Abstract
Congenital lobar emphysema (CLE) is a potentially reversible, though possibly life-threatening, cause of respiratory distress in the neonate. It poses dilemma in diagnosis and management. We are presenting a 6-week-old baby who presented with a sudden onset of respiratory distress related to CLE affecting the left upper lobe. Lobectomy was performed under general anaesthesia with one lung ventilation. The details of anaesthetic challenges and management are described here.
Keywords: Congenital lobar emphysema, lobectomy, neonatal intensive care unit
INTRODUCTION
Congenital lobar emphysema (CLE) is a developmental anomaly of the lower respiratory tract, which is characterised by hyperinflation of one or more of the pulmonary lobes.[1,2] Other terms for CLE include congenital lobar overinflation and infantile lobar emphysema.[3–5] CLE is a rare congenital malformation, with a prevalence of 1 in 20,000 to 1 in 30,000.[6] We discuss the anaesthetic management of a 6-week-old child requiring left lobectomy, who presented with respiratory distress due to CLE affecting the left lung.
CASE REPORT
A 6-week-old male term infant, weighing 3.7 kg, presented to the accident and emergency ward with a history of fever, difficulty in feeding and rapid breathing rate for last 7 days. He was delivered by forceps at 39 weeks, was breastfed and suffered no medical problems until the presenting episode. He was treated in a peripheral hospital initially for pneumonia. However, there was no improvement even after medical therapy. He was then referred to the present hospital.
On examination, the baby was pale with poor capillary return and oxygen saturation of 80% on air and was in respiratory distress with intercostal retraction and tachypnoea of 60–64 per minute. On auscultation, air entry was decreased in the left lung and bronchial breathing over right haemithorax. Blood pressure was 90/50 mm Hg and heart rate was 180 beats per minute. Capillary blood gas analysis demonstrated respiratory acidosis with pH 7.2, pCO2 60 mm Hg, pO2 101 mm Hg and base excess of –1.3. Chest radiography [Figure 1] demonstrated partial collapse of right lung with hyperinflation of left upper lobe and marked mediastinal shift across to the right. A clinical diagnosis of CLE was made and treatment started in the neonatal intensive care unit (NICU) with IV antibiotics, bronchodilators, and nebulisation and chest physiotherapy. Fluid resuscitation was commenced with 20 ml/kg N/5 dextrose solution that restored capillary return to normal, although tachypnoea remained. The baby was nursed on his left side overnight with supplemental oxygen delivered to a head box.
Figure 1.
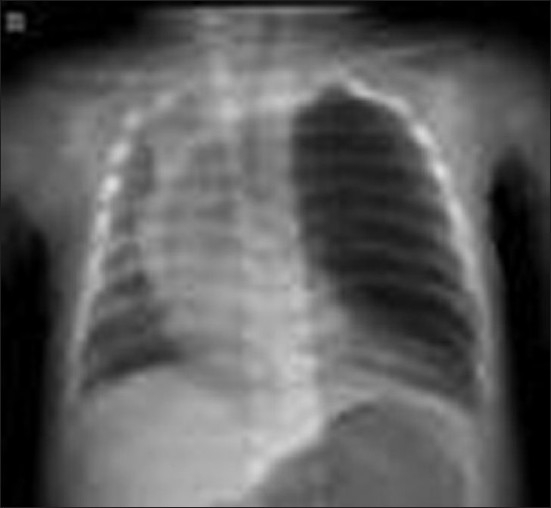
Chest X-ray PA view shows marked overdistension of the left upper lobe with mediastinal shift to the right and partial collapse of the contralateral remaining lung fields
Computed tomography (CT) scan [Figure 2] done on the next day confirmed the diagnosis of CLE of left upper lobe with gross mediastinal shift. Left upper lobectomy was planned under general anaesthesia the following day.
Figure 2.
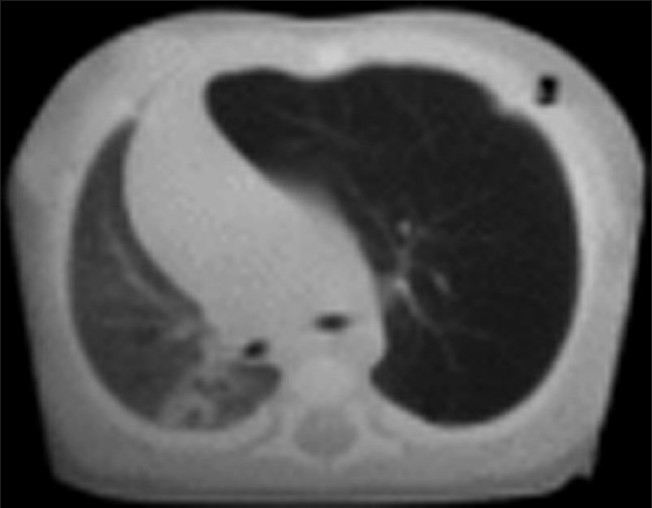
CT scan shows a hyperluscent, hyperextended lobe with midline substantial herniation and compression of the remaining lung. The mediastinum is significantly shifted away from the side of the abnormal lobe
In the operation theatre, monitoring was established with pulse oxymetry (SPO2), electrocardiogram (ECG) and noninvasive blood pressure (NIBP) monitoring. Intravenous access was checked. Patient was induced with Sevoflurane 8% in oxygen, and tracheal intubation was secured after spraying the vocal cord with 10% Lidocaine spray with a 2.5-mm portex nasal tube. The tube was passed through the vocal cord down into the right main bronchus and fixed at 12 cm at nose. Right-sided endobronchial tube position was confirmed by auscultation.
For analgesia, a mixture of 5 ml 0.25% Bupivacaine and 0.2 mg Morphine was given via caudal epidural route. Rectal Paracetamol suppositories 125 mg were also inserted. SpO2, ECG, NIBP, capnography and temperature were monitored. Once oxygen saturation and heart rate settled during intermittent positive pressure ventilation IPPV, Atracurium 3 mg and Fentantyl 3 μg were given. Anaesthesia was maintained with oxygen, air and Isoflurane (0.7–1.5%) via Drager Fabius Medical Systems ventilator, and a peak inspiratory pressure of 20 cm was maintained. During the initial period of left thoracotomy position, an oxygen concentration of 100% was required to maintain an oxygen saturation of 99%. As soon as the left upper lobe was isolated and clamped and branch pulmonary artery and vein were ligated and divided, oxygen saturation stabilised at 97% on 40%. Oxygen and N2O were then started. During the surgical procedure, tracheal suctioning was required repeatedly.
After the removal of the emphysematous left upper lobe, the tracheal tube was withdrawn up to the carina and ventilation of both the lungs was started. IV fluid was maintained with N/5 dextrose solutions 16 ml/hour. Blood loss was insignificant. A left chest drain was left in situ. The baby was electively ventilated in the NICU for 12 hours and weaned off successfully. Postoperatively, the chest radiograph [Figure 3] was repeated in the evening and revealed complete correction of the mediastinal shift and marked expansion of the right lung. Oral intake was started on the 3rd day and the baby was discharged uneventfully on the 7th postoperative day.
Figure 3.
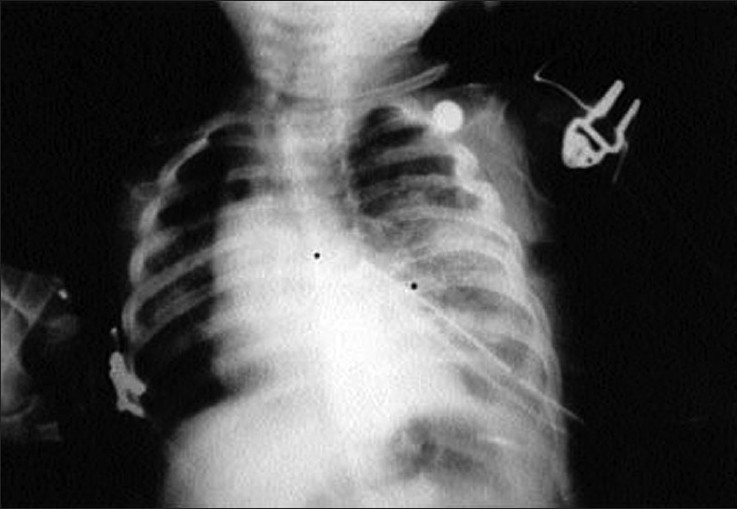
Chest X-ray AP view showing expansion of both the lung fields with no mediastinal shift on postoperative period
DISCUSSION
CLE is a rare congenital abnormality characterised by overinflation of a pulmonary lobe. It often presents a diagnostic and therapeutic dilemma. Several factors have been associated with its development. In 50% of cases, there is decreased bronchial cartilage tissue. This defect produces a ball valve effect with consequent overinflation.[7,8] Vascular abnormalities that produce compression,[9] bronchial stenosis,[8] bronchogenic cysts,[10] and congenital cytomegaloviral infection have also been associated.[11] CLE has been described in twins,[12] but in up to 40% of cases, the cause is unclear.[8] The age of onset of symptoms ranges from a few days after birth to 6 months.[13] Most patients develop symptoms in the neonatal period and the male:female ratio is 3:1.[14] As in this child, respiratory distress is the commonest mode of presentation. There is dyspnoea, wheezing, grunting respiration, tachypnoea and sometimes progressive cyanosis. Similar symptoms may occur in bronchopneumonia, cyanotic congenital heart diseases, and several congenital abnormalities of the lung.[8,15] There were no such associated anomalies in our patient. In more than 50% of cases, the left upper lobe is involved[8] and there is a shift of the mediastinum as was the case here.
The basic investigation in CLE is the chest radiograph from which a diagnosis can be made and is readily available. CT, bronchoscopy and angiopulmography are also used in the diagnosis.[7,8] In this child, a CT scan was done to exclude an extrinsic mass effect as the cause of the overinflated lung and to demonstrate the bronchial anatomy before surgery.
Operative surgery with lobectomy is the commonest mode of treatment.[8] Depending on the symptoms, conservative measures are sometimes taken, but these may fail in the presence of inter-current infections. Resection of vascular rings may be the only surgical procedure where this is the cause.[13] In over 85% of cases, the long-term outcome after surgery is excellent with complete cure. Our patient improved immediately after surgery with normalisation of the SaO2 within 12 hours.
Induction of anaesthesia is very critical in these patients as crying or struggling can increase the amount of trapped gas, and positive pressure ventilation to assist the ventilation also increases the emphysema. So, the aim should be to achieve a smooth inhalation induction. In our patient, we induced the baby with sevoflurane in oxygen without assisted ventilation. Commonly, single lumen tubes are used for the purpose of tracheal intubation. Even though lung isolation is desirable, double lumen tubes are not freely or commercially available. In small infants, lung isolation is technically difficult and not commonly practiced.[16] As an alternative, selective main stem bronchus intubation can be done.
Airway management in patients with CLE is really a difficult issue. The challenges are both respiratory and cardiovascular. In this condition, it is necessary to avoid further inflation and gas trapping in the diseased lung since this may compromise the normal lung. High intrathoracic pressure can also reduce the cardiac output. It is important to avoid IPPV until the diseased lung is isolated because further inflation of the diseased side can compromise the function of the normal lung. Some anaesthetists advocate avoiding IPPV until thoracotomy is performed and the diseased lung is isolated.[17–20] Other alternatives include placement of a bronchial blocker under fluoroscopic guidance. High frequency ventilation has been used successfully in the patients with CLE, as low airway pressures are especially suitable.[21] It is recommended if the circumstances permit and the user is familiar with the technique. Extracorporeal membrane oxygenation has been used to maintain oxygenation in children with persisting pulmonary acquired interstitial emphysema.[22] In our patient, ventilation was started once the right endobronchial intubation was successful. We used nasal intubation in view of the requirement of postoperative ventilation. An alternative induction approach, especially for the unstable infants, is sedation with intravenous ketamine and local anaesthetic infiltration of the incision site.[23] After the intrathoracic pressure has been released, general anaesthetic can then proceed with any other appropriate technique.
One-lung ventilation for small infants or neonates is challenging, both for patient and anaesthetist. The positioning of an endobronchial tube in this age group is difficult and accurate selection of tube size is essential. Commonly, single lumen tubes are used for the purpose of tracheal intubation. Even though lung isolation is desirable, double lumen tubes are not freely or Commercially available. In small infants, lung isolation is technically difficult and not commonly practiced.[24] As an alternative, selective main stem bronchus intubation can be done. A detailed discussion on the problems of one lung ventilation and lung isolation is beyond the scope of this paper.
In summary, a 6-week-old baby presented with a sudden onset of respiratory distress related to CLE affecting the left upper lobe. Lobectomy was performed after induction with Sevoflurane in oxygen, with the child breathing spontaneously. IPPV with oxygen and air was not commenced until right endobronchial intubation was achieved. Left upper lobectomy was performed uneventfully and the child was discharged from the hospital on the 7th postoperative day.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kravitz RM. Congenital malformations of the lung. Pediatr Clin North Am. 1994;41:453–72. doi: 10.1016/s0031-3955(16)38765-x. [DOI] [PubMed] [Google Scholar]
- 2.Stanton M, Davenport M. Management of congenital lung lesions. Early Hum Dev. 2006;82:289–95. doi: 10.1016/j.earlhumdev.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Stocker JT, Drake RM, Madewell JE. Cystic and congenital lung disease in the newborn. Perspect Pediatr Pathol. 1978;4:93–154. [PubMed] [Google Scholar]
- 4.Landing BH, Dixon LG. Congenital malformations and genetic disorders of the respiratory tract (larynx, trachea, bronchi, and lungs) Am Rev Respir Dis. 1979;120:151–85. doi: 10.1164/arrd.1979.120.1.151. [DOI] [PubMed] [Google Scholar]
- 5.Hernanz-Schulman M. Cysts and cystlike lesions of the lung. Radiol Clin North Am. 1993;31:631–49. [PubMed] [Google Scholar]
- 6.Thakral CL, Maji DC, Sajwani MJ. Congenital lobar emphysema: Experience with 21 cases. Pediatr Surg Int. 2001;17:88–91. doi: 10.1007/s003830000506. [DOI] [PubMed] [Google Scholar]
- 7.Doull IJ, Connett GJ, Wamer JO. Bronchoscopic appearances of congenital lobar emphysema. Pediatr Pulmonol. 1996;21:195–7. doi: 10.1002/(SICI)1099-0496(199603)21:3<195::AID-PPUL9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Mikhailova V. Congenital lobar emphysema in childhood. Khirurgiia (Sofia) 1996;49:8–12. [PubMed] [Google Scholar]
- 9.Absher DR, Kriss VM, Cotrill CM. Lobar emphysema due to anomalous aortic origin of theleft pulmonary artery. Cardiol-Young. 1999;9:327–30. doi: 10.1017/s1047951100005035. [DOI] [PubMed] [Google Scholar]
- 10.Okur H, Kucukaydin M, Ozturk A, Balkanli S, Boekurt A. Giant bronchogenic cyst presenting as a lobar emphysema in a newborn. Ann-Thorac Surg. 1996;62:276–8. doi: 10.1016/0003-4975(96)00265-2. [DOI] [PubMed] [Google Scholar]
- 11.Carrol ED, Campbell ME, Shaw BN, Pilling DW. Congenital lobar emphysema in congenital cytomegalovirus infection. Pediatr Radiol. 1996;89:900–2. doi: 10.1007/BF03178047. [DOI] [PubMed] [Google Scholar]
- 12.Thompson AJ, Reid AJ, Reid M. Congenital lobar emphysema occurring in twins.J. Perint-Med. 2000;28:155–7. doi: 10.1515/JPM.2000.022. [DOI] [PubMed] [Google Scholar]
- 13.Bappal B, Ghani SA, Chaudhary R, Sajvani MJ. Congenital lobar emphysema: a review of 10 cases. Indian J Pediatr. 1996;63:801–8. doi: 10.1007/BF02730933. [DOI] [PubMed] [Google Scholar]
- 14.Cay A, Sarihan H. Congenital malformations of the lung. J Cardiovasc Sur (Torino) 2000;41:507–10. [PubMed] [Google Scholar]
- 15.Takeda S, Miyoshi S, Inoue M, Omori K, Okumura M, Yoon HE, et al. Clinical spectrum of congenital cystic disease of the lung in children. Eur J Cardiothorac Surg. 1999;15:11–7. doi: 10.1016/s1010-7940(98)00262-0. [DOI] [PubMed] [Google Scholar]
- 16.Hammer GB, Fitzmaurice BG, Brodsky JB. Methods for single lung ventilation in paediatric patients. Anaesth Analg. 1999;89:1426–9. doi: 10.1097/00000539-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Al-Salem AH, Adu-Gyamfi Y, Grant CS. Congenital lobar emphysema. Can J Anaesth. 1990;37:377–9. doi: 10.1007/BF03005595. [DOI] [PubMed] [Google Scholar]
- 18.Lt Col DK Sreevastava, Maj S Kiran. Anaesthetic Management of Congenital Lobar Emphysema: A Report of Two Cases. Medical Journal Armed Forces India. 2005;61:79–81. doi: 10.1016/S0377-1237(05)80128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandran-Mahaldar D, Kumar S, Balamurugan K, Raghuram AR, Krishnan R, Kannan Congenital lobar emphysema. Indian J Anaesth. 2009;53:482–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Tempe DK, Virmani S, Javetkar S, Banerjee A, Puri SK, Datt V. Congenital lobar emphysema: pitfalls and management. Ann Card Anaesth. 2010;13:53–8. doi: 10.4103/0971-9784.58836. [DOI] [PubMed] [Google Scholar]
- 21.Goto H, Boozalis ST, Benson KT, Arakawa K. High frequency jet ventilation for resection of congenital lobar emphysema. Anaesth Analg. 1987;66:684–6. [PubMed] [Google Scholar]
- 22.Trento A, Thompson A, Siewers RD, Orr RA, Kochanek P, Fuhrman B, et al. Extracorporeal membrane oxygenation in children.New trends. J Thoracic Cardiovasc Surg. 1988;96:542–7. [PubMed] [Google Scholar]
- 23.Cote CJ. The anaesthetic management of congenital lobar emphysema. Anaesthesiology. 1978;49:296–8. doi: 10.1097/00000542-197810000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Hammer GB, Fitzmaurice BG, Brodsky JB. Methods for single lung ventilation in paediatric patients. Anaesth Analg. 1999;89:1426–9. doi: 10.1097/00000539-199912000-00019. [DOI] [PubMed] [Google Scholar]


