Abstract
AIM
The aim of the present study was to investigate a previously proposed interaction between quetiapine and lamotrigine resulting in reduced serum quetiapine concentrations.
METHODS
Data on 402 patients subjected to analysis of quetiapine concentration in serum were extracted from a routine therapeutic drug monitoring database. Among these patients, those concomitantly treated with lamotrigine (n = 22) were identified and matched with 22 controls receiving quetiapine while unexposed to lamotrigine. The dose-corrected quetiapine concentrations (C : D ratios) in the two groups were compared in both paired and unpaired analyses.
RESULTS
Patients co-treated with lamotrigine had a lower mean C : D ratio (0.71, 95% CI 0.46, 0.97) compared with controls (1.64, 95% CI 1.00, 2.28). Dose-corrected quetiapine concentrations were 58% lower in patients co-medicated with lamotrigine.
CONCLUSIONS
This study indicates that lamotrigine exposure is associated with substantially reduced serum concentrations of quetiapine, possibly due to induced glucuronidation. These findings need to be confirmed in experimental studies.
Keywords: drug interaction, quetiapine, lamotrigine, therapeutic drug monitoring
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Preliminary evidence from a single study indicates that co-medication with lamotrigine may reduce the serum concentration of quetiapine. Since both drugs are commonly used in bipolar disorder such a drug–drug interaction could be of great importance, but the findings need to be confirmed.
WHAT THIS STUDY ADDS
The association between lamotrigine dosing and lowered quetiapine concentrations was confirmed in a small independent patient sample. The magnitude of the reduction (58%) indicated that the proposed drug–drug interaction may be of clinical importance.
Introduction
Quetiapine is one of the most frequently prescribed antipsychotics, also approved for bipolar disorder. The anti-epileptic drug lamotrigine is used for treatment of bipolar disorder and co-medication with the two drugs is not uncommon.
In a study by Castberg and co-workers potential drug–drug interactions with quetiapine were investigated by means of data from a routine therapeutic drug monitoring database [1]. Significantly decreased quetiapine concentrations (−17%) were observed in patients (n = 147) co-medicated with lamotrigine. The authors considered this possible interaction clinically insignificant and suggested that the statistical significance might have been due to chance [1]. We have found no other publications addressing the potential interaction between lamotrigine and quetiapine.
The aim of the present study was to investigate this interaction by analyzing data from our Swedish therapeutic drug monitoring database at Karolinska University Hospital, Huddinge.
Methods
Data collection
Study data were extracted from a routine therapeutic drug monitoring (TDM) service database at the Department of Clinical Pharmacology, Karolinska University Hospital and all patients subjected to analysis of quetiapine serum concentration were identified. Serum quetiapine concentrations were measured by a LC-MS method and lamotrigine by a HPLC method. Among the quetiapine-treated patients, individuals receiving co-treatment with lamotrigine were identified by review of medication registered at the time of quetiapine analysis and by screening the above mentioned database for analyses of lamotrigine serum concentrations. For each patient receiving co-medication with quetiapine and lamotrigine (case) a control patient subjected to quetiapine analysis and unexposed to lamotrigine was identified. Cases and controls were matched for gender, age (±5 years), quetiapine preparation (instant release or slow release) and dosage interval. Patients co-medicated with other drugs known to interact with quetiapine were excluded.
Statistical analysis
To remove the potential influence of unequal sampling times, 12 h quetiapine concentrations were imputed from each measured concentration. Twelve hours was chosen since most samples (82%) where drawn approximately 12 h post-dose. For quetiapine instant release tablets, the imputation was based on an assumption of first-order elimination with a half-life of 7 h. At steady-state, the quetiapine concentration is relatively stable 10–16 h following administration of an extended release tablet [2]. Hence, no imputation was used for such samples. In accordance with published data, the concentration 12 h after intake of an extended release tablet was assumed to be twice that of the concentration measured approximately 24 h post-dose [2]. For each case and each control, a quetiapine concentration : dose (C : D) ratio was calculated by dividing the measured quetiapine serum concentration (nmol l−1) by the daily oral quetiapine dose prescribed at the time of serum analysis (mg day−1). If more than one C : D ratio was attainable in a single patient due to repeated testing, the median value of all available ratios was used in the statistical analyses.
In the main analysis, quetiapine C : D ratios were compared pair-wise between cases and matched controls using the Wilcoxon signed rank test. To validate the robustness of the results, data were re-evaluated in a secondary analysis using the Mann-Whitney U-test. Unlike the paired test used in the main analysis, this unpaired test is not dependent on the assumptions that each case and its matched control only differ with regard to lamotrigine exposure.
The potential influence of lamotrigine concentration on the strength of the proposed interaction was investigated by means of Spearman rank correlation. In this analysis, the lamotrigine area under the concentration–time curve (AUC) in each case was correlated to the quetiapine C : D ratio. The AUCs were calculated from measured lamotrigine concentrations and sampling times, assuming first-order elimination with a half-life of 24 h.
Data extraction was performed using Crystal Reports XI, Business Objects Software Ltd, and all statistical analyses were performed using R 2.10.1 [3].
Results
Study population
We identified 402 patients subjected to quetiapine analysis in the TDM service database. Among these, 23 had received lamotrigine concomitantly. One of the identified cases was excluded due to an undetectable lamotrigine concentration. For each of the remaining 22 lamotrigine-exposed patients a matched control was identified, resulting in a total of 44 patients in the final analysis (Table 1). In 68% of the included patients, quetiapine was only measured once and in the remaining patients the median interval between samplings was 36 days. Steady-state could be confirmed in 17 of the 22 cases and in 19 of the 22 controls, but according to instructions all samples should be drawn at steady-state. Lamotrigine concentrations had been measured in 20 out of the 22 included cases and in one case-control pair a match for gender could not be achieved.
Table 1.
Characteristics of included patients. Cases were treated with lamotrigine and quetiapine, controls were treated with quetiapine only
| Cases | Control | |
|---|---|---|
| Median age in years (range) | 46 (17–62) | 48 (15–60) |
| Gender (M/F) | 9/13 | 8/14 |
| % using depot quetiapine | 13.6 | 13.6 |
| Median quetiapine daily dose (mg) (range) | 300 (100–1200) | 550 (100–1100) |
| Median quetiapine sampling time (h) (range) | 12.4 (10.5–25.7) | 12.5 (4.5–26.0) |
Quetiapine concentration : dose ratios
Patients treated with lamotrigine had a lower mean C : D ratio (0.71, 95% CI 0.46, 0.97) compared with controls (1.64, 95% CI 1.00, 2.28). The median within-pair difference between cases and controls was −0.71, 95% CI −1.38, −0.20. This difference was statistically significant with a P value of 0.013. The difference in C : D ratio between individual cases and controls is presented in Figure 1 and in relative measures the difference amounted to −58% (median percentual difference between cases and controls). The C : D ratio was lower in the lamotrigine group than in the control group in 14 out of 22 of the matched pairs. Patients treated with lamotrigine had a median C : D ratio of 0.63 and in the control group the median C : D ratio was 1.34 (Figure 2). The difference in quetiapine C : D ratio remained significant when data were analyzed in an unpaired model (95% CI −1.14, −0.17, P = 0.0054). The mean lamotrigine AUC was 419 nmol l−1 h (95% CI 303, 536). We found no significant correlation between the lamotrigine AUC and the quetiapine C : D ratio (rho = 0.18).
Figure 1.
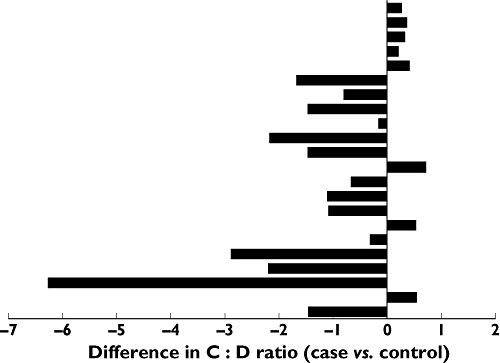
Difference in quetiapine C : D ratio (µmol l−1)/(mg day−1) between cases (co-treated with lamotrigine and quetiapine) and matched controls (unexposed to lamotrigine). Each bar illustrates the difference between the C : D ratio of an individual case and its corresponding matched control. Negative values indicate a lower C : D ratio in the lamotrigine-exposed individual
Figure 2.
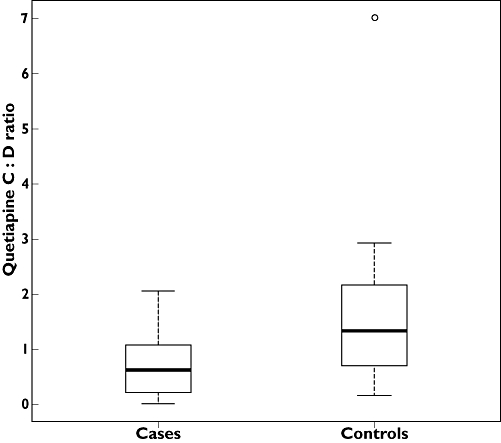
Box-plot of quetiapine C : D ratios (µmol l−1)/(mg day−1) in cases co-medicating with lamotrigine and quetiapine and in controls unexposed to lamotrigine
Discussion
In our TDM database, we found a 58% lower quetiapine C : D ratio in patients treated with lamotrigine compared with control patients. These results are in agreement with the findings of Castberg et al. who found that the quetiapine C : D ratio was reduced in lamotrigine-treated patients [1]. In fact, the effect of lamotrigine exposure was even more pronounced in our study, strongly supporting an association between lamotrigine exposure and reduced quetiapine serum concentrations.
Lamotrigine is not metabolized by cytochrome P450 (CYP) enzymes and is mainly eliminated as glucuronide conjugates. Glucuronidation is catalyzed by uridine 5'-diphospho-glucuronosyltransferase (UGT) [4]. UGT1A3 and UGT1A4 are the UGT-isoenzymes that are thought to be the major enzymes involved in the glucuronidation of lamotrigine [5]. Quetiapine is metabolized through many different pathways, mainly via CYP3A4, but some of the parent compound directly undergoes glucuronide conjugation before excretion [6]. Valproic acid, a known inhibitor of several UGT-isoenzymes, causes a 77% increase in quetiapine concentration [7], indicating that glucuronidation may be an important route for quetiapine elimination. Likewise, the metabolism of lamotrigine is inhibited by valproic acid, probably via inhibition of UGT1A3 and maybe also UGT1A4 [8].
Lamotrigine is known to induce moderately its own metabolism [9], probably via UGT induction. In addition, lamotrigine has been found to slightly decrease the concentration of levonorgestrel [10] and clonazepam [11], both of which are partially glucuronide conjugated before excretion [12].
Taken together, this indicates that induction of quetiapine glucuronidation by lamotrigine may explain or, at least, contribute to the results observed in this study.
If this theory holds true lamotrigine has the potential to interact in a clinically relevant manner with many other drugs that are glucuronidated via UGT1A-isoenzymes. This study indicates that glucuronidation may play an important role in the elimination of quetiapine. If so, other UGT1A-inhibiting and inducing agents may cause clinically relevant interactions with quetiapine.
We did not find any correlation between the lamotrigine AUC and the difference in C : D ratio between cases and controls. Although such a correlation would doubtlessly have strengthened the evidence of a causal relationship, the absence of a significant correlation should not be taken as proof against causality. For example, the concentration–effect relationship of the proposed UGT induction may be such that all lamotrigine concentrations achieved at clinically relevant doses produce a similar degree of enzyme induction. In addition, this secondary analysis may have had insufficient power to detect a potential concentration–effect relationship.
One limitation of this study is that it utilized matched controls rather than a crossover design. Although a crossover design would have further reduced the risk of bias, it was not feasible since very few patients had been subjected to quetiapine analysis both while on lamotrigine and while unexposed to the drug. This study is observational and based on data from a routine TDM database. Since TDM is commonly used in association with therapeutic difficulties (e.g. lack of effect or adverse drug reactions), the study population may not be entirely representative for quetiapine-treated patients in general. However, this should apply to both cases and controls since both groups were assembled from the same TDM database.
Although our findings indicate a drug–drug interaction between lamotrigine and quetiapine, a causal relationship can only be formally confirmed in an experimental setting. Indeed, such a clinical trial was initiated by the manufacturer of lamotrigine, but unfortunately it had to be abandoned due to unacceptable side-effects of quetiapine [13]. Hopefully, future studies with better accepted dosing regimens will provide further information but until then the potential risk for a drug interaction should be considered in patients co-medicating with lamotrigine and quetiapine. In these patients, therapeutic drug monitoring of quetiapine is advisable when lamotrigine therapy is initiated or withdrawn.
Acknowledgments
LBB is financially supported by post-doctoral grants from Karolinska Institutet, The Swedish Foundation for Clinical Pharmacology and Pharmacotherapy and Stockholm County Council, Sweden. Jonatan Lindh has received a research grant from the Karolinska Institutet.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Castberg I, Skogvoll E, Spigset O. Quetiapine and drug interactions: evidence from a routine therapeutic drug monitoring service. J Clin Psychiatry. 2007;68:1540–5. doi: 10.4088/jcp.v68n1011. [DOI] [PubMed] [Google Scholar]
- 2.Figueroa C, Brecher M, Hamer-Maansson JE, Winter H. Pharmacokinetic profiles of extended release quetiapine fumarate compared with quetiapine immediate release. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:199–204. doi: 10.1016/j.pnpbp.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 3.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 4.Cohen AF, Land GS, Breimer DD, Yuen WC, Winton C, Peck AW. Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin Pharmacol Ther. 1987;42:535–41. doi: 10.1038/clpt.1987.193. [DOI] [PubMed] [Google Scholar]
- 5.Argikar UA, Remmel RP. Variation in glucuronidation of lamotrigine in human liver microsomes. Xenobiotica. 2009;39:355–63. doi: 10.1080/00498250902745082. [DOI] [PubMed] [Google Scholar]
- 6.DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40:509–22. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- 7.Aichhorn W, Weiss U, Marksteiner J, Kemmler G, Walch T, Zernig G, Stelzig-Schoeler R, Stuppaeck C, Geretsegger C. Influence of age and gender on risperidone plasma concentrations. J Psychopharmacol. 2005;19:395–401. doi: 10.1177/0269881105053306. [DOI] [PubMed] [Google Scholar]
- 8.Lalic M, Cvejic J, Popovic J, Bozic K, Golocorbin-Kon S, Al-Salami H, Mikov M. Lamotrigine and valproate pharmacokinetic interactions in epileptic patients. Eur J Drug Metab Pharmacokinet. 2009;34:93–9. doi: 10.1007/BF03191157. [DOI] [PubMed] [Google Scholar]
- 9.GlaxoSmithKline. Summary of product characteristics, Lamictal. 2010.
- 10.Sidhu J, Job S, Singh S, Philipson R. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191–9. doi: 10.1111/j.1365-2125.2005.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson AS, Hoppu K, Nergardh A, Boreus L. Pharmacokinetic interactions between lamotrigine and other antiepileptic drugs in children with intractable epilepsy. Epilepsia. 1996;37:769–73. doi: 10.1111/j.1528-1157.1996.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 12.Levy RH, Thummel KE, Trager WF, Hansten PD, Eichelbaum M. Metabolic Drug Interactions. 1st edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 13.GlaxoSmithKline. An open-label, two cohort study to investigate the potential pharmacokinetic interaction between lamotrigine and single doses of risperidone and quetiapine in healthy volunteers. SCA 101963. 2004.


