Abstract
The aim of this study was to evaluate the efficacy, potency and side effects of clonidine as compared to tramadol in post–spinal anaesthesia shivering. In this prospective double-blind randomized controlled clinical trial, 80 American Society of Anaesthesiologists grade-l (ASAI) patients aged between 18 and 45 years scheduled for various surgical procedures under spinal anaesthesia, who developed shivering were selected.The patients were divided into two groups: Group C (n=40) comprised of patients who received clonidine 0.5mg/kg intravenously (IV) and group patients who received tramadol 0.5 mg/kg IV. Grade of shivering, disappearance of shivering, haemodynamics and side effects were observed at scheduled intervals. Disappearance of shivering was significantly earlier in group C (2.54±0.76) than in group T (5.01±1.02) (P=.0000001). Response rate to treatment in group C was higher (97.5%) than in group T (92.5%), but the difference was not significant. Nausea, vomiting and dizziness were found to be higher in group T (P=0.001, 0.005, 0.001, respectively), while the patients in group C were comparatively more sedated (sedation level, 2; group C, 25%). We conclude that clonidine gives better thermodynamics than tramadol, with fewer side effects.
Keywords: Clonidine, post-spinal anaesthesia shivering, tramadol
INTRODUCTION
Regional anaesthesia (spinal anaesthesia) is widely used as a safe anaesthetic technique for both elective and emergency operations. Shivering is known to be a frequent complication, reported in 40 to 70% of patients undergoing surgery under regional anaesthesia[1,2] Shivering is a potentially serious complication, resulting in increased metabolic rate; increased oxygen consumption (up to 100-600%) along with raised carbon dioxide (CO2) production; ventilation and cardiac output; adverse postoperative outcomes, such as wound infection; increased surgical bleeding; and morbid cardiac events. It causes arterial hypoxemia, lactic acidosis, increased intraocular pressure (IOP), increased intracranial pressure (ICP); and interferes with pulse rate, blood pressure (BP) And electrocardiographic (ECG) monitoring.[3–5]
Shivering is very unpleasant, physiologically stressful for the patient undergoing surgery, and some patients find the accompanying cold sensation to be worse than the surgical pain. Though the mechanism of origin of shivering is not clear, various hypotheses have been proposed to explain its occurrence. Perioperative hypothermia is the primary cause, which occurs due to neuraxial anaesthesia-induced inhibition of thermoregulatory mechanism. Shivering occurs as a thermoregulatory response to hypothermia or muscle activity with tonic or clonic patterns, and various frequencies have been noticed.[5] However, in the postoperative period, muscle activity may be increased even with normothermia, suggesting that mechanisms other than heat loss with subsequent decrease in the core temperature contribute to the origin of shivering. These may be uninhibited spinal reflexes, sympathetic over-activity, postoperative pain, adrenal suppression, pyrogen release and respiratory alkalosis.[5] Due to shivering and thermal discomfort, the quality of patient recovery suffers. Moreover, shivering per se may aggravate postoperative pain, simply by stretching of surgical incision.
There are various methods available to control shivering during anaesthesia, which include non-pharmacological methods and pharmacological methods using drugs which have anti-shivering properties. Non-pharmacological methods using equipment to maintain normal temperature of the body are effective but expensive and lack practicality, while the pharmacological methods using drugs like pethedine, tramadol, clonidine, doxapram, katenserin, nefopam, etc., are simple, cost-effective and easy to implement.
The aim of this prospective double-blind randomized clinically controlled study was to clinically compare the efficacy, potency, haemodynamic effects, complications and side effects of clonidine with those of tramadol for control of shivering.
METHODS
After obtaining approval of the ethics committee and written informed consent, 80 American Society of anaesthesiologists grade-l (ASAI) patients (power of study, 70%) of either sex aged 18 to 40 years scheduled for elective abdominal, orthopaedic and gynaecological surgeries, e.g., inguinal herniorraphy, abdominal and vaginal hysterectomy, K-nailing, dynamic hip screw (DHS), under spinal anaesthesia with no prior pre-medication, were included in this prospective double-blind randomized clinically controlled study. Patients with known hypersensitivity to clonidine and tramadol, known history of alcohol or substance abuse, hyperthyroidism, cardiovascular diseases, psychological disorder, severe diabetes or autonomic neuropathies and urinary tract infection (UTI) were excluded. All patients who developed post-spinal anaesthesia intraoperative shivering were randomly allocated to two groups: Group C (n=40) received clonidine 0.5 μg/kg (intravenously) IV, and group T (n=40) received tramadol 0.5 mg/kg IV.
Anaesthesiology personnel who were not involved in the study made the trial preparations and recorded group randomization separately. The anaesthesiologists conducting the case and recording the data were unaware of the preparation administered.
Subarachnoid block was given with inj. Bupivacaine 0.5% (10-15 mg) at L3-4 or L4-5 interspace using 25 gauge Quincke's needle, and blockage up to T9-10 dermatome was achieved. All operation theatres in which the operations were performed maintained constant humidity (70%) and an ambient temperature of around 21°C to 23°C. Oxygen was administered to all the patients of both groups at a rate of 5 L/min with face mask, and patients were covered with drapes but not actively warmed. No means of active re-warming were used. Intravenous fluids and anaesthetic drugs were administered at room temperature. Preloading was not done in both the groups as we did not want intravenous fluid to influence the onset of shivering mechanism. Before beginning of spinal anaesthesia, standard monitoring procedures were established. Standard monitoring of pulse rate was done, and non-invasive blood pressure (NIBP), oxygen saturation (SPO2), body temperature (axillary) were recorded before the commencement of surgery and thereafter at every 5 minutes from the baseline ie subarachnoid block (SAB), for 1 hour; and every 15 minutes, for the rest of the observation period.
Grading of shivering was done as per Wrench,[6] which is as follows:
Grade 0: No shivering
Grade 1: One or more of the following: Piloerection, Peripheral vasoconstriction, peripheral cyanosis with, but without visible muscle activity
Grade 2: Visible muscle activity confined to one muscle group
Grade 3: Visible muscle activity in more than one muscle group
Grade 4: Gross muscle activity involving the whole body
Patients who developed either grade 3 or grade 4 of shivering were included in the study. The attending anaesthetist recorded the time in minutes at which shivering started after spinal anaesthesia (onset of shivering), severity of the shivering, time to disappearance of shivering (in minutes) and response rate (shivering ceased after treatment in 15 minutes). Duration of surgery was noted, and duration of spinal anaesthesia was recorded by assessing spontaneous recovery of sensory block using pin-prick method and observing spontaneous movements of limbs in the postoperative period. If the shivering did not subside by 15 minutes, the treatment was considered to be not effective. Recurrence of shivering was also noticed until the patient left the operation theatre. Patients who did not respond or in whom recurrence of shivering occurred were treated with additional dose of clonidine (0.5 μg/kg IV) or tramadol (0.5 mg/kg IV) in the respective groups, if required. Side effects like nausea, vomiting, bradycardia (<50/min), hypotension (>20% of baseline), dizziness; and sedation score were recorded.
Sedation score was assessed with a four-point scale as per Filos:[7]
-
1:
Awake and alert
-
2:
Drowsy, responsive to verbal stimuli
-
3:
Drowsy, arousable to physical stimuli
-
4:
Unarousable
Bradycardia, hypotension and vomiting were treated with atropine, mephenteramine and metaclopramide, respectively, in titrated doses when required. Statistical analysis was done using suitable statistical software, and Student t test and Chi-square test were applied for the interpretation of results. A P value <.05 was considered statistically significant.
RESULTS
A total of 80 patients were enrolled in the present study and were randomized into two groups of 40 each (n=40), 57 of whom were male and 23 were female [Table 1]. Both the groups were comparable with respect to age, sex, weight, duration of surgery, type of surgery, volume of intravenous fluid administered and the duration of spinal block. The mean age of the patients in group C was 30.03 ± 3.80 years; and patients in Group T, 28.8 ± 3.47 years (P=.137) [Table 1].
Table 1.
Demographic data of patients in the two groups
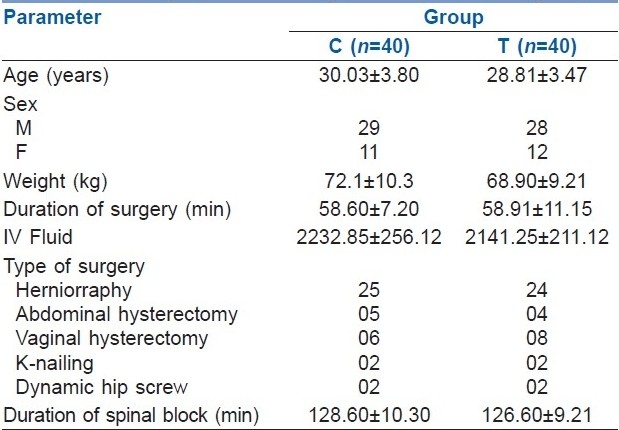
Shivering disappeared in 39 (97.5%) patients who received clonidine and 37 (92.5%) who received tramadol [Table 2]. Both the drugs were found to be effective in reducing shivering. However, severity of shivering was unchanged in 1 (2.5%) patient of group C and 3 (7.5%) patients of group T. One patient in group C (severity of shivering unchanged) and 5 patients (3- severity of shivering unchanged; 2- recurrence of shivering) in group T were given rescue doses of clonidine or tramadol, respectively. Six (7.5%) patients out of a total of 80 patients received rescue doses.
Table 2.
Post-spinal anaesthesia shivering and responses
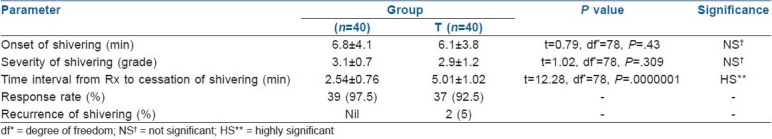
The mean interval between the injection of drug (clonidine and tramadol) and the complete cessation of shivering was 2.54±0.76 and 5.01±1.02 minutes, respectively (P=.0000001). Time for onset of shivering and severity of shivering were not statistically significantly different between the two groups. However, the time interval between administration of drug after onset of shivering and disappearance of shivering was significantly shorter in the clonidine group [Table 2](P=.0000001).
There was no statistically significant difference with respect to heart rate, mean blood pressure, axillary temperature and oxygen saturation between the two groups. Complication rates were significantly higher in group T than in group C [Table 3]. Nausea, vomiting and dizziness were higher in group T [nausea - 31; vomiting - 8; and dizziness - 22) than in group C. More patients of group C (10 patients) were sedated than of group T (5 patients).
Table 3.
Complications in both groups
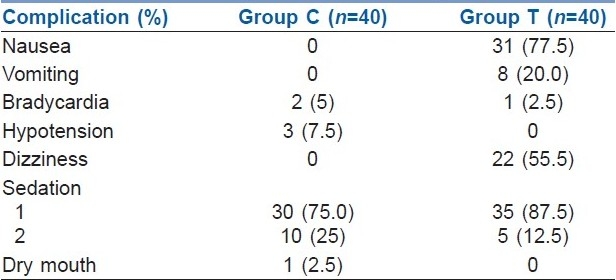
Bradycardia occurred in 2 patients of group C and 1 patient of group T. In group C, 3 patients suffered from hypotension, and 1 patient complained of dry mouth, both of which were not present in group T [Table 3].
DISCUSSION
Regional anaesthesia, either central neuraxial block or peripheral nerve block, is a safe and very popular technique used for various surgeries. However, 40% to 70% of patients undergoing regional anaesthesia develop shivering, though it is also found to occur after general anaesthesia.[1,2]
The mechanism which leads to shivering after regional anaesthesia is not very clear, but the probable mechanisms could be decrease in core body temperature secondary to sympathetic block; peripheral vasodilatation; increased cutaneous blood flow, which leads to increased heat loss through skin; cold temperature of operation theatre; rapid infusion of cold IV fluids; and effect of cold anaesthetic drugs upon the thermosensitive receptors in the spinal cord.[8,9]
There are many non-pharmacological and pharmacological methods used to prevent heat loss and decrease shivering. Non-pharmacological methods include radiant heat warmers, warming the operation theatre, blankets, warm IV fluids and using anaesthetic drugs at body temperature.[10,11] The present study was designed to standardise these possible compounding factors, while reflecting the common practice in our institution. The temperature in the operating room was maintained constant at 21°C to 23°C. IV fluids and drugs were given at room temperature. Axillary temperature was recorded at regular intervals intraoperatively.
In the present study, the factors that influence the occurrence of shivering, like temperature of IV fluids and drugs, were not tightly controlled, but this should not affect the validity of our study because the present study is focused on response to treatment used rather than incidence of shivering; and by randomization, both groups were subjected to similar degrees of influence of these factors.
Pharmacological methods to treat shivering include pethedine, tramadol, doxapram, ketanserin, nefopam, alfentanyl, doxapram, etc.
A limitation of this study is that we could not measure the core body temperature. For measurement of core body temperature, the probe needs to be put in the oesophagus or near the tympanic membrane. Both these are uncomfortable and unacceptable who has been given spinal anaesthesia. Rectal temperature monitoring was a possibility but was not tried.
In the present study, we compared the efficacy of clonidine and tramadol for treatment of shivering after spinal anaesthesia in patients undergoing various elective surgeries. Clonidine is a centrally acting selective α2 agonist. Clonidine exerts its anti-shivering effects at three levels: Hypothalamus, locus coeruleus and spinal cord. At the hypothalamic level, it decreases thermoregulatory threshold for vasoconstriction and shivering, because hypothalamus has high density of α2 adenoceptors and hence is effective in treating the established post-anaesthetic shivering.[12,13] It also reduces spontaneous firing in locus coeruleus — a pro-shivering centre in pons.[14] At the spinal cord level, it activates the α2 adrenoreceptors and release of dynorphine, norepinephrine and acetylcholine.[14] The depressor effects of these neurotransmitters at the dorsal horn modulate cutaneous thermal inputs.[15] Clonidine is highly lipid-soluble and easily crosses the blood-brain barrier.[16] Due to these merits, interaction at the α2 adrenoreceptors at spinal and supraspinal sites occurs within the central nervous system.[17]
Tramadol is an opioid analgesic with opioid action preferably mediated via μ (mu) receptor with minimal effect on kappa and delta binding sites. Tramadol also activates the mononergic receptors of the descending neuraxial inhibiting pain pathway. The anti-shivering action of tramadol is probably mediated via its opioid or serotonergic and noradrenergic activity or both.[18–20]
In the present study, we found that clonidine is as effective as tramadol in treating post–spinal anaesthesia shivering, but the time interval from the commencement of treatment to cessation of shivering is quite less with clonidine (2.54±0.76 minutes) than with tramadol (5.03±1.02 minutes) (P=.0000001).
The response rate was also higher in the clonidine group than in tramadol group, but the difference was not statistically significant (P=.03).
The complications were found to be higher in case of tramadol compared to clonidine. In the present study, the incidence of nausea was higher in tramadol group compared to clonidine group. Similar differences were noted between the two groups in relation to vomiting and dizziness. In case of group C, 10 (25%) patients had sedation of grade 2; while in group T, it was 5 (12.5%) patients who had sedation. No patient in either group had sedation of grade 3 or 4. One patient of group C had dry mouth, which was not present in group T. Two patients of group T had recurrence of shivering in postoperative period, while no patient in clonidine group suffered recurrence of shivering. These findings were similar to the findings of other researchers who compared clonidine with other drugs having anti-shivering properties.[21–23]
Bradycardia occurred in 2 patients of group C, while 1 patient of group T suffered from bradycardia. Hypotension occurred in 3 patients of group C. On overall analysis, higher complication rates were noted in group T patients compared to group C patients.
It was noted in the present study that clonidine was quicker than tramadol in providing relief in shivering. Zavaherforoush et al.[21] compared clonidine with pethedine and fentanyl for treating post–spinal anaesthesia shivering in elective Lower Segment Caesarean Section (LSCS) and also found it to be offering better thermodynamics than pethedine.
The present study also found that the patients who received clonidine were more sedated than the patients in the other group. This is similar to the findings of other researchers.[22]
CONCLUSION
In conclusion, both clonidine (0.5 μg/kg) and tramadol (0.5 mg/kg) effectively treated patients with post–spinal anaesthesia shivering, but tramadol took longer time to achieve complete cessation of shivering than clonidine, the difference being statistically significant. So we conclude that clonidine offers better thermodynamics than tramadol, with fewer side effects. The more frequent incidence of side effects of tramadol, like nausea, vomiting and dizziness, may limit it's use as an anti-shivering drug.
Further studies are needed to compare the effectiveness of various drugs in the treatment of shivering in patients undergoing surgery under spinal anaesthesia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.De Whitte, Sessler DI. Perioperative shivering: Physiology and Pharmacology. Anaesthesiology. 2002;96:467–84. doi: 10.1097/00000542-200202000-00036. [DOI] [PubMed] [Google Scholar]
- 2.Sessler DI, Ponte J. Shivering during epidural anaesthesia. Anesthesiology. 1990;72:816–21. doi: 10.1097/00000542-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar S, Saxena A, Kannan TR, Punj J, Panigrahi M, Mishra S. Tramadol for postoperative shivering: A double blind comparison with Pethedine. Anaesth Intensive care. 2001;29:149–54. doi: 10.1177/0310057X0102900209. [DOI] [PubMed] [Google Scholar]
- 4.Katyal S, Tewari A. Shivering: Anesthetic Considerations. J Anaesth Clin Pharmacol. 2002;18:363–76. [Google Scholar]
- 5.Sessler Daniel I. Temperature Monitoring. In: Millar RD, editor. Textbook of Anaesthesia. 5th ed. New York: Churchill Livingstone Inc; 1994. pp. 1367–89. [Google Scholar]
- 6.Wrench IJ, Singh P, Dennis AR, Mahajan RP, Crossley AW. The minimum effective doses of pethedine and doxapram in the treatment of post anaesthetic shivering. Anaesthesia. 1977;52:32–6. doi: 10.1111/j.1365-2044.1997.006-az006.x. [DOI] [PubMed] [Google Scholar]
- 7.Filos KS, Goudas LC, Patroni O, Polyzou V. Hemodynamic and analgesic profile after intrathecal clonidine in humans.A dose-response study. Anesthesiology. 1994;81:591–601. doi: 10.1097/00000542-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Anne Miu Han Chan, Kwok Fu. Control of shivering under regional anaesthesia in obstetric patients with Tramadol. Can J Anaesth. 1999;46(3):253–8. doi: 10.1007/BF03012605. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi S, Domkondwar G. Control of shivering under regional anaesthesia using Tramadol. Asian Archives of Anaesthesiology and Resuscitation. 2002;57:491–6. [Google Scholar]
- 10.Wrench IJ, Cavill G, Ward JE, Crossley AW. Comparison between Alfentanil, Pethedine, and placebo in the treatment of postoperative shivering. Br J Anaesth. 1997;79:541–2. doi: 10.1093/bja/79.4.541. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda T, Sessler DI, Tayefeh F, Negishi C, Turakhia M, Marder D, et al. Meperidine and Alfentanyl do not reduce the gain or maximum intensity of shivering. Anaesthesiology. 1998;88:858–65. doi: 10.1097/00000542-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Delaunay L, Bonnet F, Liu N, Beydon L, Catoire P, Sessler DI. Clonidine comparably decreases the thermoregulatory threshold for vasoconstriction and shivering in humans. Anesthesiology. 1993;79:470–4. doi: 10.1097/00000542-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Joris J, Banache M, Bonnet F, Sessler DI, Lamy M. Clonidine and ketanserin both are effective treatment for postanaesthetic shivering. Anaesthesiology. 1993;79:532–9. doi: 10.1097/00000542-199309000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Powell RM, Buggy DJ. Ondansetron given before induction of anesthesia reduces shivering after general anesthesia. Anesth Analg. 2000;90:1423–7. doi: 10.1097/00000539-200006000-00032. [DOI] [PubMed] [Google Scholar]
- 15.Alojado ME, Ohta Y, Kemmotsu O. The effect of clonidine on the activity of neurons in the rats dorsal raphe nucleus in vitro. Anesth analg. 1994;79:257–60. doi: 10.1213/00000539-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Timmermans PB, Brands A, van Zwietan PA. Lipophilicity and brain disposition of clonidine and structurally related imidazolidines. Naunyn Schmiedeberg Arch Pharmacol. 1977;300:217–26. doi: 10.1007/BF00500963. [DOI] [PubMed] [Google Scholar]
- 17.Klimecsha W, Tong C, Eisenach JC. Intrathecal alpha-2 adrenergic agonist stimulate acetylcholine and norepiriphrine release from the spinal cord dorsal horn in sheep. An in vivo microdialysis study. Anaesthesiology. 1997;87:110–6. doi: 10.1097/00000542-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Mathews S, Al Mulla A, Varghese PK, Radim K, Mumtaz S. Post-anaesthetic shivering: A new look at tramadol. Anaesthesia. 2002;57:387–403. doi: 10.1046/j.1365-2044.2002.2457_3.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsai YC, Chi KS. A comparison of tramadol, amitryptyline and meperidine for post-epidural anaesthetic shivering in parturients. Anaesth Analg. 2002;93:1288–92. doi: 10.1097/00000539-200111000-00052. [DOI] [PubMed] [Google Scholar]
- 20.Bilotta F, Pietropaoli P, Sanita R, Liberatori G, Rosa G. Nefopam and tramadol for the prevention of shivering during neuraxial anaesthesia. Reg Anaesth Pain Med. 2002;27:380–4. doi: 10.1053/rapm.2002.33563. [DOI] [PubMed] [Google Scholar]
- 21.Zavaherforoush F, Pipelzadelimto, Bagherybarma F. Comparison of clonidin, pethedin and Fentanyl for post- spinal anaesthesia shevering in elective caesarian sections. ARMAGHAN DANESH FALL. 2006;11:60–7. [Google Scholar]
- 22.Mercadante S, De Michele P, Pignataro A, Sapio M, Villari P. Effect of clonidine on post partum shivering after epidural analgesia: A randomized, controlled, double blind study. J Pain Symptom Manage. 1994;9:294–7. doi: 10.1016/0885-3924(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari A, Garg S, Katyal S, Singh A, Bansal K. Study of prophylaxis with clonidine or tramadol for shivering in SAB for TURP surgery. Anaesthesiology. 2007;107:A 835. [Google Scholar]


