 1023·38 | 53 | 5·18 (3·88–6·77) | |
| 30–34 | 1203·61 | 38 | 3·16 (2·23–4·33) | |
| 35–39 | 1584·07 | 43 | 2·71 (1·96–3·66) | |
| 40–44 | 2086·71 | 30 | 1·44 (0·97–2·05) | |
| ≥45 | 4642·58 | 35 | 0·75 (0·53–1·05) | |
| Years of education, quartiles (years) | ||||
| 1st (0–5) | 4205·94 | 93 | 2·21 (1·78–2·71) | |
| 2nd (6–9) | 4369·76 | 206 | 4·71 (4·09–5·40) | |
| 3rd (10–11) | 4718·77 | 231 | 4·89 (4·28–5·57) | |
| 4th (12) | 2452·75 | 95 | 3·87 (3·13–4·73) | |
| Wealth, tertiles | ||||
| Wealthiest | 3711·45 | 139 | 3·75 (3·15–4·42) | |
| Intermediary wealth | 7729·53 | 272 | 3·52 (3·11–3·96) | |
| Poorest | 7313·06 | 251 | 3·43 (3·02–3·88) | |
| Marital status | ||||
| Single | 14945·57 | 633 | 4·24 (3·91–4·58) | |
| Married, monogamous | 3635·14 | 51 | 1·40 (1·04–1·84) | |
| Married, polygamous | 689·83 | 9 | 1·30 (0·60–2·48) | |
| Residence | ||||
| Rural | 13723·76 | 457 | 3·33 (3·03–3·65) | |
| Peri-urban | 5242·55 | 230 | 4·39 (3·84–4·99) | |
| Urban | 309·27 | 6 | 1·94 (0·71–4·22) | |
| Partners in previous 12 months | ||||
| 0 | 1389·22 | 13 | 0·94 (0·50–1·60) | |
| 1 | 13331·53 | 595 | 4·46 (4·11–4·84) | |
| >1 | 312·56 | 37 | 11·84 (8·33–16·32) | |
Person-years are based on midpoint imputation of the date of the last negative and first positive test for HIV seroconverters and on the date of the last negative test for those who are censored.
Derived from men in the surrounding local community with a standard Gaussian kernel (radius 3 km) around each woman in the cohort (figure 2).
Table 2.
Full output from interval-censored parametric survival analysis showing the effect of community-level mean lifetime partners and prevalence of partnership concurrency in men on a woman's hazard of acquiring HIV infection (N=7284)
|
Mean lifetime partners* |
Concurrency* |
Both† |
||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |||
| Unadjusted models | ||||||||
| Mean lifetime partners | 1·09 (1·03–1·14) | 0·001 | .. | .. | 1·10 (1·04–1·16) | 0·001 | ||
| Concurrency (10% increase) | .. | .. | 1·00 (0·94–1·07) | 0·981 | 0·96 (0·90–1·03) | 0·307 | ||
| Adjusted models | ||||||||
| Community level (male) | ||||||||
| Mean lifetime partners | 1·08 (1·03–1·14) | 0·004 | .. | .. | 1·09 (1·03–1·15) | 0·004 | ||
| Concurrency (10% increase) | .. | .. | 1·02 (0·95–1·09) | 0·556 | 0·99 (0·91–1·06) | 0·730 | ||
| Prevalence (10% increase)*‡ | 1·17 (0·98–1·39) | 0·074 | 1·20 (1·01–1·42) | 0·042 | 1·17 (0·99–1·39) | 0·072 | ||
| Individual level | ||||||||
| Partners in previous 12 months (vs 0) | ||||||||
| One | 2·58 (1·43–4·64) | 0·002 | 2·56 (1·42–4·62) | 0·002 | 2·58 (1·43–4·64) | 0·002 | ||
| More than one | 4·84 (2·45–9·56) | <0·0001 | 4·84 (2·45–9·56) | <0·0001 | 4·83 (2·45–9·54) | <0·0001 | ||
| Marital status (vs single) | ||||||||
| Married, monogamous | 0·60 (0·43–0·83) | 0·002 | 0·60 (0·44–0·84) | 0·002 | 0·60 (0·44–0·84) | 0·002 | ||
| Married, polygamous | 0·67 (0·34–1·33) | 0·250 | 0·67 (0·34–1·34) | 0·257 | 0·67 (0·34–1·33) | 0·250 | ||
| Urban (vs rural) | ||||||||
| Peri-urban | 1·17 (0·95–1·45) | 0·147 | 1·19 (0·96–1·47) | 0·118 | 1·16 (0·94–1·44) | 0·166 | ||
| Urban | 0·71 (0·31–1·63) | 0·418 | 0·72 (0·31–1·66) | 0·444 | 0·71 (0·31–1·63) | 0·415 | ||
| Wealth tertile (vs wealthiest) | ||||||||
| Intermediary wealth | 0·94 (0·76–1·15) | 0·553 | 0·94 (0·77–1·16) | 0·581 | 0·94 (0·76–1·15) | 0·551 | ||
| Poorest | 0·87 (0·70–1·09) | 0·220 | 0·89 (0·71–1·10) | 0·283 | 0·87 (0·70–1·08) | 0·217 | ||
| Years of education | 0·96 (0·93–0·98) | 0·001 | 0·96 (0·93–0·98) | 0·001 | 0·96 (0·93–0·98) | 0·001 | ||
| Age (vs 15–19 years) | ||||||||
| 20–24 years | 1·52 (1·25–1·84) | <0·0001 | 1·50 (1·24–1·82) | <0·0001 | 1·52 (1·25–1·84) | <0·0001 | ||
| 25–29 years | 1·11 (0·82–1·49) | 0·498 | 1·10 (0·82–1·47) | 0·539 | 1·11 (0·82–1·49) | 0·499 | ||
| 30–34 years | 0·72 (0·50–1·03) | 0·069 | 0·71 (0·49–1·01) | 0·057 | 0·72 (0·50–1·03) | 0·069 | ||
| 35–39 years | 0·55 (0·39–0·79) | 0·001 | 0·55 (0·38–0·79) | 0·001 | 0·55 (0·39–0·79) | 0·001 | ||
| 40–44 years | 0·29 (0·19–0·44) | <0·0001 | 0·29 (0·19–0·44) | <0·0001 | 0·29 (0·19–0·44) | <0·0001 | ||
| ≥45 years | 0·19 (0·12–0·28) | <0·0001 | 0·19 (0·12–0·28) | <0·0001 | 0·19 (0·12–0·28) | <0·0001 | ||
HR=hazard ratio.
Derived from male sexual behaviour in the surrounding local community with a standard Gaussian kernel (radius 3 km) around each woman in the cohort (figure 2).
Includes both male community-level mean lifetime partners and prevalence of concurrent partnerships covariates.
Unadjusted hazard ratio, 1·26 (p<0·0001).
The mean number of reported lifetime sexual partners in men in the surrounding local community strongly predicted risk of HIV acquisition in HIV-negative women (both before and after adjustment for individual-level sexual behaviour, demographic, socioeconomic, and environmental factors associated with HIV acquisition; table 2). For every unit increase in mean number of lifetime partners for men at a community level, the corresponding hazard of HIV acquisition in women increased by 8% (p=0·004). In other words, an HIV-negative woman living in a community with high numbers of reported lifetime partners in men (>12) has nearly double the hazard of HIV acquisition, after adjustment for other factors, compared with a woman living in a community with low numbers of reported lifetime sexual partners (fewer than four). By contrast, living in a community with high levels of male concurrent partnerships was not associated with an increased risk of HIV acquisition to the woman even after adjustment for individual-level factors and reported numbers of lifetime partners in the surrounding community (p=0·730).
The results were robust to the size and shape of the virtual community (kernel) and an alternative population-level indicator of partnership concurrency—mean numbers of concurrent partners (table 3). Restriction of the analysis only to women who had been resident in the study area for the full period of follow-up (n=6534) similarly had no effect on the findings (for mean lifetime partners, p=0·001; for concurrency prevalence, p=0·644). The results also remained consistent in a secondary analysis that assessed the effect of standardised community-level male and female sexual behaviour on the hazard of HIV acquisition of 11 861 repeat HIV-testers of both sexes (webappendix pp 3–4).
Table 3.
Sensitivity analysis of the effect of size and shape of the kernel (used to derive the male community-level sexual behaviour variables) and an alternative community-level concurrency measure on a woman's hazard of HIV acquisition (N=7284)
| HR (95% CI) | p value | ||
|---|---|---|---|
| Gaussian 3·8 km radius (SD 1) | |||
| Mean lifetime partners | |||
| Unadjusted | 1·12 (1·06–1·20) | 0·0002 | |
| Adjusted A | 1·11 (1·04–1·18) | 0·002 | |
| Adjusted A+B | 1·12 (1·04–1·20) | 0·002 | |
| Prevalence of concurrency, 10% increase | |||
| Unadjusted | 1·00 (0·93–1·08) | 0·967 | |
| Adjusted A | 1·02 (0·94–1·11) | 0·568 | |
| Adjusted A+B | 0·97 (0·89–1·06) | 0·567 | |
| Gaussian 3 km radius (SD 3) | |||
| Mean lifetime partners | |||
| Unadjusted | 1·17 (1·08–1·28) | 0·0003 | |
| Adjusted A | 1·11 (1·01–1·23) | 0·035 | |
| Adjusted A+B | 1·14 (1·02–1·27) | 0·019 | |
| Prevalence of concurrency, 10% increase | |||
| Unadjusted | 0·96 (0·87–1·07) | 0·501 | |
| Adjusted A | 0·99 (0·88–1·10) | 0·831 | |
| Adjusted A+B | 0·94 (0·83–1·06) | 0·291 | |
| Gaussian 2·5 km radius (SD 1) | |||
| Mean lifetime partners | |||
| Unadjusted | 1·07 (1·02–1·12) | 0·003 | |
| Adjusted A | 1·07 (1·02–1·12) | 0·006 | |
| Adjusted A+B | 1·07 (1·02–1·13) | 0·007 | |
| Prevalence of concurrency, 10% increase | |||
| Unadjusted | 0·99 (0·93–1·05) | 0·888 | |
| Adjusted A | 1·02 (0·96–1·08) | 0·628 | |
| Adjusted A+B | 1·00 (0·94–1·06) | 0·718 | |
| Gaussian 3 km radius (SD 1), mean number of concurrent partners* | |||
| Unadjusted | 1·06 (0·87–1·29) | 0·553 | |
| Adjusted A | 1·10 (0·90–1·35) | 0·329 | |
| Adjusted A+B | 0·88 (0·69–1·14) | 0·332 | |
HR=hazard ratio. The unadjusted model includes only the male community-level sexual behaviour covariate. The adjusted A model includes one of the community-level sexual behaviour covariates and community level HIV prevalence (male), partners in past 12 months, marital status, years of education, urban locale, wealth tertile, and age. The adjusted A + B model included all the independent variables included in model A (in the row immediately above) and the community-level sexual behaviour covariate not included in model A.
An alternative community-level indicator of partnership concurrency.
Discussion
Our work constitutes a formal test of the effect of concurrent partnerships on HIV incidence in this setting (panel). By shifting the focus away from an individual's own sexual behaviour patterns and onto the sexual behaviour profiles of the surrounding local community, we have tested the transmission dimension of the concurrency theory. The study took place in an area with one of the highest population-based prevalences of HIV documented worldwide19 and where there are large variations in the level of male partnership concurrency across the population. However, these differences in the prevalence of male concurrency did not translate into detectable differences in prospective incidence of HIV in women. The results were robust to differing definitions of community and held after we controlled for demographic, socioeconomic, behavioural, and environmental factors. Furthermore, the results also held at an ecological level and for the secondary analysis that quantified the effect of combined male and female sexual behaviour at a community level on the incidence of all participants (male and female) in the cohort. We were therefore unable to find any evidence to support the belief that concurrent partnerships are an important driver of the rate of spread of HIV infection in this hyperendemic setting. At the same time, the relation between numbers of lifetime partners in the community and risk of new infection provides strong and robust evidence of the effect of multiple partnering on HIV transmission and emphasises the importance of the characteristics of local community on spread of the virus (over and above an individual's characteristics and behaviours). This strong independent association is indicative of the fact that mean number of lifetime partners proxies for rate of partner turnover during the study period and thus men living in communities with high numbers of lifetime partners (relative to the age-profile of the community) continue to have (on average) higher numbers of sexual partnerships.
Panel. Research in context.
Systematic review
Although theoretical mathematical models suggest that concurrent sexual partnerships could account for the rapid spread of HIV in sub-Saharan Africa, a recent systematic review13 concluded that there is no empirical evidence to show that the kinds of concurrent partnerships found in Africa produce more rapid spread of HIV than do other forms of sexual behaviour. Provision of such evidence is not straightforward, however, because partnership concurrency is a risk factor for disease transmission and spread through a population and not of individual risk of disease acquisition (provided that having concurrent partners does not increase an individual's cumulative number of sex partners or unprotected sex acts).
Interpretation
Our study is the first to examine the effect of prevalence of concurrency in the surrounding local community on an individual's risk of HIV acquisition. By shifting the focus away from an individual's own sexual behaviour patterns and onto the sexual behaviour profiles of the surrounding local community, our study has tested the transmission dimension of the concurrency theory in a typical rural South African population with high HIV prevalence. Although the mean number of lifetime partners of men in the immediate local community was independently predictive of hazard of HIV infection in women, a high prevalence of partnership concurrency in the same local community was not associated with any increase in risk of HIV acquisition. Our data therefore provide no evidence to suggest that the high rate of new HIV infections is being driven by the segment of the sexually active population reporting concurrent sexual partners (29% of men and 2% of women). Our findings suggest that in similar hyperendemic sub-Saharan African settings, there is a need for clear messages aimed at the reduction of multiple partnerships, irrespective of whether those partnerships overlap in time. However, the absence of an effect of concurrency on HIV incidence in this setting should not be taken to necessarily mean that high levels of concurrent partnerships could not have played an important part in the initial stages of the HIV epidemic in this population or continue to play a part in other specific epidemic settings.
As in any observational study involving collection of data for sexual behaviour and HIV acquisition, the possibility of bias affecting the results must be considered and discussed. Importantly, selection on the independent variables in the multivariable survival analysis would not have biased the hazard coefficient estimates. Thus, the hazard coefficients will not be affected by selection on age, education, wealth, urban versus rural residence, marital status, HIV prevalence, community-level concurrency, and partners reported in the last 12 months. Of course, a randomised controlled trial could further improve the strength of the evidence for concurrency effects, because it would allow us to control for selection on both known or unknown factors, but such a study is not feasible. By using a community-level estimate of concurrency as a surrogate measure for the concurrency practices of a participant's partner or partners in our analyses, we would naturally expect some attenuation of any concurrency effect. However, this attenuation would also apply to the community lifetime partners covariate, which is a significant predictor of HIV acquisition in our analysis, and thus attenuation would be unlikely to account for the null finding on the effect of concurrency on HIV acquisition.
A limitation of the study is that data for concurrency and lifetime partners were obtained only at the beginning of the study in 2004. We therefore assumed that no large systematic shifts in patterns of male sexual behaviour at a community level have taken place during the study. Such systematic shifts are unlikely, but nevertheless the theoretical possibility remains. Another limitation is that we were unable to control for all exposures outside an individual's geographically defined community—for example, women with migrant partners who return home periodically. However, since partner choice has a strong local geographical dimension, we would still expect to be able to show an effect (at an ecological or individual level) if concurrent partnerships were playing an important part in transmission.
The evidence for the concurrency hypothesis has been reviewed extensively elsewhere.13 However, our findings using individual-level longitudinal data for HIV seroconversion are in agreement with those of other studies that have not shown any association between partnership concurrency and HIV prevalence at a city or a country level.30,31 Similarly, a study in South Africa32 on intimate partner violence and HIV incidence in women found no relation between respondents' reports of the likelihood that their partner had other partners and risk of HIV acquisition. A small study in Malawi33 that attempted to investigate the association between partnership measured the correlation between HIV serodiscordance and concurrency in couples in Likoma Island. The results suggested that concurrent partnerships increased exposure to HIV infection. However, the use of prevalent (as opposed to incident) infection, the small sample size (142 couples), and the possibility of selection bias (in only 23% of concurrent partnerships were both partners tested for HIV) make it difficult to draw any robust inferences.
To spread widely, HIV infection has to have a high basic reproductive number, which will depend on the likelihood of transmission and the contact pattern throughout the population.34 Theoretically, concurrency along with high rates of partner turnover in key individuals can increase the spread of infection, as can factors such as high viral loads35 and the presence of other sexually transmitted infections.36 The present high incidence of infection in this population is possible because of the high prevalence of infectious individuals and the number of partners they have over time. What initially drove the rapid spread of infection is uncertain, but it has to have been a function of contacts and transmission patterns to which concurrency might have contributed. Hence, the absence of a positive concurrency finding in this hyperendemic setting should not be taken to necessarily mean that high levels of concurrent partnerships could not have played an important part in the initial stages of the HIV epidemic in this area or indeed continue to play a part in other specific epidemic settings. Additionally, as noted previously, formal polygamous marriages (a form of partnership concurrency that could be protective for acquisition of HIV37) occur in the study area although fairly rare (3% of women in the cohort). Therefore, application of our methods to other study sites where the local epidemiology of HIV differs will be important. In the early stages of the HIV epidemic, when the disease is concentrated mainly in high-risk populations, it seems plausible that partnership concurrency could increase the rate at which HIV spreads outside these groups to the general population.
There is a growing debate about the relative merits of, and empirical evidence for, the effect of concurrent partnerships on the HIV epidemic in Africa.2,10,12,13,15 A systematic review of the empirical evidence for the concurrency theory concludes, “promoters of the concurrency hypothesis have failed to establish that concurrency is unusually prevalent in Africa or that the kinds of concurrent partnerships found in Africa produce more rapid spread of HIV than other forms of sexual behaviour.”13 Currently, several countries are planning or implementing HIV prevention strategies that specifically target the reduction of concurrent sexual partnerships.25 The dwindling funds available for HIV programmes worldwide38 and the difficulties of design and implementation of culturally sensitive messages around partnership concurrency, combined with the absence of empirical evidence for an effect, make these interventions a difficult investment to justify. Furthermore, there is a danger of such messages inadvertently giving the impression that having many serially monogamous partnerships does not place an individual (and his or her partners) at significant risk of infection. Thus, rather than “developing a hierarchy of messages beneath the core theme of concurrent partners reduction”,11 messages should be limited to specific behaviours and biological factors with proven effect on HIV acquisition or transmission as part of a combination prevention approach. Even where campaigns refer to both multiple and concurrent partnerships, the unnecessary appeal to reduction of concurrent partnerships is likely to dilute the message. Conversely, simplifying the public health message to reduction in multiple partnerships alone is likely to improve message clarity and effectiveness.39
Our results provide clear evidence of the effect of multiple partnering on HIV transmission in this typical rural, high-prevalence African population. However, we find no evidence to suggest that the high rate of new infections is being driven by the segment of the sexually active population reporting concurrent sexual partners (29% of men and 2% of women). Our findings suggest that in similar hyperendemic sub-Saharan African settings, there is a need for straightforward, unambiguous messages aimed at the reduction of multiple partnerships, irrespective of whether those partnerships overlap in time.
Acknowledgments
Acknowledgments
This research was supported by grant 1R01-HD058482-01 from the National Institute of Child Health and Human Development. Funding for the Africa Centre's Demographic Surveillance Information System and Population-based HIV Survey was received from the Wellcome Trust, UK (grant 082384/Z/07/Z). NM was supported by a Wellcome Trust fellowship (grant WT083495MA).
Contributors
FT and TB designed the study. NM designed the sexual behaviour survey. FT did the spatial analyses and took primary responsibility for writing the report. LH, FT, and TB did the statistical analyses; LH drafted the statistical methods section. All authors contributed to data analysis and interpretation and writing and critiquing of the report.
Conflicts of interest
We declare that we have no conflicts of interest.
Web Extra Material
References
- 1.Halperin DT, Epstein H. Concurrent sexual partnerships help to explain Africa's high HIV prevalence: implications for prevention. Lancet. 2004;364:4–6. doi: 10.1016/S0140-6736(04)16606-3. [DOI] [PubMed] [Google Scholar]
- 2.Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS Behav. 2010;14:11–16. doi: 10.1007/s10461-008-9433-x. [DOI] [PubMed] [Google Scholar]
- 3.Shelton JD. A tale of two-component generalised HIV epidemics. Lancet. 2010;375:964–966. doi: 10.1016/S0140-6736(10)60416-3. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS . 2008 Report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2008. [Google Scholar]
- 5.Ferguson NM, Garnett GP. More realistic models of sexually transmitted disease transmission dynamics: sexual partnership networks, pair models, and moment closure. Sex Transm Dis. 2000;27:600. doi: 10.1097/00007435-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Watts CH, May RM. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math Biosci. 1992;108:89–104. doi: 10.1016/0025-5564(92)90006-i. [DOI] [PubMed] [Google Scholar]
- 8.Shelton JD. Ten myths and one truth about generalised HIV epidemics. Lancet. 2007;370:1809–1811. doi: 10.1016/S0140-6736(07)61755-3. [DOI] [PubMed] [Google Scholar]
- 9.Potts M, Halperin DT, Kirby D. Public health. Reassessing HIV prevention. Science. 2008;320:749–750. doi: 10.1126/science.1153843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein H. AIDS and the irrational. BMJ. 2008;337:a2638. doi: 10.1136/bmj.a2638. [DOI] [PubMed] [Google Scholar]
- 11.UNAIDS . Strategic considerations for communications on multiple and concurrent partnerships within broader HIV prevention in Southern Africa. Joint United Nations Programme on HIV/AIDS, World Bank, and Harvard University; Geneva, Switzerland: 2008. [Google Scholar]
- 12.Lurie MN, Rosenthal S. Concurrent partnerships as a driver of the HIV epidemic in sub-Saharan Africa? The evidence is limited. AIDS Behav. 2010;14:17–24. doi: 10.1007/s10461-009-9583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawers L, Stillwaggon E. Concurrent sexual partnerships do not explain the HIV epidemics in Africa: a systematic review of the evidence. J Int AIDS Soc. 2010;13:34. doi: 10.1186/1758-2652-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux AV Diez, Aiello AE. Multilevel analysis of infectious diseases. J Infect Dis. 2005;191(suppl 1):S25–S33. doi: 10.1086/425288. [DOI] [PubMed] [Google Scholar]
- 15.Morris M. Barking up the wrong evidence tree. AIDS Behav. 2010;14:31–33. doi: 10.1007/s10461-009-9639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanser F, Hosegood V, Bärnighausen T. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2008;37:956–962. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanser F, Bärnighausen T, Cooke GS, Newell ML. Localized spatial clustering of HIV infections in a widely disseminated rural South African epidemic. Int J Epidemiol. 2009;38:1008–1016. doi: 10.1093/ije/dyp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNAIDS/WHO . Guidelines for using HIV testing technologies in surveillance: selection, evaluation, and implementation. Joint United Nations Programme on HIV/AIDS/World Health Organization; Geneva, Switzerland: 2001. [PubMed] [Google Scholar]
- 19.Welz T, Hosegood V, Jaffar S, Batzing-Feigenbaum J, Herbst K, Newell ML. Continued very high prevalence of HIV infection in rural KwaZulu-Natal, South Africa: a population-based longitudinal study. AIDS. 2007;21:1467–1472. doi: 10.1097/QAD.0b013e3280ef6af2. [DOI] [PubMed] [Google Scholar]
- 20.Bärnighausen T, Tanser F, Newell ML. Lack of a decline in HIV incidence in a rural community with high HIV prevalence in South Africa, 2003–2007. AIDS Res Hum Retroviruses. 2009;25:405–409. doi: 10.1089/aid.2008.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bärnighausen T, Wallrauch C, Welte A. HIV incidence in rural South Africa: comparison of estimates from longitudinal surveillance and cross-sectional cBED assay testing. PLoS One. 2008;3:e3640. doi: 10.1371/journal.pone.0003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosegood V, McGrath N, Moultrie TA. Dispensing with marriage: marital trends in rural KwaZulu-Natal, South Africa 2000–2006. Demogr Res. 2009;20:279–312. doi: 10.4054/DemRes.2009.20.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleland J, Boerma JT, Carael M, Weir SS. Monitoring sexual behaviour in general populations: a synthesis of lessons of the past decade. Sex Transm Infect. 2004;80(suppl 2):ii1–ii7. doi: 10.1136/sti.2004.013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waller LA, Gotway CA. Applied spatial statistics for public health data. Wiley; Hoboken, NJ, USA: 2004. [Google Scholar]
- 25.UNAIDS Reference Group on Estimates, Modelling, and Projections: Working Group on Measuring Concurrent Sexual Partnerships HIV: consensus indicators are needed for concurrency. Lancet. 2009;375:621–622. doi: 10.1016/S0140-6736(09)62040-7. [DOI] [PubMed] [Google Scholar]
- 26.Morris M, Epstein H, Wawer M. Timing is everything: international variations in historical sexual partnership concurrency and HIV prevalence. PLoS One. 2010;5:e14092. doi: 10.1371/journal.pone.0014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royston P. Flexible parametric alternatives to the Cox model, and more. Stata J. 2001;1:1–28. [Google Scholar]
- 28.Bärnighausen T, Tanser F, Gqwede Z, Mbizana C, Herbst K, Newell ML. High HIV incidence in a community with high HIV prevalence in rural South Africa: findings from a prospective population-based study. AIDS. 2008;22:139–144. doi: 10.1097/QAD.0b013e3282f2ef43. [DOI] [PubMed] [Google Scholar]
- 29.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 30.Lagarde E, Auvert B, Carael M. Concurrent sexual partnerships and HIV prevalence in five urban communities of sub-Saharan Africa. AIDS. 2001;15:877–884. doi: 10.1097/00002030-200105040-00008. [DOI] [PubMed] [Google Scholar]
- 31.Mishra V, Bignami-Van Assche S. Concurrent sexual partnerships and HIV infection: evidence from national population-based surveys. Macro International Inc; Calverton, MD, USA: 2009. [Google Scholar]
- 32.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet. 2010;376:41–48. doi: 10.1016/S0140-6736(10)60548-X. [DOI] [PubMed] [Google Scholar]
- 33.Helleringer S, Kohler H-P, Kalilani-Phiri L. The association of HIV serodiscordance and partnership concurrency in Likoma Island (Malawi) AIDS. 2009;23:1285–1287. doi: 10.1097/QAD.0b013e32832aa85c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson R, May R. Infectious diseases of humans: dynamics and control. Oxford University Press; New York, NY, USA: 1991. [Google Scholar]
- 35.Boily MC, Baggaley RF, Wang L. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng B, Butler L, Horvath T, Rutherford G. Population-based biomedical sexually transmitted infection control interventions for reducing HIV infection. Cochrane Database Syst Rev. 2011;3 doi: 10.1002/14651858.CD001220.pub3. CD001220. [DOI] [PubMed] [Google Scholar]
- 37.Reniers G, Watkins S. Polygny and the spread of HIV in sub-Saharan Africa: a case of benign concurrency. AIDS. 2010;24:299–307. doi: 10.1097/QAD.0b013e328333af03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Bank . The global economic crisis and HIV prevention and treatment programmes: vulnerabilities and impact. Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2009. [Google Scholar]
- 39.Randolph W, Viswanath K. Lessons learned from public health mass media campaigns: marketing health in a crowded media world. Annu Rev Public Health. 2004;25:419–437. doi: 10.1146/annurev.publhealth.25.101802.123046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


