Abstract
Inhalational agents are used routinely for maintenance of anaesthesia. Post anaesthesia hepatic failure has been documented following exposure to halothane. However, there are very few reports of such complications following isoflurane anaesthesia. A 6-year-old child developed fulminant hepatic failure 2 days following craniotomy under general anaesthesia. There was no evidence of viral, autoimmune, or metabolic causes of hepatitis. No other medications known to cause hepatitis, except low dose paracetamol, were administered. The clinical and histological picture of our case is similar to that of halothane hepatitis, which has a significant mortality rate. We report this as a possible fulminant hepatic failure resulting from exposure to isoflurane anaesthesia.
Keywords: Children, fulminant, hepatic failure, isoflurane
INTRODUCTION
Isoflurane is the agent of choice in neurosurgical procedures due to its minimal effects on cerebral dynamics. Its safety profile on hepatic function has been established. However, there are few reports of occurrence of fulminant hepatic failure following exposure to isoflurane anaesthesia. We report one such case of fulminant hepatic failure due to repeated isoflurane exposure.
CASE REPORT
A 6-year-old male child weighing 17 kg underwent vermian pilocytic astrocytoma excision under general anaesthesia. The intraoperative course was uneventful. The child developed Cerebrospinal fluid (CSF) leak in the postoperative period which was managed by lumbar drain insertion and subsequent removal. The patient improved symptomatically and was discharged home. Two months later, the child presented with pseudomeningocoele at the operative site and cystoperitoneal shunt was placed under general anaesthesia. All the above surgeries were done by intravenous induction with thiopentone, and isoflurane was used as the inhalation anaesthetic agent during maintenance. The postoperative course was uneventful.
One-and-a-half years later, the child presented with history of imbalance while walking, decreased activity, and vomiting after food. Repeat computed tomography (CT) brain showed recurrence of the tumour for which he was readmitted. All blood investigations were normal. He underwent middle suboccipital craniectomy and near total excision of the tumour under general anaesthesia using isoflurane as the inhalation agent for maintenance. The child withstood the surgery well and received 200 ml of packed RBC. He was extubated and transferred to postoperative ward. Over the next 24 hours, the child was conscious, talking and taking oral feeds. On the second postoperative day, the child vomited twice in the early morning and he became drowsy and unresponsive to painful stimuli. Child was febrile. His blood glucose was <30 mg/dl. It was corrected immediately with 25% dextrose. The patient improved a little but still remained confused and was taken for repeat CT brain. CT brain showed adequate decompression of tumour and no fresh bleed or oedema. Sepsis was suspected at this time and relevant serological investigations were asked for. As the child deteriorated neurologically, trachea was intubated and ventilated.
There was marked increase in hepatic enzyme levels with evidence of coagulopathy. Total WBC count was 18,200/mm3, serum bilirubin 4.5 mg/dl, alkaline phosphatase 512 IU/l, aspartate transaminase (AST) 7304 IU/l, alanine transaminase (ALT) 5665 IU/l, international normalized ratio (INR) >10. Over the next 12 hours, his renal function started to deteriorate and liver function worsened. Haemodialysis was done for his acute kidney injury. He tolerated the dialysis well but later started developing hypotension. Inotropic support was initiated to maintain his blood pressure. The child's condition continued to deteriorate and succumbed to severe metabolic disturbances 24 hours after the onset of symptoms. All cultures were negative for infection, and marker for sepsis was also negative. Serological studies were negative for viral hepatitis. Post-mortem liver biopsy revealed preserved architecture of Zone 1, and necrotic changes in areas around the central vein (Zone 3) suggestive of drug induced lesion [Figure 1a and b]. The histological pattern of severe centrilobular necrosis (Zone 3) seen in our case is in concordance with the changes described with halothane hepatitis.
Figure 1.
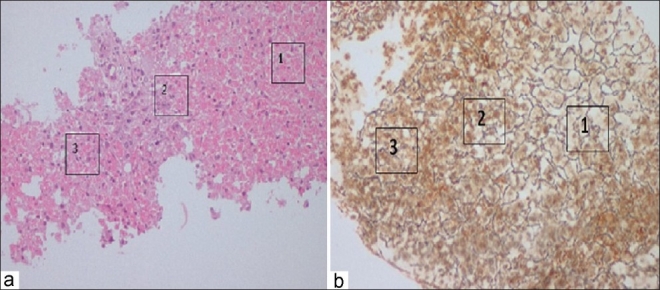
(a) Low-power view of the liver biopsy core showing preserved hepatocytes around the portal areas (Zone 1) and necrosis in Zones 2 and 3. (b) The reticulin stain showing the preserved framework in Zone 1 and destroyed framework in Zones 2 and 3
DISCUSSION
Isoflurane is less hepatotoxic compared to halothane and enflurane. In susceptible patients, halothane, enflurane, isoflurane, and desflurane can produce severe hepatic injury by an immune-mediated response directed against reactive anaesthetic metabolites covalently bound to hepatic proteins. The incidence of hepatotoxicity appears to correlate directly with the anaesthetic metabolism catalysed by cytochrome P450 2E1 to trifluoroacetylated hepatic proteins.[1] Production of acylated proteins may be an important mediator of anaesthetic-induced hepatotoxicity. Proteins modified by acetylation may constitute neo-antigens with a potential for triggering an antibody-mediated immune response. The likelihood of suffering postoperative immune hepatitis depends on the amount of the anaesthetic metabolised and is thereby considerably less with enflurane, isoflurane or desflurane compared with halothane.[2] Approximately 20% of halothane is oxidatively metabolised compared to only 2% of enflurane and 0.2% of isoflurane. Sera from patients with a clinical diagnosis of halothane hepatitis showed antibody reactivity against hepatic proteins from rats exposed to halothane or enflurane. No reactivity was detected in rats exposed to isoflurane, desflurane, or oxygen alone.[1]
Isoflurane was hailed as the anaesthetic of the 1980s. Since its release by the Food and Drug Administration in 1979, controversy has existed about the extent to which isoflurane is capable of producing hepatotoxic effects.[3] Isoflurane was initially thought to be incapable of liver damage secondary to its minimal metabolism and lack of reactive metabolites. Several case reports[3–7] have since been appearing in literature suspecting the role of isoflurane as the cause of post-anaesthetic hepatic injury, and in most cases, the diagnosis was after exclusion of various possible causes. Ihtiyar et al.[8] reported fatal hepatotoxicity following single exposure to isoflurane. Postoperative hepatitis is usually associated with factors such as blood product transfusions, hypovolemic shock, and other surgical stress.
In the present case report, the patient had repeated surgeries under general anaesthesia using isoflurane. The present incident happened when the previous exposure was 1½ years back. The clinical picture was that of an acute hepatitis leading to fulminant hepatic failure. Serological markers for viral hepatitis were negative. Presence of fever, hypotension and renal failure led to the possibility of sepsis induced multiorgan dysfunction syndrome. But the blood and urine cultures did not show any growth after sufficient time of incubation. Serological marker of infection confirmed by polymerase chain reaction (PCR) was negative. The child received only two doses of paracetamol as suppository for pain relief. The total dose received was 160 mg, i.e., less than 10 mg/kg (far less than the toxic single dose of 200 mg/kg or rectal dose of 75–100 mg/kg/day). Hence, the possibility of paracetamol induced hepatotoxicity is unlikely. This is similar to a case reported by Brunt, et al.,[9] who developed fulminant hepatic failure following repeated exposure to isoflurane anaesthesia.
Immediate post-mortem liver biopsy showed severe centrilobular hepatic necrosis commonly seen with halothane hepatitis Type II. As the other possible causes for the hepatic failure were ruled out, isoflurane was thought to have induced fulminant hepatic failure leading to death.
CONCLUSION
Isoflurane, a common anaesthetic agent, can cause severe fulminant hepatic failure following repeated exposures. In the event of postoperative hepatic dysfunction, one should consider the possibility of inhalational agent induced injury. In children, where repeated procedures are performed, avoidance of re-exposure to inhalational agents may be worth considering in future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Njoku D, Laster MJ, Gong DH, Eger EI, 2nd, Reed GF, Martin JL. Biotransformation of halothane, enflurane, isoflurane and desflurane to trifluroacetylated liver proteins: Association between protein acylation and hepatic injury. Anesth Analg. 1997;84:173–8. doi: 10.1097/00000539-199701000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Reichle FM, Conzen PF. Halogenated inhalational anesthetics. Best Pract Res Clin Anaesthesiol. 2003;17:29–46. doi: 10.1053/bean.2002.0265. [DOI] [PubMed] [Google Scholar]
- 3.Sinha A, Clatch RJ, Stuck G, Blumenthal SA, Patel SA. Isoflurane hepatotoxicity: A case report and review of literature. Am J Gastroenterol. 1996;91:2406–9. [PubMed] [Google Scholar]
- 4.Weitz J, Kienle P, Böhrer H, Hofmann W, Theilmann L, Otto G. Fatal hepatic necrosis after isoflurane anesthesia. Anesthesia. 1997;52:892–5. doi: 10.1111/j.1365-2044.1997.187-az0319.x. [DOI] [PubMed] [Google Scholar]
- 5.Malnick SD, Mahlab K, Borchardt J, Sokolowski N, Attali M. Acute cholestatic hepatitis after exposure to Isoflurane. Ann Pharmacother. 2002;36:261–3. doi: 10.1345/aph.1A009. [DOI] [PubMed] [Google Scholar]
- 6.Gelven PL, Cina SJ, Lee JD, Nichols CA. Massive hepatic necrosis and death following repeated Isoflurane exposure: Case report and review of literature. Am J Forensic Med Pathol. 1996;17:61–4. doi: 10.1097/00000433-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Turner GB, O’Rourke D, Scott GO, Beringer TR. Fatal hepatotoxicity after reexposure to isoflurane: A case report and review of literature. Eur J Gastroenterol Hepatol. 2000;12:955–9. doi: 10.1097/00042737-200012080-00017. [DOI] [PubMed] [Google Scholar]
- 8.Ihtiyar E, Algin C, Haciolu A, Isiksoy S. Fatal isoflurane hepatotoxicity without re-exposure. Indian J Gastroenterol. 2006;25:41–2. [PubMed] [Google Scholar]
- 9.Brunt EM, White H, Marsh JW, Holtmann B, Peters MG. Fulminant hepatic failure after repeated exposure to isoflurane anesthesia: A case report. Hepatology. 1991;13:1017–21. [PubMed] [Google Scholar]


