Abstract
Nutrapharmacology, or the use of bioactive food compounds at pharmacological doses is emerging as a therapeutic approach to target the complex metabolic dysregulations in ageing and obesity-related chronic disease. Resveratrol, a polyphenol found in the skin of grapes, and other edible plants and related food products, has received extensive attention through the link with the French paradox, and later with its chemopreventive activity demonstrated in vitro and in animal cancer models. A plethora of laboratory investigations has provided evidence for the multi-faceted properties of resveratrol and suggests that resveratrol may target ageing and obesity-related chronic disease by regulating inflammation and oxidative stress. A number of obstacles stand in the path to clinical usage however, not least the lack of clinical evidence to date, and the myriad of doses and formulations available. Further, data on the effects of resveratrol consumption in a capsule vs. food form is conflicting, and there are uncertain effects of long term dosing. The review will summarize the human pharmacokinetic and pharmacodynamic published data, and the topics for research if resveratrol is to become a multi-target therapeutic agent addressing chronic disease.
Keywords: bioactive food compounds, nutraceuticals, nutrapharmacology, polyphenols, resveratrol
Introduction: nutrapharmacology, the role of food compounds in disease prevention
The use of bioactive food compounds (nutrients and phytochemicals found in fruit, vegetables and spices) at pharmacological doses, is emerging as a therapeutic approach to address the complex metabolic dysregulations in ageing and obesity-related chronic diseases. This is termed nutrapharmacology and the compounds are nutraceuticals. The evidence comes from basic science reports, demonstrating that these compounds can efficiently modulate the oxidative, inflammatory and apoptotic imbalances in chronic disease metabolic pathways [1, 2].
Dietary agents originate from daily food. Therefore they may be more acceptable to consumers who may erroneously perceive them as having fewer side effects than pharmaceutical agents. In contradistinction, people may need to consume large amounts of foods containing these compounds for therapeutic benefit. Whilst it may not be plausible to consume such large amounts, nutraceuticals offer a convenient alternative at a time when, in spite of scientific evidence suggesting their benefits in health and disease prevention, these compounds are poorly represented in our daily menu. However food sources are complex, and are likely to contain combinations of compounds, possibly acting synergistically to enhance/reduce their bioavailability and/or activity [1, 3, 4]. The benefits of dietary constituents may therefore be different from the putative benefits of nutraceutical formulations at pharmacological dose. Additionally, bioactive phytochemicals may have dose-related toxic effects. Thus clinical evidence of both the food and the nutraceutical sources is required before considering these agents in the management of chronic disease.
Resveratrol (RSV) is one compound of interest. It is 13 years since the first paper reported the initial in vitro and in vivo evidence of cancer chemopreventative activity of RSV [5]. The abundance of research providing promising data brings this phytochemical to the era of clinical testing [6–8]. This review will summarize the pharmacology of RSV, as evidenced in the first published clinical observations, and discuss its clinical relevance.
Resveratrol
History
Since its identification in the 1940s from the white hellebore by Takaoka, and in 1963 in the Japanese knotweed Polygonum cuspidatum by Nonomura [9] RSV has gained notoriety, helped by media attention in the 1990s when speculated to explain the French paradox [10, 11]. On closer investigation, it appeared that RSV was perhaps the active phytochemical in red wine. The concept of the French paradox has since been challenged, but the apparent cardiovascular protective properties of RSV have been explained by the inhibition of LDL cholesterol peroxidation, free-radical scavenging activity, and modulation of nitric oxide production [12–15]. RSV, as grape and Polygonum cuspidatum extracts, is present in Ayur-vedic and traditional Chinese medicine formulae, prescribed for fungal infection, cardiovascular disease, gastrointestinal disorders, diabetes and inflammation [16, 17]. Figure 1 summarizes the last decade of research invested in understanding RSV's biochemical effects, listing genes and products affected by exposure to RSV. Some of the proteins thought to mediate the effects of RSV in the context of multifactorial chronic diseases include AMP-activated protein kinase (AMPK), silent mating type information regulation 2 homolog 1 (SIRT1), N-ribosyldihydronicotinamide:quinone oxidoreductase (NQO2), NFE2-related factor 2 (Nrf2) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [18–24]. The extensive range of affected proteins raises the possibility of off-target deleterious effects, further emphasizing the need for formal clinical toxicity and efficacy studies of chronic RSV intake.
Figure 1.
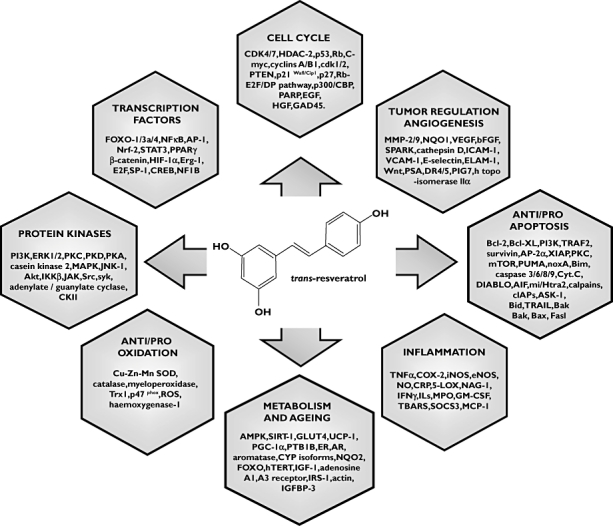
Structure of resveratrol. Genes and products affected by exposure to the compound [7, 17, 25, 56]. CDK – 1/2/4/7, cyclin-dependant kinase 1/2 /4/7; HDAC-2, histone deacetylase 2; P53, tumour protein 53; Rb, retinoblastoma tumour suppressor gene; c-Myc, gene myelocytomatosis; PTEN, phosphatase and tensin homolog; p21Waf1/Cip1, cyclin-dependent kinase inhibitor 1A; P27, cyclin dependant kinase inhibitor 27; Rbf-E2F/DP pathway, Retinoblastoma-family protein/ E2F transcription factor/ DP transcription factor; P300/CBP, CREB binding protein; PARP, poly(ADP)-ribose polymerase; EGF, endothelial growth factor; HGF, human growth factor; GAD45, DNA damage inducible gene 45; FOXO-1/3a/4, forkhead transcription factor 1/3a/4; NFκB, nuclear factor kappa B; AP-1, activating protein-1;NRF-2, nuclear respiratory factor-2; STAT3, signal transducer and activator of transcription 3; PPARγ, peroxisome proliferator activated receptor gamma; HIF-1α, hypoxia inducible factor 1-alpha; Erg-1, ets-related gene; E2F, transcription factor E2F; SP-1, Sp1 transcription factor; CREB, cyclic-AMP response element binding proteins; NF1B, neurofibromin 1b; PI3K, phosphoinositide 3 kinases; ERK1/2, extracellular signal regulated kinase 1 /2; PKC, protein kinase C; PKD, protein kinase D; PKA, protein kinase A; MAPK, mitogen activated protein kinase; JNK-1, c-Jun N-terminal kinases; Akt, serine/threonine protein kinase; IKKβ, IkB kinase beta; JAK, Janus kinase; Src, sarcoma pro-oncogenic tyrosine kinase; syk, spleen tyrosine kinase gene; CKII, casein kinase 2; Cu-Zn-Mn SOD, copper-zinc-manganese superoxide dismustase; Trx1, cytosolic thioredoxin; p47 phox, enzyme for production of superoxide; ROS, reactive oxygen species; AMPK, adenosine monophosphate activated protein kinase; GLUT4, glucose transporter 4; UCP-1, uncoupling protein 1; PGC-1α, PPARgamma coactivator 1 proliferator-activated receptor-gamma; PTB1B, protein tyrosine phosphatase 1 B; ER, eostrogen receptor; AR, andogen receptor; CYP isoforms, cytochrome P450 isoforms; NQO2, NADPH dehydrogease quinone 2; hTERT, human telomerase reverse transcriptase; IGF-1, insulin like growth factor-1; IRS-1, insulin receptor substrate 1; IGFBP-3, Insulin-like growth factor-binding protein 3; TNFα, tumour necrosis factor alpha; COX-2, cyclo-oxygenase 2; iNOS, inducible nitric oxide synthase, eNOS, endothelial nitric oxide synthase; NO, nitric oxide; CRP, C- reactive protein; 5-LOX, 5-lipoxygenase- activating protein; NAG-1, nonsteroidal anti-inflammatory drug-activated gene; IFNγ, interferon gamma; ILs, interleukins; MPO, myeloperoxidase; GM-CSF, granulocyte-macrophage colony stimulating factor; TBARS, thiobarbituric acid reactive substances; SOCS3, Suppressor of cytokine signaling 3; MCP-1, monocyte chemotactic protein-1; TGFβ, transforming growth factor beta; Bcl-2, B-cell lymphoma 2; Bcl-XL, BCL2-like 1; TRAF2, TNF receptor-associated factor 2; AP-2α, activating protein 2; XIAP, X-linked Inhibitor of apoptosis protein; mTOR, mammalian target of rapamycin; PUMA, BCL2 binding component 3; noxA, nitrate reductase, NADH oxidase subunit; Bim, BCL2-like 11 apoptosis facilitator; Cyt. C, cytochrome C; DIABLO, diablo homolog; AIF, apoptosis inducing factor; mi/Htra2, HtrA serine peptidase 2; cIAPs, inhibitor of apoptosis proteins; ASK-1, Apoptosis signal-regulating kinase 1; Bid, BH3 interacting domain death agonist; TRAIL, TNF-related apoptosis-inducing ligand; Bak, BCL2-antagonist/killer 1; Bax, Bcl-2 associated protein; Fasl, Fas antigen ligand; MMP-2/9, matrix metalloproteinases 2/9; NQO1, human NAD(P)H quinone 1; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; ICAM-1, inter-cellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1; ELAM-1, endothelium leukocyte adhesion molecule 1; Wnt, wingless-type MMTV integration site family, member; PSA, prostate-specific antigen; DR4/5, tumour necrosis factor receptor superfamily, member 10a/10b; PIG7, p53-induced gene 7.
Chemical description
RSV is a lipophillic polyphenol. The styrene double-bond allows for cis and trans conformations [9, 25]. The glucose-bound form of RSV is piceid, dominant in food sources and converted to trans-RSV by hydrolysis [26]. Three hydrogen atoms per RSV molecule are available for transfer to reactive species and interrupt oxidative cascades [27]. RSV is produced in response to environmental stress such as UV rays, drought, parasitic or fungal attack [28, 29].
Food sources vs. nutraceuticals
RSV has been found in over 100 plants, often described as abundant in nature. Few edible plants contain RSV however, with minimal amounts provided as shown in Table 1. Cultivars, soil and growing conditions result in large variations in concentrations [30]. Measures are influenced by the inclusion/exclusion of the piceid and cis-forms in assays [26]. The nutraceutical form of RSV is usually 99% purified trans-RSV, extracted from the rhizome of Polygonum cuspidatum or grapes.
Table 1.
Some sources of resveratrol and amount provided in standard serves
| Total resveratrol content | |||
|---|---|---|---|
| Food sources | Average per standard units | Average equivalent in standard serving | Reference |
| Red wines | 0.1–14.3 mg l−1 | 150 ml glass: 0.015–2.15 mg | [88, 89] |
| Pinot noir | 10.5 mg l−1 | 150 ml glass: 1.57 mg | |
| White wines | <0.1 to 1.2 mg l−1 | 150 ml glass: 0.015−0.18 mg | |
| Riesling | up to 1.2 mg l−1 | 150 ml glass: up to 0.32 mg | |
| Red grape juice | 0.5 mg 100 ml−1 | 250 ml glass: 1.25 mg | [90, 91] |
| White grape juice | 0.05 mg 100 ml−1 | 250 ml glass: 0.125 mg | |
| Cranberry juice | 0.2 mg 100 ml−1 | 250 ml glass: 0.5 mg | |
| Fresh grape skin | 5–10 mg 100 g−1 | ||
| Grapes (dry sample) | 0.64 mg 100 g−1 | 250 g (1 cup): 1.6 mg | [30, 92, 93] |
| Blueberry (dry sample) | 0.4 mg 100 g−1 | 150 g punnet: 0.6 mg | |
| Strawberries (frozen) | 0.375 mg 100 g−1 | 150 g punnet: 1.56 mg | |
| Red currant (frozen) | 1.5 mg 100 g−1 | 125 g (1/2 cup): 1.87 mg | |
| Cranberry (frozen) | 1.9 mg 100 g−1 | 125 g (1/2 cup): 2.41 mg | |
| Bilberry (frozen) | 0.678 mg 100 g−1 | 125 g (1/2 cup): 1.7 mg | |
| Raw peanuts | 0.15 mg 100 g−1 | 250 g (1 cup): 0.37 mg | [88, 94, 95] |
| Roasted peanuts | 0.006 mg 100 g−1 | 250 g (1 cup): 0.015 mg | [96–98] |
| Boiled peanuts | 0.52 mg 100 g−1 | 250 g (1 cup): 1.3 mg | |
| 100% peanut butter | 0.047 mg 100 g−1 | 1 tablespoon: 0.01 mg | |
| Cocoa powder | 0.185 mg 100 g−1 | 1 tablespoon: 0.019 mg | |
| Dark chocolate | 0.124 mg 100 g−1 | 50 g: 0.063 mg | |
| Milk chocolate | 0.001 mg 100 g−1 | 50 g: 0.0005 mg | |
| Polygonum cuspidatum | 181–350 mg 100 g−1 | [88] | |
| Itadori tea | 0.97 mg 100 ml−1 | 200 ml: 1.94 mg | [98] |
| Nutraceuticals formulae | Capsule: 20 to 500 mg as pure trans-RSV, or from 50 µg to 50 mg as part of antioxidant formulae | ||
| Darakchasava (Ayur-vedic formula) | 0.36 mg/100 ml−1 | [16] | |
A daily menu, including safe wine drinking, could provide 6 to 8 mg of RSV, including numerous other phytochemicals. In contrast, one nutraceutical capsule provides between 20 to 500 mg of pure trans-RSV, or 3- to 83-fold the supply of a daily diet. The disparity between the dosages is remarkable, and raises questions on the physiological implications of nutraceutical doses. The scarcity of clinical data and understanding of effects of a dietary vs. nutraceutical dose need addressing, considering the wide over the counter availability.
Tolerability and toxicity
Tolerability to nutraceutical RSV has been investigated in healthy lean subjects, from a single up to 29 repeated doses. Tolerability to RSV appears reasonable, with nausea and mild headaches occasionally reported, and mild to moderate diarrhoea reported at larger doses (not always placebo controlled) [31, 32]. These side effects occurred with single daily dosing regimens. If related to peak concentrations, it is possible that split-dosing may improve tolerance. In a study administering 2000 mg twice daily over 1 week [33], there was statistically, but not clinically significant, raised serum bilirubin and potassium concentrations. Daily dosing of 1000 mg for 4 weeks did not change bilirubin concentrations [34]. Data on chronic dosing, e.g. over 90 days, are not available, nor are there data in obese subjects, who are highly represented in the population with chronic disease.
The inhibition and induction by RSV of hepatic P450 isoenzymes involved in phase 1 and 2 detoxification have been observed in subjects receiving 1000 mg RSV nutraceutical daily over 4 weeks. This may be of relevance in patients medicated for co-morbidities due to increased or decreased effect, and drug interactions [34].
Pharmacokinetics
Initial pharmacokinetic (PK) studies used enzymatic hydrolysis to reconvert conjugates to parent RSV for quantification. HPLC with MS/MS is now commonly used to quantify plasma parent and conjugated RSV. Comparison of results is however difficult because different minimal detectable concentrations are used, and the different fractions are not always measured. Definitions of ‘total RSV’ vary in reports, from unspecified to include parent and/or metabolites detected, or include protein-bound and/or unbound [26, 35]. Table 2 summarizes the PK parameters reported in human studies with administration of nutraceutical forms of RSV.
Table 2.
Reported nutraceutical RSV pharmacokinetics parameters in humans
| 99% pure nutraceutical RSV | ||||||||
|---|---|---|---|---|---|---|---|---|
| Single dosing | ||||||||
| Dose | Cmax of total or [parent] RSV | Cmax of dominant conj. | tmaxof total or parent | Half-life of total, parent or [dominant conj.] | AUC of total, parent or [dominant conj.] (ng ml−1 h) | Tissue uptake of parent, or [dominant conj.] | CV for Cmax and AUC | Reference |
| 25 mg labelled RSV/70 kg BW | 2 µm[<0.02 µm] | 0.9–14 µm (C2, C3) | 60 min; 2nd peak at 360 min | 7.2 to 11.8 h | 6240 | [41] | ||
| 500 to 5000 mg | [0.3 to 2.4 µm] | 5 to 18.8 µm (C1) | 50 to 90 min | 2.8 to 8.9 h [3.2 to 11.5 h] | 224 to 1319 [4049 to 30 898] | Up to 80% | [44] | |
| 400 mg Fed | 0.18 µm | Not measured | 2 h | – | – | 128% | [39] | |
| 400 mg Fasting | 0.2 µm | 30 min | ||||||
| 85.5 mg piceid form equivalent to 50 mg RSV | n.d. | 0.94 µm (C3) | 360 to 480 min | – | – | [26] | ||
| 250 mg | [0.024 µm] | 1.3 µm | 90 min | Not reported | Not reported | [84] | ||
| 500 mg | [0.062 µm] | 3.1 µm (sulphates, unspecified) | ||||||
| Multiple dosing | ||||||||
| 25 mg | Dose ratio: 50 mg:25 mg = 6 | Not measured | 50 to 90 min | 1–3 h at dose | AUC ratio:150 mg/25 mg = 39 | [43] | ||
| 50 mg | 1 | |||||||
| 100 mg | Cmaxratio: 150 mg:25 mg = 25 | 2–5 h at dose | ||||||
| 150 mg | 13 | |||||||
| 13 doses over 48 h | ||||||||
| 200 mg × 3/day for 3 days | 0.15 µm | Not measured | 1 to 1.5 h | 2.9 to 4.7 h | 77 to 116 | 40% | [99] | |
| 2000 mg ×2/day | [33] | |||||||
| D7 SDBF | 5.6 µm | Not measured | 3 h | 2.4 h | 3558 | |||
| D8 HFBF | 3 µm | 5 h | 2.4 h | 1966 | ||||
| D15 SDQ | 5.6 µm | 4 h | 2.2 h | 4025 | ||||
| D16 SDQetoh | 5.7 µm | 3 h | 2.1 h | 3800 | ||||
| 500 mg – 8 days | ND | 13.4 µm (C6) | Not reported | Not reported | Not reported | 18.6 [86 C4] nmol g−1 | 200% | [45] |
| 1000 mg – 8 days | ND | 22.3 µm (C6) | 674 [67.2 C1] nmol g−1 | 200% | ||||
| 500 mg 29 days | [0.19 to 4.24 µm] | 2.5 to 18.3 µm (C1) | 60 to 70 min (0.25 to 5 h inter-subject variability) | 4.8 to 9.7 h [3.09 to 8.14 h] non dose-dependent | 175 to 4097 [3558 to 38 900] non dose-dependent | 35 to 107% | [31] | |
| 1000 mg 29 days | ||||||||
| 2500 mg 29 days | ||||||||
| 5000 mg 29 days | ||||||||
| 1000 mg 28 days | [0.3 µm] | 10.4 µm (C1) | Not reported | Not reported | Not reported | 400% | [34] | |
ND not detected; BW body weight; Cmax, maximal concentration; tmax, time of maximal concentration; AUC, area under the concentration vs. time curve; Total RSV, parent and conjugated RSV; CV, coefficient of variability; Conj. conjugates; C1, resverarol-3-O-sulphate; C2, resveratrol- 4′-O-sulphate; C3, resveratrol disulfate, C4; resveratrol 3-O-glucuronide; C5, resveratrol 4′-O- glucuronide; C6, resveratrol sulphate glucuronide; SDBF, standard breakfast; HFBF, high fat breakfast; SDQ, standard breakfast and quercetin; SDQetoh, standard breakfast and quercetin and ethanol.
Absorption
Human absorption seems rapid, via simple intestinal trans-epithelial diffusion [36–38]. The rate but not the extent of absorption following a 400 mg RSV nutraceutical dose was significantly impaired when taken with food compared with the fasted state [39]. Similarly, the PK of 2000 mg twice daily showed delayed and decreased absorption with a high fat breakfast [33]. In contrast, RSV absorption when taken as red wine was not reduced by a meal compared with the fasted state [40].
Metabolism and bioavailability
Following ingestion, most RSV undergoes rapid metabolism in enterocytes, resulting in up to a 20-fold higher concentration of circulating conjugates, and less than 1% of parent RSV [36, 41]. Seven sulphation and glucuronidation conjugates have been identified [26, 42]. Entero-hepatic recirculation and intestinal de-conjugation of metabolites was evidenced in animals [37, 41]. Extreme inter-individual and inconsistent non-dose-dependent variability were observed in all PK parameters, possibly explained by assay sensitivity, individual enzymatic polymorphism and distinction, or lack of, between parent, cis, trans and conjugated forms in Cmax measures. Non-proportional changes in bioavailability were evidenced by non-proportional changes in the Cmaxand AUC with increasing doses (Table 2). Additionally, morning intake demonstrated greater bioavailability, perhaps due to the influence of the circadian rhythm on drug metabolism enzyme activity and the entero-hepatic circulation [43]. These considerations are important when drawing conclusions about RSV bioavailability and correlations with efficacy.
Clinicians are interested in the effects of repeated dosing on bioavailability, especially in the context of chronic disease, where a steady-state concentration of a drug is targeted and intake is chronic. No studies have investigated the benefits of split vs. single daily dosing on RSV bioavailability. However repeated intake was observed to increase plasma parent concentration. Cmax was 2.4 µm after single 5000 mg dose administration [44]. The same investigators reported Cmax of 4.24 µm with 5000 mg administered daily for 29 days. Surprisingly this increase did not occur at all doses investigated (500 to 5000 mg) [31]. Additionally, clearance appears to decrease with repeated dosing, possibly demonstrating saturable metabolism and altered bioavailability, which is concerning for chronic intake.
Overall, bioavailability of parent RSV is poor due to the rapid metabolism resulting in high concentrations of circulating conjugates. Tissue accumulation of total RSV [45] and activity of conjugates were recently evidenced [46, 47], suggesting that circulating parent concentration may not be the sole mode of exposure. Plasma parent RSV Cmax was undetectable at 500 mg and 1000 mg doses in colorectal cancer patients after 8 days intake, but total RSV was found in large concentrations in colorectal tumour and nearby healthy tissue, and bioactivity demonstrated [45]. It is unclear whether these concentrations are due to direct absorption of the parent or the absorption of cleaved glucuronide conjugates.
Distribution and excretion
In vitro, 50% to 98% of total RSV was observed non-covalently bound to albumin, LDL and haemoglobin [42, 48, 49]. In humans, close to 50% of total RSV was found to be bound to plasma proteins [26]. Evidence of total RSV accumulation in the healthy colon mucosa and tumour tissue demonstrates tissue uptake, identifying the colonic tissue as a target [45]. The kidney is the dominant excretion pathway with urinary and faeces recovery of total RSV between 70 to 98% within 24 h [44].
Pharmacodynamics and clinical evidence
Obesity and ageing related chronic diseases are emerging as a new pandemic, with few solutions [50–52]. At the crossroads of these disorders stands the combination of inflammation and oxidative stress [4, 53]. Through its ability to up-regulate host antioxidant capacity and activity, and inhibit the NFkB and cyclo-oxygenase pro-inflammatory pathways, RSV is potentially in a favourable position to address clinically carcinogenesis, atherogenesis, neurodegeneration, mitochondrial dysfunction and insulin signalling [22, 25, 54–56]. In vitro, ex vivo and animal model experiments have provided evidence for bioactivity with clinical potential in cancer chemoprevention [57] and therapy [58], cardiovascular disease and obesity [24, 54, 59–61], hepatic alcoholic or metabolic dysregulations [62–67], diabetes [54, 66], arthritis, osteoporosis [68], and neuroprotection [69, 70]. Figure 2 outlines this clinical potential.
Figure 2.
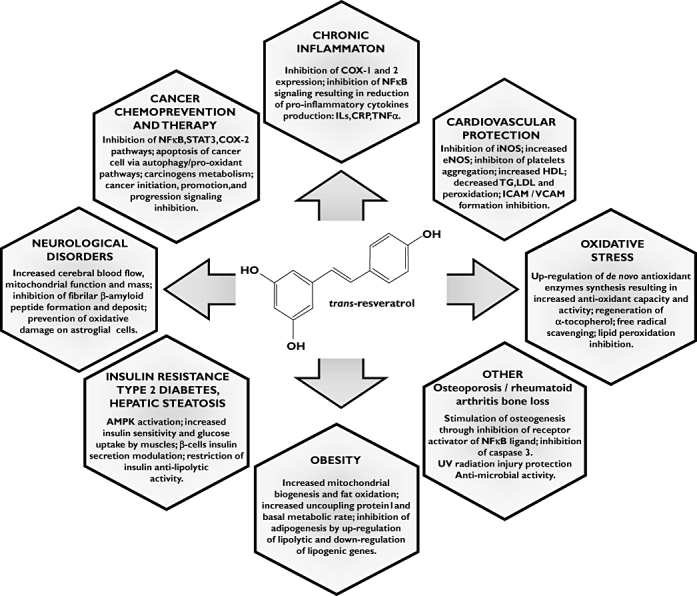
The potential clinical applications of resveratrol and main proposed mechanisms of action [6, 7, 24, 56, 70]. NFκB, nuclear factor kappa B; STAT3, signal transducer and activator of transcription 3; COX-1/2, cyclo-oxygenase 1 /2; IL's, interleukins; CRP, C-reactive protein; AMPK, adenosine monophosphate activated protein kinase; TNFα, tumour necrosis factor alpha; TG, triglycerides; HDL, high density lipoproteins; LDL, low density lipoproteins; ICAM, inter-cellular adhesion molecule 1; VCAM, vascular cell adhesion molecule 1; iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase.
It is still the dawn of clinical investigations, because the successful translation in humans of in vitro and in vivo findings is thought to depend largely on parent RSV plasma bioavailability. Basic science investigations have demonstrated efficacy at parent concentrations physiologically difficult to replicate (>5 µm) [35, 56, 71], considering that daily 5000 mg resulted in plasma parent concentration of 4.24 µm[31].
Animals model experiments have however also shown efficacy at very low concentrations [8], and diametrically opposing activity depending on dose [72]. A possible dose-related target-specific bioactivity allows for anti- or pro-oxidant effects, and apoptotic or survival signalling [71]. This is relevant in the context of multifactorial chronic diseases if this characteristic is confirmed in humans, and if RSV is to become a therapeutic agent.
Table 3 outlines the interventions listed on clinicaltrials.gov and currently underway. The broad range of designs and targeted outcomes reflects the multifaceted potential of RSV, the lack of consensus on adequate dosage, and the debate on the most adequate source: food vs. nutraceuticals.
Table 3.
Currently recruiting and ongoing clinical trials investigating the effects of RSV (Source: http://www.clinicaltrials.gov)
| Investigator | Population | Length and RSV daily dosage | Endpoints |
|---|---|---|---|
| Manini | Aged 65–100 years | 12 weeks, 300 mg or 1000 mg | Safety, cognitive and physical performance |
| Dandona | Obese and type 2 diabetes | 12 weeks, 40 mg three times daily or 500 mg | NFκB activity, oxidative stress, GIP, GLP-1 secretions |
| Poulsen | Obese | 5 weeks, daily 500 mg three times daily | Description of the molecular biology underpinning the interplay between calorie restriction, SIRT1, STAT5 and the GH/IGF-I axis |
| Vita | Overweight, type 2 diabetes | 1 week for each 90 and 270 mg | Arterial flow-mediated dilation, inflammation and oxidative stress markers, insulin resistance |
| Holcombe | Colon cancer, surgery scheduled | 4 weeks, 20, 80 or 160 mg | Wnt signaling in cancerous and normal colonic mucosa |
| Oka | Overweight, ≥50 years, insulin resistant | 28 days, 5000 mg | Insulin sensitivity, IGF-1 concentrations, cholesterol, physical activity levels |
| Kerwin | Clinical diagnosis Alzheimer's disease | 52 weeks, 215 mg | Cognition, function and behaviour |
| Holcombe | Healthy adults | 28 days, 1/3, 2/3 or 1 pound fresh grapes | Colonic mucosa cell proliferation. Expression of beta-catenin and Wnt pathway genes. RSV content variation in grapes |
| Klein | Post-menopausal women | 12 weeks, 75 mg or 30% calorie restriction diet | Muscle tissue gene expression, insulin resistance, inflammation, intra-hepatic lipids, body composition |
| Fu | Malignancy, failed therapies, or no standard care available | 28 days cycles, starting at 3000 mg alone or combined with curcumin | Safety-efficacy study, maximum tolerated dose finding |
| Timmers | Obese, sedentary, 45–65 years | 30 days, 150 mg | Fat oxidation, mitochondrial biogenesis and function, adipose and skeletal muscle lipolysis |
| Hummel | ≥50 years, heart failure symptoms | 6 weeks 300 mg twice daily dried grape mixed polyphenols | Artery flow-mediated dilation + seven other CVD health markers |
| Holte | Follicular lymphoma grade I or II | 16 weeks, Merlot-grape juice 660 ml or 495 ml | Apoptosis, proliferation of tumour cells, pro- inflammatory cytokines |
| Sabaté | ≥6 months diagnosis of types 2 diabetes with HbA1c >9% | 24 weeks, moderate fat diet with or without 32 g peanuts or 2 tablespoons peanut butter | HDL cholesterol, plasma lipids, glucose, HbA1c, anthropometrics, blood pressure |
NFκB, nuclear factor kappa B; GIP, gastric inhibitory peptide; GLP-1, glucagon like peptide 1; SIRT1, sirtuin 1; STAT5, signal transducer and activator of transcription 5; GH/IGF-I axis, growth hormone /insulin like growth factor 1 axis; Wnt, wingless-type MMTV integration site family pathway; IGF-1, insulin like growth factor 1; HbA1c, glycosylated haemoglobin A1c; CVD, cardiovascular disease.
Cardiovascular health
Initial clinical data on cardiovascular protection provides some evidence that low RSV dosages, either through phytochemicals synergy in food sources or at lower nutraceutical doses, are sufficient to induce putative beneficial effects, consistent with animal observations of anti-oxidant, anti-inflammatory and vasorelaxation activity [73, 74].
Red grape polyphenols (containing 0.9 mg RSV) acutely and significantly increased flow mediated dilation (FMD) in males with coronary heart disease, indicating activity on endothelial function [75]. Compared with baseline, powdered mixed grape polyphenols intake (containing 7 µmol kg−1 RSV) for 4 weeks significantly decreased triglycerides, LDL cholesterol, apo-lipoproteins B/E, and oxidative stress measured by plasma TNFα and urine isoprostane concentrations, in pre- and post-menopausal women [76]. When compared with water, beer or vodka, red wine intake (100 ml daily) for 3 weeks significantly increased FMD and promoted endothelial progenitor cells level and function, through increased nitric oxide bioavailability [77]. Acute red wine intake (400 ml) with a high fat meal delayed and reduced the peroxidation of post-prandial LDL in healthy subjects when compared with plain ethanol [15]. An acute dose-dependent effect on FMD was also demonstrated in overweight and obese subjects receiving nutraceutical RSV (30, 90 or 270 mg) against placebo [78]. However these dose and intake-dependent benefits remain to be confirmed with chronic intake, and can certainly not be attributed to RSV alone, when whole grape products were administered.
Cancer chemoprevention
Initial oncology trials suggest that both food source RSV as part of a synergy of phytochemicals and large nutraceutical RSV dosages may have chemopreventive activity.
Grape extract (containing 0.073 to 0.114 mg RSV) administered for 14 days pre-surgical resection to colon cancer patients, inhibited the expression of genes implicated in cancer initiation in the healthy mucosa but not cancerous tissue (measured by qRT-PCR) [79, 80]. In contrast, nutraceutical RSV doses of 500 and 1000 mg resulted in a dose-dependent decrease in cell proliferation by up to 5.6% in colorectal tumours after 8 days intake, quantified by immune-histochemical staining of the surrogate marker of cell proliferation Ki-67, in biopsy and surgically removed tissue. This study also provided evidence that the parent and conjugated RSV accumulate in tumour tissue and nearby healthy mucosa, by up to 674 nmol g−1, with a dominance of parent compound [45]. The same investigators explored the effects of repeated dosing (500 mg to 5000 mg) over 29 days in healthy subjects on circulating insulin-like growth factor (IGF)-1 and IGF-binding protein-3 (IGFBP-3), both involved in carcinogenesis pathways [31, 81]. The plasma concentrations of IGF-1 and IGFBP-3, measured by enzyme-linked immunosorbent assay, were significantly decreased from baseline, with the 2500 mg regimen, but interestingly not at lower or higher dosages. The authors suggested the modulation of the IGF axis as a mechanism of action for RSV's chemoprevention activity.
These studies provide some evidence for chemoprevention potential in humans, without clarification on adequate dosages.
Other
Inflammation and oxidative stress were significantly reduced in peripheral blood mononuclear cells of healthy subjects receiving Polygonum cuspidatum (containing 40 mg RSV), for 6 weeks against placebo [82]. Reactive oxygen species generation, P47 phox protein expression (subunit in the enzyme converting O2to O2- species), and NFκB DNA binding capability were significantly blunted demonstrating modulation of oxidative stress and pro-inflammation signalling. Expression of IL-6, SOCS3, TNFα, plasma C-reactive protein and TNFα concentrations were significantly reduced.
The same group investigated similar biomarkers, endotoxin concentrations and the induction of Nrf2 following a high fat/high carbohydrate meal. Nutraceutical RSV (100 mg) combined with grape extract (75 mg polyphenols) was administered acutely before the meal to healthy individuals. Supplementation significantly blunted the meal-induced inflammatory and oxidative stress, and increased antioxidant capacity compared with placebo (measured by Western blotting and RT-PCR) [83].
Acute nutraceutical RSV dosing (250 or 500 mg) dose-dependently increased cerebral blood flow during cognitive tasking in healthy subjects, indexed by total concentration of haemoglobin and near-infrared-spectroscopy [84].
These studies suggest clinical potential in the modulation of key drivers of chronic diseases. However clinical efficacy remains to be demonstrated with patients in phase 3 trials.
Areas of concern
Poor systemic bioavailability of resveratrol
Enhancing the bioavailability of RSV is the subject of extensive biotechnology research, because poor bioavailability remains a major obstacle to replicate successfully pre-clinical evidence [85]. Target plasma concentrations, and therefore dosages prescribed, may however differ for different applications, as shown in initial clinical investigations [75, 76].
Exposure to conjugates may play a role in efficacy. In fact, sulphation conjugates were recently reported to modulate inflammation pathways in vitro with a similar efficacy to the parent compound in some cases [46, 47], whilst glucuronidation conjugates were reported inactive in vitro at concentrations up to 300 µm. Authors have proposed that metabolites may constitute an abundant pool of RSV, via β-glucuronidase and sulphatase deconjugation [36, 86]. Recent evidence of parent RSV tissue accumulation, and bioactivity in spite of undetectable plasma concentrations suggests that efficacy may not necessarily depend on parent RSV circulating concentration alone [45]. It is possible that reaching in vitro concentrations may not be necessary, but that the excursion of total RSV (parent and metabolites) under the concentration vs. time curve is of importance.
Localization of tissue uptake, what determines target tissues and the target/dose relationship are clearly topics for future research.
Food vs. nutraceutical dose
Dietary intake can reach 6 to 8 mg daily. As such, nutraceutical dosages (20 mg to 500 mg per capsule) may appear disproportionate. In key studies on obesity and ageing dysregulations, the animals received between 20 to 400 mg kg−1 body weight [62–65, 67]. Converted to the human equivalent with the body surface area normalization method [87], this equates to 243 to 4875 mg for a 75 kg adult, providing some justification for nutraceutical dosages. In contrast, recent clinical data show bioactivity at µg doses, when provided as part of a synergy of phytochemicals in foods. This fuels the debate on the synergy or antagonism of bioactive dietary compounds, a phenomenon difficult both to ignore and measure.
It is too soon to comment on the most suitable dose and source, or the effects and safety of chronic intake, until further clinical data are available. In the mean time, nutraceutical RSV is easily available commercially, and uncontrolled self-prescribing is encouraged by claims of calorie-restriction mimetic and anti-ageing activity, following initial in vivo reports [7].
Conclusions
RSV is an intriguing molecule worth investigating for its multi-target bioactivity, but many questions need answering. These include queries over the long term effects of nutraceutical dosages 100- to 1000-fold higher than dietary sources, the consequences of tissue accumulation and chronically down-regulating inflammatory pathways, the consequences of large circulating concentrations of metabolites which seem bioactive, why there are such large differences in PK between subjects, should disease-prevention and therapeutic doses be different and lastly do apparent saturable kinetics in parent RSV bioavailability and clearance in repeated dosing have toxicity implications?
RSV has encouraging potential to address systemically and at the genomic level inflammation and oxidative stress, two drivers of ageing and chronic diseases [8, 18]. The in vitro, in vivo and first clinical evidence have certainly confirmed a role for nutrapharmacology in health, and have provided justification for the daily consumption of RSV and other phytochemical containing food products as part of disease prevention, which may ultimately be the reason why these bioactive compounds do exist in nature [3]. Clinical evidence examining the effect of metabolites, the correlation between dose, concentration and effect, especially in the context of chronic disease, is awaited.
Acknowledgments
The authors thank Brian Gabrielli and Jonathan Whitehead for proof reading Figure 1
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173–99. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76:1044–63. doi: 10.1055/s-0030-1250111. [DOI] [PubMed] [Google Scholar]
- 3.Anand P, Kunnumakara A, Sundaram C, Harikumar K, Tharakan S, Lai O, Sung B, Aggarwal B. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cave M, Hurt R, Frazier T, Matheson P, Garrison R, McClain C, McClave S. Obesity, inflammation, and the potential application of pharmaconutrition. Nutr Clin Pract. 2008;23:16–34. doi: 10.1177/011542650802300116. [DOI] [PubMed] [Google Scholar]
- 5.Jang M, Cai L, Udeani G, Slowing K, Thomas C, Beecher C, Fong H, Farnsworth N, Kinghorn A, Mehta R. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 6.Brown L, Kroon P, Das D, Das S, Tosaki A, Chan V, Singer M, Feick P. The biological responses to resveratrol and other polyphenols from alcoholic beverages. Alcohol Clin Exp Res. 2009;33:1513–23. doi: 10.1111/j.1530-0277.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris B. Everitt AV, Rattan SIS, Couteur DG, de Cabo R, editors. Calorie restriction mimetics and aging. Calorie Restriction, Aging Longevity. 2010:141–75. The Netherlands: D Springer Science+Business Media B.V. [Google Scholar]
- 8.Kroon A, Iyer A, Chunduri P, Chan V, Brown L. The cardiovascular nutrapharmacology of resveratrol: pharmacokinetics, molecular mechanisms and therapeutic potential. Curr Med Chem. 2010;17:2442–55. doi: 10.2174/092986710791556032. [DOI] [PubMed] [Google Scholar]
- 9.Orallo F. Comparative studies of the antioxidant effects of cis-and trans-resveratrol. Curr Med Chem. 2006;13:87–98. [PubMed] [Google Scholar]
- 10.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–26. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 11.Criqui M, Ringel B. Does diet or alcohol explain the French paradox? Lancet. 1994;344:1719–23. doi: 10.1016/s0140-6736(94)92883-5. [DOI] [PubMed] [Google Scholar]
- 12.Siemann E, Creasy L. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic. 1992;43:49–52. [Google Scholar]
- 13.Frankel E, German J, Kinsella J, Parks E, Kanner J. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–57. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- 14.Gresele P, Pignatelli P, Guglielmini G, Carnevale R, Mezzasoma A, Ghiselli A, Momi S, Violi F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr. 2008;138:1602–08. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- 15.Natella F, Ghiselli A, Guidi A, Ursini F, Scaccini C. Red wine mitigates the postprandial increase of LDL susceptibility to oxidation. Free Radic Biol Med. 2001;30:1036–44. doi: 10.1016/s0891-5849(01)00504-4. [DOI] [PubMed] [Google Scholar]
- 16.Paul B, Masih I, Deopujari J, Charpentier C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J Ethnopharmacol. 1999;68:71–6. doi: 10.1016/s0378-8741(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 17.Shakibaei M, Harikumar K, Aggarwal B. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–28. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 18.Csiszar A. Anti inflammatory effects of resveratrol: possible role in prevention of age related cardiovascular disease. Ann NY Acad Sci. 2011;1215:117–22. doi: 10.1111/j.1749-6632.2010.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal B, Baur JA. Resveratrol and life extension. Ann NY Acad Sci. 2011;1215:138–43. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu JM, Hsieh T. Resveratrol: a cardioprotective substance. Ann NY Acad Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- 21.Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann NY Acad Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- 22.Pezzuto J. Resveratrol as an inhibitor of carcinogenesis. Pharm Biol. 2008;46:443–573. [Google Scholar]
- 23.Cantó C, Jiang L, Deshmukh A, Mataki C, Coste A, Lagouge M, Zierath J, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–19. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Um J, Park S, Kang H, Yang S, Foretz M, McBurney M, Kim M, Viollet B, Chung J. AMP-activated protein kinase–deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–63. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pervaiz S, Holme A. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–97. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 26.Burkon A, Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides-Two novel resveratrol metabolites in human plasma. Mol Nutr Food Res. 2008;52:549–57. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- 27.Caruso F, Tanski J, Villegas-Estrada A, Rossi M. Structural basis for antioxidant activity of trans-resveratrol: ab initio calculations and crystal and molecular structure. J Agric Food Chem. 2004;52:7279–85. doi: 10.1021/jf048794e. [DOI] [PubMed] [Google Scholar]
- 28.Langcake P, Cornford C, Pryce R. Identification of pterostilbene as a phytoalexin from Vitis vinifera leaves. Phytochemistry. 1979;18:1025–27. [Google Scholar]
- 29.Langcake P, Pryce R. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Pathol. 1976;9:77–86. [Google Scholar]
- 30.Lyons M, Yu C, Toma R, Cho S, Reiboldt W, Lee J, van Breemen R. Resveratrol in raw and baked blueberries and bilberries. J Agric Food Chem. 2003;51:5867–70. doi: 10.1021/jf034150f. [DOI] [PubMed] [Google Scholar]
- 31.Brown V, Patel K, Viskaduraki M, Crowell J, Perloff M, Booth T, Vasilinin G, Sen A, Schinas A, Piccirilli G. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–11. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cottart C, Nivet Antoine V, Laguillier Morizot C, Beaudeux J. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 33.la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, Cameron D. Steady-state pharmacokinetics and tolerability of trans-resveratrol 2000mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49:449–54. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Chow H, Garland L, Hsu C, Vining D, Chew W, Miller J, Perloff M, Crowell J, Alberts D. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res. 2010;3:1168–75. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gescher A, Steward W. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prevent. 2003;12:953–57. [PubMed] [Google Scholar]
- 36.Walle T. Bioavailability of resveratrol. Ann NY Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 37.Brisdelli F, D'Andrea G, Bozzi A. Resveratrol: a natural polyphenol with multiple chemopreventive properties (review) Curr Drug Metab. 2009;10:530–46. doi: 10.2174/138920009789375423. [DOI] [PubMed] [Google Scholar]
- 38.Kuhnle G, Spencer J, Chowrimootoo G, Schroeter H, Debnam E, Srai S, Rice-Evans C, Hahn U. Resveratrol is absorbed in the small intestine as resveratrol glucuronide. Biochem Biophys Res Commun. 2000;272:212–17. doi: 10.1006/bbrc.2000.2750. [DOI] [PubMed] [Google Scholar]
- 39.Vaz-da-Silva M, Loureiro A, Falcao A, Nunes T, Rocha J, Fernandes-Lopes C, Soares E, Wright L, Almeida L, Soares-da-Silva P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int J Clin Pharmacol Ther. 2008;46:564–70. doi: 10.5414/cpp46564. [DOI] [PubMed] [Google Scholar]
- 40.Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Vescovi P, Fogliano V, Marchelli R. Bioavailability of trans resveratrol from red wine in humans. Mol Nutr Food Res. 2005;49:495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 41.Walle T, Hsieh F, DeLegge M, Oatis J, Walle U. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel E, Somoza V. Metabolism and bioavailability of trans resveratrol. Mol Nutr Food Res. 2005;49:472–81. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 43.Almeida L, Vaz da Silva M, Falcão A, Soares E, Costa R, Loureiro A, Fernandes Lopes C, Rocha J, Nunes T, Wright L. Pharmacokinetic and safety profile of trans resveratrol in a rising multiple dose study in healthy volunteers. Mol Nutr Food Res. 2009;53:7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 44.Boocock D, Faust G, Patel K, Schinas A, Brown V, Ducharme M, Booth T, Crowell J, Perloff M, Gescher A. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prevent. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 45.Patel K, Brown V, Jones D, Britton R, Hemingway D, Miller A, West K, Booth T, Perloff M, Crowell J. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–99. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calamini B, Ratia K, Malkowski M, Cuendet M, Pezzuto J, Santarsiero B, Mesecar A. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem J. 2010;429:273–82. doi: 10.1042/BJ20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoshino J, Park E, Kondratyuk T, Marler L, Pezzuto J, van Breemen R, Mo S, Li Y, Cushman M. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem. 2010;53:5033–43. doi: 10.1021/jm100274c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jannin B, Menzel M, Berlot J, Delmas D, Lançon A, Latruffe N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: plasmatic protein binding and cell uptake. Biochem Pharmacol. 2004;68:1113–18. doi: 10.1016/j.bcp.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 49.Lu Z, Zhang Y, Liu H, Yuan J, Zheng Z, Zou G. Transport of a cancer chemopreventive polyphenol, resveratrol: interaction with serum albumin and hemoglobin. J Fluoresc. 2007;17:580–87. doi: 10.1007/s10895-007-0220-2. [DOI] [PubMed] [Google Scholar]
- 50.Christensen K, Doblhammer G, Rau R, Vaupel J. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 52.Dixon J. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316:104–08. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Reuter S, Gupta S, Chaturvedi M, Aggarwal B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635:1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 55.Harikumar K, Aggarwal B. A multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–35. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 56.Baur J, Sinclair D. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 57.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res. 2009;2:409–18. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 58.Harikumar K, Kunnumakkara A, Sethi G, Diagaradjane P, Anand P, Pandey M, Gelovani J, Krishnan S, Guha S, Aggarwal B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int J Cancer. 2010;127:257–68. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Csiszar A, Labinskyy N, Pinto J, Ballabh P, Zhang H, Losonczy G, Pearson K, De Cabo R, Pacher P, Zhang C. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Csiszar A, Wang M, Lakatta E, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-{kappa} B. J Appl Physiol. 2008;105:1333–41. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Das S, Das D. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Pat Cardiovas Drug Discov. 2007;2:133–38. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 62.Baur J, Pearson K, Price N, Jamieson H, Lerin C, Kalra A, Prabhu V, Allard J, Lopez-Lluch G, Lewis K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 [alpha] Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 64.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 65.Pearson K, Baur J, Lewis K, Peshkin L, Price N, Labinskyy N, Swindell W, Kamara D, Minor R, Perez E. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–63. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 67.Dal-Pan A, Blanc S, Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol. 2010;10:1–10. doi: 10.1186/1472-6793-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shakibaei M, Csaki C, Nebrich S, Mobasheri A. Resveratrol suppresses interleukin-1 [beta]-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol. 2008;76:1426–39. doi: 10.1016/j.bcp.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 69.Rocha-González H, Ambriz-Tututi M, Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci Ther. 2008;14:234–47. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markus M, Morris B. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008;3:331–39. [PMC free article] [PubMed] [Google Scholar]
- 71.de la Lastra C, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007;35:1156–60. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 72.Dudley J, Das S, Mukherjee S, Das DK. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J Nutr Biochem. 2009;20:443–52. doi: 10.1016/j.jnutbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Das M, Das D. Resveratrol and cardiovascular health. Mol Aspects Med. 2010;31:503–12. doi: 10.1016/j.mam.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Bhat K, Kosmeder J, Pezzuto J. Biological effects of resveratrol. Antioxid Redox Signal. 2001;3:1041–64. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- 75.Lekakis J, Rallidis L, Andreadou I, Vamvakou G, Kazantzoglou G, Magiatis P, Skaltsounis A, Kremastinos D. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:596–600. doi: 10.1097/00149831-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Zern T, Wood R, Greene C, West K, Liu Y, Aggarwal D, Shachter N, Fernandez M. Grape polyphenols exert a cardioprotective effect in pre-and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135:1911–17. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- 77.Huang PH, Chen YH, Tsai HY, Chen JS, Wu TC, Lin FY, Sata M, Chen JW, Lin SJ. Intake of red wine increases the number and functional capacity of circulating endothelial progenitor cells by enhancing nitric oxide bioavailability. Arterioscler Thromb Vasc Biol. 2010;30:869–77. doi: 10.1161/ATVBAHA.109.200618. [DOI] [PubMed] [Google Scholar]
- 78.Wong R, Howe P, Buckley J, Coates A, Kunz I, Berry N. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.03.003. Epub ahead of print. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Holcombe R. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. 2009;1:25–37. [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez M, Hope C, Planutis K, Planutiene M, Pontello A, Duarte B, Albers C, Holcombe R. Dietary grape-derived resveratrol for colon cancer prevention. J Clin Oncol 28:15s, 2010 (suppl; abstr 3622) [Google Scholar]
- 81.Ibrahim Y, Yee D. Insulin-like growth factor-I and cancer risk. Growth Horm IGF Res. 2004;14:261–69. doi: 10.1016/j.ghir.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Ghanim H, Sia C, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A, Dandona P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab. 2010;95:E1–E8. doi: 10.1210/jc.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghanim H, Sia CL, Korzeniewski K, Lohano T, Abuaysheh S, Marumganti A, Chaudhuri A, Dandona P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab. 2011;96 doi: 10.1210/jc.2010-1812. Published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kennedy D, Wightman E, Reay J, Lietz G, Okello E, Wilde A, Haskell C. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010;91:1590–97. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- 85.Szekeres T, Fritzer-Szekeres M, Saiko P, Jäger W. Resveratrol and resveratrol analogues – structure – activity relationship. Pharm Res. 2010;27:1042–48. doi: 10.1007/s11095-010-0090-1. [DOI] [PubMed] [Google Scholar]
- 86.Wang LX, Heredia A, Song H, Zhang Z, Yu B, Davis C, Redfield R. Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti HIV activity. J Pharm Sci. 2004;93:2448–57. doi: 10.1002/jps.20156. [DOI] [PubMed] [Google Scholar]
- 87.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 88.Burns J, Yokota T, Ashihara H, Lean M, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–40. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 89.Goldberg D, Yan J, Ng E, Diamandis E, Karumanchiri A, Soleas G, Waterhouse A. A global survey of trans-resveratrol concentrations in commercial wines. Am J Enol Vitic. 1995;46:159–65. [Google Scholar]
- 90.Wang Y, Catana F, Yang Y, Roderick R, van Breemen R. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J Agric Food Chem. 2002;50:431–35. doi: 10.1021/jf010812u. [DOI] [PubMed] [Google Scholar]
- 91.Rimando A, Kalt W, Magee J, Dewey J, Ballington J. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem. 2004;52:4713–19. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 92.Wang S, Chen C, Wang C, Chen P. Resveratrol content in strawberry fruit is affected by preharvest conditions. J Agric Food Chem. 2007;55:8269–74. doi: 10.1021/jf071749x. [DOI] [PubMed] [Google Scholar]
- 93.Wang S, Chen C, Sciarappa W, Wang C, Camp M. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J Agric Food Chem. 2008;56:5788–94. doi: 10.1021/jf703775r. [DOI] [PubMed] [Google Scholar]
- 94.Sanders T, McMichael JR, Hendrix K. Occurrence of resveratrol in edible peanuts. J Agric Food Chem. 2000;48:1243–46. doi: 10.1021/jf990737b. [DOI] [PubMed] [Google Scholar]
- 95.Sobolev V, Cole R. trans-Resveratrol content in commercial peanuts and peanut products. J Agric Food Chem. 1999;47:1435–39. doi: 10.1021/jf9809885. [DOI] [PubMed] [Google Scholar]
- 96.Hurst W, Glinski J, Miller K, Apgar J, Davey M, Stuart D. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J Agric Food Chem. 2008;56:8374–78. doi: 10.1021/jf801297w. [DOI] [PubMed] [Google Scholar]
- 97.Udenigwe C, Ramprasath V, Aluko R, Jones P. Potential of resveratrol in anticancer and anti inflammatory therapy. Nutr Rev. 2008;66:445–54. doi: 10.1111/j.1753-4887.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 98.Counet C, Callemien D, Collin S. Chocolate and cocoa: new sources of trans-resveratrol and trans-piceid. Food Chem. 2006;98:649–57. [Google Scholar]
- 99.Nunes T, Almeida L, Rocha J, Falcão A, Fernandes-Lopes C, Loureiro A, Wright L, Vaz-da-Silva M, Soares-da-Silva P. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J Clin Pharmacol. 2009;49:1477–82. doi: 10.1177/0091270009339191. [DOI] [PubMed] [Google Scholar]


