Abstract
Sepsis can sometimes be difficult to substantiate, and its distinction from non-infectious conditions in critically ill patients is often a challenge. Serum procalcitonin (PCT) assay is one of the biomarkers of sepsis. The present study was aimed to assess the usefulness of PCT assay in critically ill patients with suspected sepsis. The study included 40 patients from the intensive care unit with suspected sepsis. Sepsis was confirmed clinically and/or by positive blood culture. Serum PCT was assayed semi-quantitatively by rapid immunochromatographic technique (within 2 hours of sample receipt). Among 40 critically ill patients, 21 had clinically confirmed sepsis. There were 12 patients with serum PCT ≥10 ng/ml (8, blood culture positive; 1, rickettsia; 2, post-antibiotic blood culture sterile; and 1, non-sepsis); 7 patients with PCT 2-10 ng/ml (4, blood culture positive; 1, falciparum malaria; 2, post-antibiotic blood culture sterile); 3 patients with PCT of 0.5 to 2 ng/ml (sepsis in 1 patient); and 18 patients with PCT < 0.5 ng/ml (sepsis in 2 patients). Patients with PCT ≥ 2 ng/ml had statistically significant correlation with the presence of sepsis (P<0.0001). The PCT assay revealed moderate sensitivity (86%) and high specificity (95%) at a cut-off ≥ 2 ng/ml. The PCT assay was found to be a useful biomarker of sepsis in this study. The assay could be performed and reported rapidly and provided valuable information before availability of culture results. This might assist in avoiding unwarranted antibiotic usage.
Keywords: Blood culture, immunochromatographic assay , procalcitonin, sepsis
INTRODUCTION
Sepsis can be difficult to distinguish from non-infectious conditions in critically ill or comatose patients in the early stages; and diagnosis, treatment and outcomes greatly differ between patients with and without sepsis.[1]
Positive bacteriological cultures, including blood cultures, may not be available before 24 to 48 hours; interpretation of local colonization may be ambiguous; and traditional markers of infection, such as body temperature and white blood cell (WBC) count, may not be specific.[2] Furthermore, there are concerns about possible blood culture–negative clinical sepsis, particularly in the setting of increased prophylactic and empirical antibiotic use.[3] Conversely, differentiating true infection from contamination after growth of common skin commensals in blood cultures, poses a diagnostic problem.[4]
Since early identification of infections and sepsis is crucial for patient management, an effective marker specific for bacterial infection is very useful in the critical care settings. There are several markers of sepsis, like C-reactive protein, serum procalcitonin (PCT), IL-6, IL-8, lactate, etc., of which PCT has been found to be the most effective.[1,4] PCT has been proposed as an indicator of the presence of infection and as a useful marker of the severity of sepsis.[2] The present study was an attempt to assess the usefulness of serum PCT as a marker of sepsis in critically ill patients, using the semi-quantitative, rapid immunochromatographic kit, in Apollo Hospitals, Bangalore.
METHODS
This study was carried out from July 2007 to June 2008 in the Department of Microbiology, Apollo Hospitals, Bangalore. The study included patients from the intensive care unit (ICU) with suspected sepsis. Sepsis was confirmed clinically and by positive blood culture (BacT/Alert system). Laboratory and clinical findings helped identify patients as having “sepsis syndrome” (sepsis, severe sepsis, septic shock) or no sepsis based on the ACCP (American College of Chest Physicians) recommendations.[5]
Serum PCT was assayed semi-quantitatively by rapid immunochromatographic technique using a commercially available test kit (PCT-Q, B.R.A.H.M.S. Diagnostica GmbH, Berlin, Germany). The result was independently read by two microbiologists to minimize reading bias and interpreted as per the manufacturer's recommendations:
-
i)
PCT >10 ng/ml: Severe bacterial sepsis or septic shock
-
ii)
PCT 2 to 10 ng/ml: Severe systemic inflammatory response, most likely due to sepsis unless other causes are known
-
iii)
PCT 0.5 to 2 ng/ml: A systemic infection cannot be excluded
-
iv)
PCT <0.5 ng/ml: Local bacterial infection possible; sepsis unlikely
The clinical condition, signs and symptoms of sepsis, antibiotics used, blood culture and final outcome of patients were recorded for all patients.
Statistic analysis
Sensitivity and specificity of the PCT assay were analyzed using two different cut-off values for PCT (≥0.5 ng/ml and ≥2 ng/ml); sensitivity being PCT positives/total no. of sepsis cases × 100 and specificity being PCT positives/total no. of cases without sepsis × 100. Statistical analysis was carried out using Chi-square test to evaluate the correlation between PCT levels and sepsis. A P value <.05 was considered statistically significant.
RESULTS
The study included 40 ICU patients with suspected sepsis. Patient ages ranged from 18 to 84 years [male-female ratio, 28:12]. Among these patients, PCT above 10 ng/ml was seen in 12 patients; 2-10 ng/ml, in 7 patients; 0.5-2 ng/ml, in 3 patients; and <0.5 ng/ml, in 18 patients [Table 1].
Table 1.
Serum procalcitonin values in the 40 patients with suspected sepsis
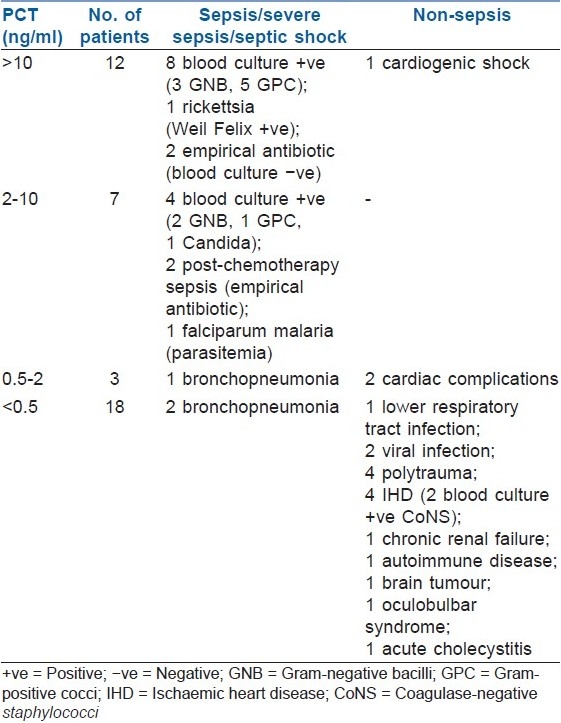
Among the 12 patients with PCT >10 ng/ml, 1 patient in shock did not have any signs of sepsis or infection and recovered with inotropic support only (no antibiotics were required; cardiogenic shock). Of the 11 patients with clinically diagnosed sepsis, 8 yielded positive blood cultures subsequently (2 patients with septic shock had Staphylococcus aureus bacteremia); 1 patient had rickettsial disease with a typical clinical presentation (fever, skin rashes, altered sensorium), which was confirmed by a positive Weil Felix reaction which showed elevated titres and a rise in titre within a week, and responded well to doxycycline and supportive therapy; and 2 patients with negative blood cultures were on empiric antimicrobial therapy. Four patients in this group expired due to complications of sepsis (mortality, 33.3%). One of the patients with renal transplant and with features of sepsis had a serum PCT >10 ng/ml (day 1). It was confirmed by blood culture to be due to coagulase-negative staphylococci. He responded to parenteral meropenem and teicoplanin, and his subsequent serum PCT levels reduced to <0.5 ng/ml on day 3 and day 5.
All 7 patients with PCT of 2-10 ng/ml had some form of sepsis (sepsis, 4 patients; severe sepsis, 2; and septic shock, 1 patient). Four of them yielded positive blood cultures; 1 patient had parasitemia; and 2 malignancy patients with febrile neutropenia who were on empiric antimicrobial therapy had negative blood cultures. Two (28.5%) patients in this group expired due to complications of sepsis.
Of the 3 patients with PCT of 0.5-2 ng/ml, 1 patient had clinical evidence of sepsis secondary to culture-proven Pseudomonas bronchopneumonia, though his blood culture did not yield any organism. He responded well to a combination therapy of cefoperazone-sulbactam and amikacin and was stable at discharge. The other 2 patients had no evidence of infection (congestive cardiac failure and unstable angina), and 1 of them expired due to cardiac complications.
Of the 18 patients with PCT <0.5 ng/ml, only 2 patients with bronchopneumonia had evidence of sepsis. Among 3 other patients with infections, 1 patient had lobar pneumonia but without sepsis; 1, pyrexia of unknown origin (possibly a viral infection); and 1, probable viral encephalitis but no signs of sepsis. The remaining 13 patients did not have any signs of infection or sepsis. Two patients (post-myomectomy hypertrophic obstructive cardiomyopathy and acute myocardial infarction) with positive blood cultures (S. epidermidis and S. haemolyticus) were concluded to have cardiogenic shock and not septic shock, as they responded well to inotropic support. In 1 of them, antibiotics were given additionally, considering his postoperative status (single-vessel stenting done 3 days prior to PCT testing). In this cohort, in a patient with a differential diagnosis of food botulism, a low PCT assisted in excluding an infective aetiology, and the patient was subsequently diagnosed as having acute oculobulbar syndrome.
In 8 out of the 9 fatal cases, cause of death was attributed to sepsis. One patient succumbed to congestive cardiac failure.
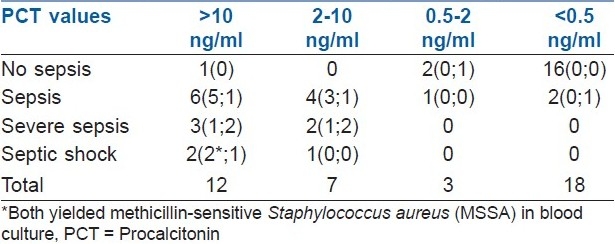
Serum PCT cut-offs of ≥0.5 ng/ml and 2 ng/ml were analyzed separately for their sensitivity and specificity as biomarkers for sepsis among the 21 patients with sepsis and 19 patients without sepsis. There was a statistically significant correlation with the presence of sepsis determined using either PCT ≥0.5 ng/ml or ≥2 ng/ml (P<0.0001 in both cases).
Using 0.5 ng/ml or more as cut-off, 19 of the 21 patients with sepsis could be detected, but 3 out of the 19 patients without sepsis showed PCT above 0.5 ng/ml (sensitivity, 90%, i.e., 19/19+2; and specificity, 84%, i.e., 16/16+3). Using PCT cut-off of 2 ng/ml or more as a marker of sepsis, 18 of the 21 patients with sepsis could be detected by PCT, and only 1 out of the 19 patients without sepsis showed PCT above 2 ng/ml (sensitivity, 85.7%, i.e., 18/18+3; and specificity, 94.7%, i.e., 18/18+1) [Table 2].
Table 2.
Relation of serum PCT levels with sepsis, number of patients (blood cultures; mortality)
DISCUSSION
The aim of this study was to assess the usefulness of serum PCT as a marker of sepsis in critically ill patients in our hospital. Early identification of infection and sepsis in critically ill patients is a challenge for clinicians. Serum PCT has been found to be a very good indicator of sepsis syndrome.[1]
To the best of our knowledge, there are very few studies on serum PCT and sepsis from India. These include studies on its use in determining bacterial sepsis in children with febrile neutropenia, its use as a marker of renal parenchymal infection and its use as a marker of the severity of acute pancreatitis.[6–8]
Most studies using PCT for interpretation of bacterial infection and sepsis have used the quantitative PCT assay (immunoluminometric method). Several studies have achieved high sensitivity and modest specificity with a cut-off of 1 to 1.2 ng/ml.[1,2,4,6,9,10] Others have used 2 ng/ml as the diagnostic threshold for infection and sepsis.[9,11] Various studies have found the PCT values in sepsis ranging from 0.5 to 3.5 ng/ml; in severe sepsis, 6.2-9.1 ng/ml; and in septic shock, 12.8 to 38.5 ng/ml.[1,11,12]
On the other hand, semi-quantitative PCT assay is a rapid immunochromatographic assay and is easy to perform. The method shows good sensitivity and specificity in diagnosing bacterial sepsis at PCT levels of ≥2 ng/ml.[13–15] Using PCT levels above 2 ng/ml as the cut-off to indicate sepsis syndrome, the present study showed sensitivity of 86% and specificity of 95%. Using a cut-off of above 0.5 ng/ml revealed higher sensitivity but with a reduction in the specificity to 84%. Hence serum PCT of 2 ng/ml or more using the rapid immunochromatographic method is an effective marker of sepsis and may help in aggressive management of such patients along the lines of sepsis.
Despite improved methods, blood cultures are not always positive in clinically confirmed sepsis. Presumed bacterial cause of fever cannot be detected in 60% to 80% of patients with suspected primary and secondary bloodstream infections.[4] In this study, excluding the 2 cases of malaria and rickettsial fever, the sensitivity of blood culture was 63% (12 of 19); and in all these patients, serum PCT was above 2 ng/ml.
Differentiating true infection from contamination or probable skin commensals in blood cultures poses a diagnostic problem.[3] The 2 blood cultures yielding coagulase-negative staphylococci were probable skin commensals, as both patients did not have any infective foci and both responded well with inotropes alone. In both these patients, the serum PCT was less than 2 ng/ml.
Falsely high PCT is known to occur in cardiogenic shock, major surgery, accidental trauma, pancreatitis and burn patients.[2] Of patients with cardiogenic shock, 1 patient had PCT above 10 ng/ml, 2 had PCT less than 0.5 ng/ml and 1 patient with coexisting sepsis had PCT of 2-10 ng/ml. PCT levels are significantly higher in patients with septic shock than in those with cardiogenic shock, and hence a cut-off of above 10 ng/ml is recommended to discriminate between septic and cardiogenic shocks.[10]
PCT secretion begins within 4 hours after stimulation and peaks at 8 hours.[16] Serum PCT increases early after trauma and peaks within 48 to 72 hours post-trauma and declines thereafter in the absence of infection or sepsis.[3,17] In the present study, out of 13 patients with major surgery or trauma, 4 patients had surgery or trauma with in 72 hours of suspected sepsis and PCT testing; 3 of them with PCT levels below 0.5 ng/ml did not have sepsis, and 1 patient had septic shock (PCT >10 ng/ml). In contrast, initial falsely low PCT levels, typically seen during the early course or localized state of infection, often show a gradual increase during follow-up measurements after 6-24 hours.[4] Reduction in concentration of PCT has been described in response to antibiotic administration and may be used to detect antibiotic responsiveness.[9,15,18] In the present study, serial serum PCT levels were not estimated for most patients. However, one of the patients (post-renal transplant status) from this cohort for whom 3 serum PCT levels were performed showed a marked decrease in the PCT levels in response to the antibiotics administered. This is in concordance with the several clinical-management protocols, like the “PCT-guided antimicrobial stewardship”, that have been used to either withhold or continue antibiotics based on the PCT levels.[3,19]
The above facts emphasize the importance of follow-up measurements of PCT. A limitation of the present study is that serial measurements of PCT were not done for most patients. Also the number of patients evaluated in this study was not very large. More Indian studies evaluating PCT on a larger number of patients with suspected sepsis are needed to conclusively corroborate the findings of this study, and such studies will be useful in an age of overuse of antibiotics and emerging antibiotic resistance. Another limitation of the study was that C-reactive protein (CRP) levels were not estimated for most patients. However, most studies have found superiority of PCT in comparison to CRP as a marker of infection and sepsis.[2,4,16]
CONCLUSION
The PCT assay was found to be a promising biomarker of sepsis in this small exploratory study and provided valuable and early information before the culture results were available. The semi-quantitative, rapid assay revealed moderate sensitivity (86%) and high specificity (95%) at a cut-off above 2 ng/ml. The assay is simple to perform, and results are available soon. PCT assay might assist in avoiding unwarranted antibiotic usage in critically ill patients who present with symptoms similar to those in infective conditions. However, the assay result must be interpreted in association with the clinical findings and other laboratory parameters.
Acknowledgments
Our sincere thanks to the technical staff of the Department of Microbiology and the staff in the intensive care unit for their prompt action, notification and technical support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164:396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]
- 2.Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care. 2004;8:234–42. doi: 10.1186/cc2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller B, Schuetz P, Trampuz A. Circulating biomarkers as surrogates for bloodstream infections. Int J Antimicrob Agents. 2007;30:S16–23. doi: 10.1016/j.ijantimicag.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 4.Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–83. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 5.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 6.Sachdeva A, Yadav SP, Anjan M, Srivastava LM, Kharya G. Serial measurement of interleukin-6 and procalcitonin for determination of bacterial sepsis and outcome in children with febrile neutropenia. J Clin Oncol. 2008;26:20633. [Google Scholar]
- 7.Singh JC, Kekre NS. Procalcitonin: A marker of renal parenchymal infection in children? Ind J Urol. 2006;22:162–3. [Google Scholar]
- 8.Shafiq N, Malhotra S, Bhasin DK, Rana S, Siddhu S, Pandhi P. Estimating the diagnostic accuracy of procalcitonin as a marker of the severity of acute pancreatitis: A meta-analytic approach. JOP. 2005;6:231–7. [PubMed] [Google Scholar]
- 9.Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA. Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med. 2000;28:2591–4. doi: 10.1097/00003246-200007000-00068. [DOI] [PubMed] [Google Scholar]
- 10.Aouifi A, Piriou V, Bastien O, Blanc P, Bouvier H, Evans R, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med. 2000;28:3171–6. doi: 10.1097/00003246-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Suprin E, Camus C, Gacouin A, Le Tulzo Y, Lavoue S, Feuillu A, et al. Procalcitonin: A valuable indicator of infection in a medical ICU? Intensive Care Med. 2000;26:1232–8. doi: 10.1007/s001340000580. [DOI] [PubMed] [Google Scholar]
- 12.Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000;26:148–52. doi: 10.1007/BF02900728. [DOI] [PubMed] [Google Scholar]
- 13.Meisner M, Brunkhorst FM, Reith HB, Schmidt J, Lestin HG, Reinhart K. Clinical experiences with a new semi-quantitative solid phase immunoassay for rapid measurement of procalcitonin. Clin Chem Lab Med. 2000;38:989–95. doi: 10.1515/CCLM.2000.147. [DOI] [PubMed] [Google Scholar]
- 14.Boo NY, Nor Azlina AA, Rohana J. Usefulness of a semi-quantitative procalcitonin test kit for early diagnosis of neonatal sepsis. Singapore Med J. 2008;49:204–8. [PubMed] [Google Scholar]
- 15.Fioretto JR, Borin FC, Bonatto RC, Ricchetti SM, Kurokawa CS, de Moraes M, et al. Procalcitonin in children with sepsis and septic shock. J Pediatr (Rio J) 2007;83:323–8. doi: 10.2223/JPED.1644. [DOI] [PubMed] [Google Scholar]
- 16.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–17. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 17.Wanner GA, Keel M, Steckholzer U, Beier W, Stocker R, Ertel W. Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit Care Med. 2000;28:950–7. doi: 10.1097/00003246-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohoun C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–8. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chirouze C, Schuhmacher H, Rabaud C, Gil H, Khayat N, Estavoyer JM, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Inf Dis. 2002;35:156–61. doi: 10.1086/341023. [DOI] [PubMed] [Google Scholar]


