In January 2010, a 39-year-old Sri Lankan man, resident in Switzerland for 15 years, presented to the emergency department with constipation and abdominal pain from which he had suffered intermittently during the previous weeks. On physical examination, the abdomen was soft without tenderness or organomegaly. An abdominal X-ray confirmed stool retention without perforation or bowel obstruction. Laboratory testing revealed a haemoglobin level of 9.1 g dl−1 with normal indices and elevated reticulocytes. The blood film showed poikilocytosis, polychromasia, anisocytosis and basophilic stippling (Figure 1). Liver enzymes, bilirubin and LDH were mildly elevated. Haptoglobin was undetectable and the Coombs test was negative. A blood count performed in 2008 had been unremarkable. The diagnosis of a Coombs negative haemolytic anaemia was made. Despite further investigations, its origin remained unclear: infections, drugs, sickle cells or other haemoglobinopathies, glucose-6-phosphate dehydrogenase or pyruvate kinase deficiency and microangiopathic haemolytic anaemia were ruled out. Because of the abdominal colics, porphyria testing was performed. Increased concentrations of coproporphyrine III, delta-aminolevulinic-acid (urine) and elevated free- and zinc-protoporphyrin (erythrocytes) were observed. These results were not consistent with classical porphyrias because, instead of a single enzyme, a number of enzymes were inhibited. As heavy metals are known to inhibit enzymes such as ALA dehydratase, coproporphyrinogen oxidase and ferrochelatase [1], a blood lead concentration was obtained and found to be 63.2 µg dl−1 (normal < 5 µg dl−1). Reasons for lead exposure such as occupation, food, environment or illicit drugs were not detected. Ceramics with lead-containing glazing were not used in the patient's household and none of the family members had elevated blood lead concentrations. On repeated questioning, the patient admitted having taken five ayurvedic pills per day for 2 months until the end of December 2009 to increase his fertility. The pills had been ordered from India and were labelled ‘ashwagandha/mucuna’, which are sold as aphrodisiacs. Left over pills were examined at the Official Food Law Enforcement Authority, where concentrations up to 7.3 mg of lead per pill and traces of arsenic, chromium and mercury were found. Treatment with the oral heavy metal chelator, dimercaptosuccinic acid (DMSA), was started at a dose of 30 mg kg−1 bodyweight per day for 5 days followed by 20 mg kg−1 for 14 days. After 3 weeks, the blood lead concentration had decreased to 17 µg dl−1; anaemia and red blood cell morphology had normalized and the patient had become asymptomatic.
Figure 1.
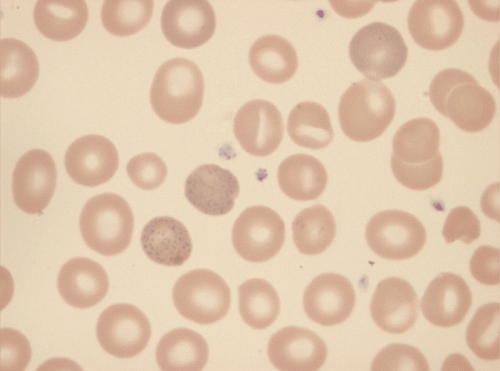
The blood film shows poikilocytosis, polychromasia, anisocytosis and basophilic stippling
The majority of cases of adult lead poisoning originate from workplace exposures. Inorganic lead is absorbed from the lungs, especially in adults, or from the gastrointestinal tract, which represents the predominant exposure route in children. Lead from ‘herbal medicines’, especially from Asian countries, is an emerging source of heavy metal poisoning. The blood lead concentration correlated with the symptoms at presentation. Lead concentrations below 50 µg dl−1 may cause symptoms such as asthenia, arthralgia, hypertension, headache and even infertility [2, 3]. Above these concentrations abdominal colics, kidney dysfunction, haemolytic anaemia and encephalopathy may occur. Much of the toxicity of lead can be attributed to interference with calcium-mediated signalling or the distortion of enzymes and structural proteins, in this case leading to symptoms of acute porphyria by impairing enzymes of porphyrinbiosynthesis [1]. Measurement of the blood lead concentration is the mainstay of diagnosis, but careful history taking remains of paramount importance. Basophilic stippling in the blood smear suggests lead intoxication but is non-specific. The key first step in management is to stop exposure. Medical treatment consists of chelation [4, 5] by substances such as DMSA or calcium EDTA. In conclusion, lead poisoning shares symptoms with other conditions and may be easily missed. The importance of a detailed drug history including alternative medicines should be emphasized.
Competing Interests
There are no competing interests to declare. There was no financial support for this study.
Authors' contributions
Martin Toniolo: Patient management, literature search, writing; Alessandro Ceschi: Literature search, writing; Marianne Meli: Literature search, writing; Andreas Lohri: Literature search, writing; Geneviève Favre: Patient management, literature search, writing.
REFERENCES
- 1.Hiroyoshi F. Lead, chemical porphyria and heme as biological mediator. Tohoku J Exp Med. 2002;196:53–64. doi: 10.1620/tjem.196.53. [DOI] [PubMed] [Google Scholar]
- 2.Doumouchtsis KK, Doumouchtsis SK, Doumouchtsis EK, Perrea DN. The effect of lead intoxication on endocrine functions. J Endocrinol Invest. 2009;32:175–83. doi: 10.1007/BF03345710. [DOI] [PubMed] [Google Scholar]
- 3.Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–22. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- 4.Kosnett MJ, Wedeen RP, Rothenberg SJ. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115:463–71. doi: 10.1289/ehp.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradberry S, Vale A. A comparison of sodium calcium edetate (edetate calcium disodium) and succimer (DMSA) in the treatment of inorganic lead poisoning. Clin Toxicol (Phila) 2009;47:841–58. doi: 10.3109/15563650903321064. [DOI] [PubMed] [Google Scholar]


