Abstract
AIMS
S/GSK1349572 is an unboosted, once daily, next generation integrase inhibitor with potent activity, low pharmacokinetic (PK) variability and a novel resistance profile. As the primary route of metabolism is via glucuronidation, the effects of atazanavir (ATV, a UGT1A1 inhibitor) and atazanavir/ritonavir (ATV/RTV) on S/GSK1349572 PK were evaluated.
METHODS
A randomized, open label, two period, crossover study was conducted in healthy adult subjects. Twenty-four subjects received S/GSK1349572 30 mg every 24 h for 5 days. Subjects then were administered S/GSK1349572 30 mg every 24 h in combination with either ATV/RTV 300/100 mg every 24 h (n = 12) or ATV 400 mg every 24 h (n = 12) for 14 days. Serial PK samples and safety assessments were obtained throughout the study.
RESULTS
The combination of S/GSK1349572 with ATV/RTV or ATV was generally well tolerated. All adverse events were mild or moderate, and no subject withdrew because of an adverse event. The AE of highest frequency was ocular icterus, observed only during combination of S/GSK1349572 and ATV or ATV/RTV. Co-administration with ATV/RTV resulted in increased plasma S/GSK1349572 area under the concentration–time curve during a dosing interval (AUC(0,τ)), observed maximal concentration (Cmax), and concentration at the end of dosing interval at steady state (Cτ) by 62%, 34% and 121%, respectively. Co-administration with ATV resulted in increased plasma S/GSK1349572 AUC(0,τ), Cmax, and Cτ by 91%, 50% and 180%, respectively.
CONCLUSIONS
Co-administration of ATV/RTV and ATV was generally well tolerated and produced a modest, non-clinically significant increase in S/GSK1349572 exposure. No dose adjustment for S/GSK1349572 is necessary when co-administered with ATV and ATV/RTV.
Keywords: atazanavir, drug interaction, HIV integrase, ritonavir, S/GSK1349572
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
S/GSK1349572, an investigational HIV integrase inhibitor, is primarily metabolized via glucuronidation by UGT1A1, with a minor component by CYP3A4. As such, inhibitors of UGT1A1 have the potential to increase S/GSK1349572 concentrations. Because S/GSK1349572 and atazanavir (ATV) may be used in combination and because ATV is a potent inhibitor of UGT1A1, this study evaluated the effect of ATV and ritonavir-boosted ATV (ATV/RTV) on the pharmacokinetics of S/GSK1349572.
WHAT THIS STUDY ADDS
The effects of concomitant atazanavir (ATV, a UGT1A1 inhibitor) and S/GSK1349572 pharmacokinetics were previously unknown and were evaluated in this study. Co-administration of ATV/RTV and ATV was generally well tolerated and produced a modest, non-clinically significant increase in S/GSK1349572 exposure. No dose adjustment for S/GSK1349572 is necessary when it is co-administered with ATV and ATV/RTV.
Introduction
The HIV integrase inhibitors (INIs) are a promising new class of antiretrovirals. Studies in treatment-naive and experienced patients demonstrate durable responses with a favourable toxicity profile [1, 2]. However, clinical resistance to both raltegravir and elvitegravir has been reported from phase II and III studies in treatment-naive and treatment-experienced patients [2–4]. Therefore, development of new INIs with different resistance profiles is desirable.
S/GSK1349572 is an investigational HIV INI currently in phase IIb clinical trials. This next generation agent demonstrates a number of favourable characteristics compared with other compounds in this class. S/GSK1349572 possesses potent antiviral activity with an in vitro protein-adjusted IC90 of 152 nm (0.064 µg ml−1). Pharmacokinetics (PK) in humans support once daily dosing without a requirement for boosting with ritonavir (RTV) and without regard for food [5]. The PK are characterized by low intersubject variability and a well-characterized PK/pharmacodynamic (PD) relationship [6]. These characteristics demonstrate potential improvements against other drugs in the integrase inhibitor class. A predictable PK/PD relationship and once daily dosing are advantages compared with raltegravir and the use of GSK1349572 without a PK booster is an advantage over elvitegravir. A mean 2.5 log decrease in plasma HIV RNA at a 50 mg once daily dose was observed in a 10 day monotherapy study in HIV-infected subjects [7]. Finally, S/GSK1349572 demonstrates a different resistance profile, in vitro, compared with the Food and Drug Administration approved INI raltegravir and the investigational agent elvitegravir [8]. These attributes make S/GSK1349572 an attractive compound for further development.
S/GSK1349572 is primarily metabolized via glucuronidation by UGT1A1, with a minor component by CYP3A4. As such, inhibitors of UGT1A1 have the potential to increase S/GSK1349572 concentrations. Atazanavir (ATV) is an HIV protease inhibitor approved for use in treatment-naive and experienced patients. Since these drugs may be used in combination and because ATV is a potent inhibitor of UGT1A1, this study evaluated the effect of ATV and RTV-boosted ATV (ATV/RTV) on the PK of S/GSK1349572.
Methods
This was a randomized, open-label, two-period, crossover study in healthy subjects. Eligibility was assessed by physical examination, medical history and laboratory testing. Adult males or females of nonchildbearing potential were enrolled. Subjects were excluded for a positive result for HIV or hepatitis C antibody or hepatitis B surface antigen. Subjects were not allowed to receive any prescription or nonprescription drugs, including vitamins or herbal products, within 7 days prior to the first dose and throughout the study. Subjects had a screening visit within 30 days prior to the first dose of the study drug, two treatment periods, and a follow-up visit 7 to 14 days after the last dose of the study drug.
The trial was conducted at a single clinical site, and 24 subjects were housed as inpatients for the study duration. In the first treatment period, all subjects received S/GSK1349572 30 mg every 24 h for 5 days (treatment A). In the second treatment period, subjects received S/GSK1349572 30 mg every 24 h in combination with either ATV/RTV 300/100 mg every 24 h (treatment B, n = 12) or ATV 400 mg every 24 h (treatment C, n = 12) for 14 days. All doses of the study drugs were administered in the morning within 30 min after the start of a moderate fat meal. There was no washout between treatment periods. Safety evaluations (clinical chemistry and haematology, urinalysis, vital signs, electrocardiogram) were performed throughout the study. Serial blood samples for determination of plasma concentrations of S/GSK1349572 and ATV were collected over the dosing interval on day 5 for treatment A and on day 14 for treatments B and C. Subjects were questioned regarding adverse events (AEs) and use of concomitant medications in an open-ended fashion on a frequent basis. Written informed consent was obtained from all subjects, and the protocol was approved by the institutional review board, IntegReview, Inc., of Austin, TX.
Bioanalytical methods
Following extraction from plasma by protein precipitation, S/GSK1349572 and ATV concentrations were determined by validated high performance liquid chromatography/tandem mass spectrometry methods using TurboIonSpray® (AB SCIEX, Foster City, CA) and multiple reaction monitoring at GlaxoSmithKline (computer systems used to acquire and quantify data included AB SCIEX Analyst® Version 1.4.2 and Brothersoft SMS 2000 Version 2.1). For analysis of S/GSK1349572 and ATV, [2H7,15N]S/GSK1349572 and [2H5]ATV were used as internal standards. The validated linear concentration ranges were 5 to 5000 ng ml−1 and 10 to 10 000 ng ml−1 for S/GSK1349572 and ATV, respectively. Three concentrations of quality control (QC) samples for each analyte were included in each run (20, 400, and 4000 ng ml−1 for S/GSK1349572; 40, 400 and 800 ng ml−1 for atazanavir). Based on the results of the analysis of the S/GSK1349572 QC samples, the bias ranged from −8.2 to −3.5%, and the within-run and between-run precision were less than or equal to 8.3% and 3.1%, respectively. The results of the analysis of the ATV QC samples indicated a bias of 0.5% to 3.9%, a within-run precision of less than 2.6% and a negligible between-run variance.
Pharmacokinetic analysis
A noncompartmental PK analysis of the concentration–time data was performed with WinNonlin® (Version 5.2, Pharsight Corporation, Mountain View, CA). Plasma PK parameters for S/GSK1349572 and ATV were calculated using actual recorded times for each treatment. Parameters that were determined included area under the concentration–time curve during a dosing interval (AUC(0,τ)), observed maximal concentration (Cmax), concentration at end of dosing interval at steady state (Cτ), apparent oral clearance and elimination half-life. The parameter AUC(0,τ) was calculated using the linear-up/log-down trapezoidal method.
Statistical analysis
For S/GSK1349572, the statistical analysis was performed on the log-transformed PK parameters, AUC(0,τ), Cτ, and Cmax. Analysis of variance was performed using the SAS Mixed Linear Models procedure (SAS Institute Inc, Cary, NC) to assess the effect of ATV/RTV and ATV on the PK of S/GSK1349572. Subject was fitted as a random effect and treatment was fitted as a fixed effect in the model. The ratio of geometric least squares means and associated 90% confidence interval was estimated for the PK parameters of interest. S/GSK1349572 given alone was considered to be the reference treatment and S/GSK1349572 co-administered with ATV/RTV and ATV was considered to be the test treatment.
Results
A total of 24 subjects enrolled in the study and completed all evaluations. The majority of subjects were male (88%) and White (79%). Their mean age was 37.2 years with a range of 18 to 61 years. The mean (SD) body mass index was 25.9 (2.9) kg m−2. The ATV and ATV/RTV treatment groups were similar with regard to demographic characteristics.
Safety
Increases in indirect bilirubin were observed during ATV dosing periods, but no other clinically significant trends in laboratory values, vital signs or electrocardiograms were reported during study drug dosing. No serious adverse events or deaths were reported, and no subject withdrew from the study because of an AE. The most frequently reported drug-related AE during S/GSK1349572 alone dosing was nausea (two subjects; 8%). During dosing with ATV/RTV + S/GSK1349572, the most frequent drug-related AEs were ocular icterus (eight subjects; 67%), pruritis (two subjects; 17%) and maculopapular rash (two subjects; 17%). The most frequently reported drug-related AE in the S/GSK1349572 + ATV group was ocular icterus (three subjects; 25%). All AEs were mild, with the exception of two subjects with moderate rash who were observed during co-administration of S/GSK1349572 and ATV/RTV (9 and 10 days into co-administration). The rashes did not progress and both subjects were able to complete dosing in the study.
Total bilirubin was increased in eight (67%) subjects during co-administration of ATV and S/GSK1349572 and in 12 (100%) subjects during co-administration of ATV/RTV and S/GSK1349572. The mean (range) of total bilirubin on GSK1349572 alone was 0.65 (0.4–1.2) mg dl−1. During co-administration with ATV, the highest mean total bilirubin value was observed on day 4 at 2.3 (1.4–3.7) mg dl−1 and for ATV/RTV, the highest mean value was on day 10 at 5.1 (2.5–7.9) mg dl−1. Fractionation of the bilirubin demonstrated that the abnormalities were related to an increase in indirect bilirubin, an anticipated and benign effect of atazanavir. These laboratory changes in total and indirect bilirubin returned to baseline at follow-up. No bilirubin increases were observed in the S/GSK1349572-alone treatment period. No clinically significant trends in other laboratory values, vital signs or electrocardiograms were reported during study drug dosing.
Pharmacokinetics
Mean concentration–time profiles of S/GSK1349572 alone and with ATV or ATV/RTV are shown in Figure 1. S/GSK1349572 PK parameters are shown in Table 1. Co-administration with ATV/RTV resulted in an increase in plasma S/GSK1349572 exposures with AUC(0,τ), Cmax and Cτ increased by 62%, 34% and 121%, respectively (Table 2). A larger increase was observed when S/GSK1349572 was co-administered with ATV, resulting in an increase in plasma S/GSK1349572 exposures for AUC(0,τ), Cmax and Cτ of 91%, 50% and 180%, respectively (Table 2).
Figure 1.
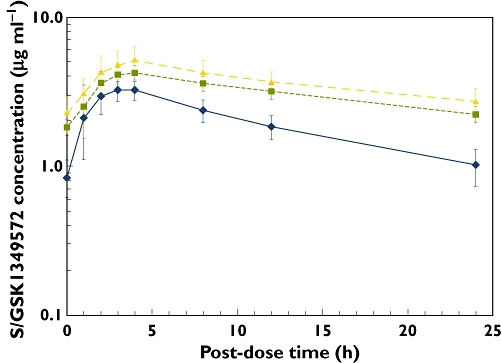
Mean concentration–time profile of S/GSK1349572 alone ( , n = 24) and with concomitant ATV (
, n = 24) and with concomitant ATV ( , n = 12) and ATV/RTV (
, n = 12) and ATV/RTV ( , n = 12). ATV, atazanavir; RTV, ritonavir
, n = 12). ATV, atazanavir; RTV, ritonavir
Table 1.
S/GSK1349572 pharmacokinetic parameters
| Treatment regimen | n | Cmax(µg ml−1) | tmax(h) | AUC(0,τ) (µg ml−1 h) | Cτ(µg ml−1) | t1/2(h) | CL/F(l h−1) |
|---|---|---|---|---|---|---|---|
| S/GSK1349572 every 24 h | 24 | 3.32 | 3.0 | 45.5 | 0.98 | 13.0 | 0.66 |
| (16) | (1.0–4.0) | (19) | (29) | (19) | (19) | ||
| S/GSK1349572 every 24 h + ATV/RTV every 24 h | 12 | 4.29 | 4.0 | 73.3 | 2.21 | 24.1 | 0.41 |
| (19) | (2.0–4.0) | (16) | (16) | (11) | (16) | ||
| S/GSK1349572 every 24 h + ATV every 24 h | 12 | 5.11 | 4.0 | 87.4 | 2.67 | 23.8 | 0.34 |
| (20) | (2.0–4.0) | (17) | (21) | (21) | (17) |
Data reported as geometric mean (coefficient of variance, %) except tmax, which is reported as median (range). ATV, atazanavir; AUC(0,τ), area under the concentration–time curve during a dosing interval; CL/F, apparent oral clearance; Cmax, observed maximal concentration; Cτ, concentration at end of dosing interval at steady-state; RTV, ritonavir; t1/2, elimination half-life; tmax, time to maximum concentration.
Table 2.
Summary of treatment comparisons
| GLS mean ratio (90% CI) | ||
|---|---|---|
| Plasma S/GSK1349572 PK parameter | ATV/RTV + S/GSK1349572 vs. S/GSK1349572 (n = 12) | ATV + S/GSK1349572 vs. S/GSK1349572 (n = 12) |
| AUC(0,τ) | 1.62 | 1.91 |
| (1.50, 1.74) | (1.80, 2.03) | |
| Cmax | 1.34 | 1.50 |
| (1.25, 1.42) | (1.40, 1.59) | |
| Cτ | 2.21 | 2.80 |
| (1.97, 2.47) | (2.52, 3.11) | |
| CL/F | 0.618 | 0.523 |
| (0.574, 0.667) | (0.494, 0.555) | |
| t1/2 | 1.81 | 1.90 |
| (1.64, 1.99) | (1.68, 2.14) | |
ATV, atazanavir; AUC(0,τ), area under the concentration–time curve during a dosing interval; CI, confidence interval; CL/F, apparent oral clearance; Cmax, observed maximal concentration; Cτ, concentration at end of dosing interval at steady state; GLS, geometric least squares; PK, pharmacokinetic; RTV, ritonavir; t1/2, elimination half-life.
Atazanavir concentrations measured during co-administration with S/GSK1349572 were similar to historical data [9]. Geometric mean (coefficient of variation, %) AUC(0,τ), Cmax and Cτ of ATV given with S/GKS1349572 were 27.5 (45) µg ml−1 h, 4.85 (42) µg ml−1 and 0.19 (84) µg ml−1, respectively. Corresponding values for ATV co-administered with RTV and S/GSK1349572 were 53.1 (31) µg ml−1 h, 5.39 (26) µg ml−1 and 1.08 (42) µg ml−1. There was no correlation between ATV exposure and treatment ratio of S/GSK1349572 exposure with or without ATV and ATV/RTV.
Discussion
With numerous options currently available for HIV-infected subjects, the overall profile of a new therapeutic agent must now be considered. New drugs must demonstrate comparable long-term safety and efficacy with gold standard treatments, but additional factors such as tolerability, drug interactions and dosing convenience are important considerations. S/GSK1349572 is an investigational INI with a number of favourable characteristics. Its attributes include low dose, once daily administration, a unique resistance profile and low potential for drug interactions. In particular, the ability to co-administer without dosing adjustments due to drug interactions is a significant advantage for new HIV therapies.
Atazanavir is an inhibitor of UGT1A1, which is the primary metabolic pathway for S/GSK1349572. Thus, ATV has the potential to increase S/GSK1349572 exposures. Although the dose of S/GSK1349572 is 50 mg in phase III trials with integrase-naive subjects, a 30 mg dose was used in this study since the magnitude of the interaction was unknown. This study demonstrated that ATV caused only a modest increase in S/GSK1349572 AUC of less than two-fold. The magnitude of the increase was larger with ATV alone compared with ATV/RTV. The difference in exposure between treatment groups is likely related to the CYP3A4- and UGT-inducing properties of the RTV component. RTV induction of CYP3A4 and UGT at steady-state is well described and likely reduces the UGT inhibitory effect of ATV, resulting in lower S/GSK1349572 exposure compared with ATV alone [10]. The elimination half-life of S/GSK1349572 was also prolonged during co-administration. The magnitude of effect on exposure and half-life were quite similar, indicating that ATV and ATV/RTV mainly affected S/GSK1349572 absolute clearance, not oral bioavailability, which is consistent with the fact that S/GSK1349572 has low clearance and low hepatic extraction. A similar fold-increase in GSK1349572 exposure would be expected with the 50 mg dose (the dose currently evaluated in phase 3 trials) vs. the 30 mg dose studied, since S/GSK1349572 PK are linear between 25 and 50 mg [11].
As this study was conducted in healthy subjects, the results may not necessarily relate to patients. Exposures of ATV in healthy subjects have been reported to be higher than those in HIV-infected subjects [9]. If HIV-infected subjects do achieve lower ATV concentrations, then the increases in S/GSK1349572 exposure noted in this study may not be as large when the drug is used in the clinical setting. A statistical analysis was performed in this study and revealed that there was no correlation between ATV exposure observed and the ratio of S/GSK1349572 pharmacokinetic parameters (with or without ATV or ATV/RTV) in this study. Nevertheless, a more modest increase would not significantly impact the interpretation of these data.
The primary PK parameter associated with efficacy for S/GSK1349572 is trough concentration [6]. Thus, the modestly increased S/GSK1349572 trough concentrations demonstrated in this study may be beneficial to patients receiving treatment with S/GSK1349572 and ATV or ATV/RTV. The increased exposure of S/GSK1349572 also supports this combination as a two drug regimen for treatment-naive subjects. The low dose and high potency of S/GSK1349572, combined with the potency of a protease inhibitor, make the combination of S/GSK1349572 and ATV an attractive potential option for a two drug RTV-sparing and nucleoside-sparing treatment regimen. The favourable interaction shown in this study may warrant a study to evaluate the safety and efficacy of this combination in HIV-infected patients. Despite the effect of ATV and ATV/RTV, the PK variability of S/GSK1349572 remains relatively low.
Literature values of ATV PK were similar to those in this study suggesting that S/GSK1349572 does not have a significant effect on ATV. This was to be expected as in vitro studies demonstrated that S/GSK1349572 is not an inhibitor or inducer of CYP isozymes and is not an inhibitor of UGT1A1, UGT2B7, P-glycoprotein or organic anion-transporting polypeptide (unpublished data). In vivo, a previous study demonstrated that S/GSK1349572 did not affect the PK of midazolam, the probe substrate for CYP3A, which is the primary metabolic pathway for ATV [5].
The combination of ATV or ATV/RTV and S/GSK1349572 was well tolerated in this short-term study. No subject withdrew from the study because of an AE. The most common AE was benign ocular icterus, a well-described side effect of ATV, which was only reported during ATV/RTV or ATV concomitant dosing with S/GSK1349572 [9]. All AEs were mild, with the exception of two subjects with moderate rash who were observed during co-administration of S/GSK1349572 and ATV/RTV (9 and 10 days into co-administration). Both subjects were able to complete the study. The incidence of rash is consistent with the prescribing information for ATV [9]. Asymptomatic indirect bilirubin abnormalities were noted during co-administration of S/GSK1349572 and ATV/RTV or ATV, as have been reported for ATV [12]. No other clinically significant laboratory abnormalities were observed.
The combination of S/GSK1349572 and ATV or ATV/RTV all given once daily resulted in a modest increase in S/GSK1349572 exposure. This effect is not considered clinically significant, and no dose adjustment is necessary when S/GSK1349572 is co-administered with ATV/RTV or ATV in phase III clinical trials.
Acknowledgments
Funding for this study was provided by Shionogi-GlaxoSmithKline, LLC. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to acknowledge the following individual for her editorial assistance during the development of this manuscript: Christine Levesque.
Competing Interests
Drs Song, Chen, Peppercorn, Min, Piscitelli and Ms Borland and Lou are employees of GlaxoSmithKline and own stock in GlaxoSmithKline. Dr Wajima is an employee of Shionogi & Co, Ltd.
REFERENCES
- 1.Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, Prada G, Morales-Ramirez JO, Crumpacker CS, Isaacs RD, Campbell H, Strohmaier KM, Wan H, Danovich RM, Teppler H. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naïve patients with HIV-1 infection. J Acquir Immune Defic Syndr. 2009;52:350–6. doi: 10.1097/QAI.0b013e3181b064b0. [DOI] [PubMed] [Google Scholar]
- 2.Steigbigel RT, Cooper DA, Teppler H, Eron JJ, Gatell JM, Kumar PN, Rockstroh JK, Schechter M, Katlama C, Markowitz M, Yeni P, Loutfy MR, Lazzarin A, Lennox JL, Clotet B, Zhao J, Wan H, Rhodes RR, Strohmaier KM, Barnard RJ, Isaacs RD, Nguyen BY. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trials. Clin Infect Dis. 2010;50:605–12. doi: 10.1086/650002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennox JL, DeJesus E, Berger DS, Lazzarin A, Pollard RB, Ramalho Madruga JV, Zhao J, Wan H, Gilbert CL, Teppler H, Rodgers AJ, Barnard RJ, Miller MD, Dinubile MJ, Nguyen BY, Leavitt R, Sklar P. Raltegravir versus efavirenz regimens in treatment-naïve HIV-1 infected patients: 96 weeks efficacy, durability, subgroup, safety, and metabolic analysis. J Acquir Immune Defic Syndr. 2010;55:39–48. doi: 10.1097/QAI.0b013e3181da1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McColl DJ, Fransen S, Gupta S, Parking N, Margot N, Chuck S, Cheng A, Miller M. Resistance and cross-resistance to first generation integrase inhibitors: insights from a phase 2 study of elvitegravir (GS-9137) [Abstract 9]. XVI International HIV Drug Resistance Workshop. Antivir Ther. 2007;12:11. [Google Scholar]
- 5.Min S, Song I, Borland J, Chen S, Lou Y, Fujiwara T, Piscitelli SC. Pharmacokinetics and safety in healthy subjects of S/GSK1349572, a next generation HIV integrase inhibitor in healthy volunteers. Antimicrob Agents Chemother. 2010;54:254–8. doi: 10.1128/AAC.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song I, Chen S, Lou Y, Borland J, Fujiwara T, Piscitelli S, Min S. Pharmacokinetic and pharmacodynamic relationship of S/GSK1349572, a next generation integrase inhibitor, in HIV-1 infected patients. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; July 19–22, 2009; Cape Town, South Africa. Abstract WEPEB250.
- 7.Lalezari J, Sloan L, DeJesus E, Hawkins T, Mccurdy L, Song I, Borland J, Stroder R, Chen S, Lou Y, Underwood M, Fujiwara T, Piscitelli S, Min S. Potent antiviral activity of S/GSK1349572, a next generation integrase inhibitor (INI), in INI-naïve HIV-1-infected patients. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; July 19–22, 2009; Cape Town, South Africa.
- 8.Sato A, Kobayashi M, Yoshinaga T, Fujiwara T, Underwood M, Johns B, Foster S, Hazen R, Ferris R, Brown K, Garvey E. S/GSK1349572 is a potent next generation HIV integrase inhibitor. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; July 19–22, 2009; Cape Town, South Africa. Abstract WEPEA097.
- 9.Reyataz [Package Insert] Princeton, NJ: Bristol Myers Squibb; 2010. [Google Scholar]
- 10.Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–59. doi: 10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- 11.Rockstroh J, Felizarta F, Maggiolo F, Pulido F, Stellbrink HJ, Tsybakova O, Yeni P, Almond S, Brothers C, Song I, Min S. Once-daily S/GSK1349572 combination therapy in antiretroviral-naive adults: rapid and potent 24-week antiviral response in SPRING-1 (ING11276) Tenth International Congress on Drug Therapy in HIV Infection. November 7–11, 2010. Glasgow. Abstract O434.
- 12.Torti C, Lapadula G, Antinori A, Quirino T, Maserati R, Castelnuovo F, Maggiolo F, De Luca A, Paraninfo G, Antonucci F, Migliorino G, Lazzarin A, Di Perri G, Rizzardini G, Esposito R, Carosi G. Hyperbilirubinemia during atazanavir treatment in 2,404 patients in the Italian expanded access program and MASTER cohorts. Infection. 2009;37:244–9. doi: 10.1007/s15010-008-8010-6. [DOI] [PubMed] [Google Scholar]


