Abstract
Objective
To compare assessment of regional cerebral metabolic changes with DTBZ-PET measurement of regional cerebral blood flow (K1) and FDG-PET measurement of regional cerebral glucose uptake (CMRglc) in a clinically representative sample of mild dementia and mild cognitive impairment (MCI) subjects. We hypothesized that DTBZ-PET K1 and FDG-PET CMRglc provide equivalent information.
Design, Setting, Participants
DTBZ-PET K1 measurement of regional cerebral blood flow and FDG-PET CMRglc measurement of regional cerebral glucose uptake was performed in 50 subjects with either mild dementia (MMSE ≥ 18) or MCI drawn from a university based Cognitive Disorders Clinic. Results were compared with 80 normal control subjects.
Main Outcome Measures
DTBZ-PET regional K1 measurements and FDG-PET CMRglc measurements were compared with standard correlation analysis. The overall patterns of DTBZ-PET K1 deficits and FDG-PET CMRglc deficits were assessed with stereotaxic surface projections (SSP) of parametric images.
Results
DTBZ-PET regional K1 measurements and FDG-PET CMRglc measurements were highly correlated, both within and between subjects. SSP maps of deficits in DTBZ-PET regional K1 measurements and FDG-PET CMRglcs were markedly similar. DTBZ-PET K1 SSP maps exhibited a mild decrease in sensitivity relative to FDG-PET CMRglc maps.
Conclusion
DTBZ-PET K1 and FDG-PET CMRglc provide comparable information in assessment of regional cerebral metabolic deficits in mild dementia and MCI. K1 measures can assess regional cerebral metabolism deficits accurately in mild dementia and MCI. K1 assessments of regional cerebral metabolic deficits can be combined with tracer binding results to improve of utility of PET imaging in mild dementia and MCI.
Introduction
The present approach to diagnosis of dementia relies on clinical characterization, psychometric evaluation, and application of standardized clinical criteria1. There is overlap in clinical criteria for the three major forms of degenerative dementia - Alzheimer disease (AD), Lewy Body dementia (DLB), and Frontotemporal dementias (FTDs) – and clinical diagnosis has relatively poor specificity1,2,3,4,5. Differentiation is particularly difficult in individuals with early, mild dementia. Positron emission tomography (PET) is a useful technique for evaluation of dementias. [18F]Fluoro-deoxyglucose PET (FDG-PET) assessment of regional cerebral metabolic rate for glucose (CMRglc) is the most widely studied PET method for evaluation of dementias6,7,8. FDG-PET is useful in determining whether individuals have an underlying cerebral problem, differentiating types of dementia, identifying cerebral abnormalities in individuals with mild cognitive impairment (MCI), predicting the likelihood of progression from MCI, and predicting the rate of progression of dementia9-28,. FDG-PET identifies characteristic patterns of regional cerebral metabolic changes in AD, DLB, FTDs, and vascular dementia (VaD)29-39.
PET methods identifying characteristic pathologic features of dementias are being explored. Diminished dopaminergic nigrostriatal terminals are a feature of DLB40,41. Single site studies and a recent multicenter clinical trial indicate that PET or single photon emission computed tomography methods measuring nigrostriatal dopaminergic terminal density are useful in establishing a diagnosis of DLB42,43,44. Tracers binding to characteristic histopathologic features of dementias, such as the amyloid ligand 2-(4′methylaminophenyl) benzothiazole (BTA-1; Pittsburgh B [PiB]), may provide an in vivo analogue of histopathologic diagnosis45-54. Determining patterns of altered regional cerebral metabolism and abnormal retention of tracers aimed at characteristic pathologic features should provide complementary information. This can be accomplished by studying patients with FDG-PET and a second PET study using one of the nigrostriatal dopaminergic terminal ligands or [11C]PiB55. This approach requires at least 2 scans with accompanying increased expense, radiation exposure, and demands on patients.
Regional cerebral metabolism and regional cerebral blood flow are tightly coupled56,57,58. Data analysis for some PET tracers allows determination of the regional cerebral tracer blood to brain transport rate (K1) as a measure of regional cerebral blood flow. We reported previously that assessment of regional cerebral K1 of the dopaminergic terminal ligand [11C]dihydrotetrabenazine (DTBZ) produced results closely comparable to measurement of CMRglc with FDG-PET59. Our prior study was a retrospective analysis of subjects drawn from a well characterized research cohort not representative of clinical practice. These subjects had moderate AD (mean MMSE score =15), DLB (mean MMSE score = 17, and FTD (mean MMSE score = 23), not the early, mild dementia/MCI subjects in whom accurate clinical diagnosis is most difficult. To confirm the utility of K1 assessments, we report comparison of FDG-PET assessment of CMRglc with measurements of [11C]DTBZ-PET K1s in a prospective series of representative subjects undergoing initial characterization of mild dementia and MCI.
Methods
Subjects
Fifty subjects were recruited through the Michigan Alzheimer Disease Research Center as part of an ongoing project studying utility of PET imaging in early dementia and MCI. Subjects are drawn from the Cognitive Disorders Clinic at the University of Michigan. Subjects are excluded if their Mini-Mental State Score (MMSE) was lower than 18 or if they had a confounding neurologic or psychiatric disorder that prevented establishment of a clear diagnosis of dementia or MCI. Subjects were excluded also if clinical evaluation (including routine structural imaging) indicated a diagnosis of VaD. While this is not a population based cohort, subjects enrolled for this study are representative of patients seen for initial evaluation in tertiary referral cognitive disorders clinics. There is no overlap in subjects between those enrolled in this study and our prior study of DTBZ K1 and FDG-PET CMRglc.
All subjects underwent standardized clinical and psychometric evaluations. Clinical evaluations consisted of a history and standard neurologic examination performed by a neurologist experienced in the evaluation of dementias. All subjects underwent evaluation for treatable causes of dementia with recommended serum and structural imaging studies1. Psychometric evaluation included the Alzheimer Disease Research Center Unified Data Set measures (Digit Span, Category Fluency, Trails Test, Digit Symbol, Wechsler Memory Scale Revised Logical Memory Story A, Boston Naming Test-30-item, Geriatric Depression Scale, Neuropsychiatric Inventory Questionnaire) plus additional standard measures of memory, executive function, attention, language, visuospatial function, mood, and anxiety. Clinical and psychometric data were abstracted into a standard form stripped of identifiers by one of the authors (JFB). Standardized data forms were reviewed in a consensus conference by three authors (RLA, KAF, BG) to assign a consensus diagnosis. Standard criteria were used to assign diagnoses of AD, DLB, and FTD60,61,62. For diagnosis of MCI, standard clinical and psychometric criteria were applied63. Subjects with MCI were classified as single domain MCI (amnestic or non-amnestic; aMCI or naMCI) or multi-domain MCI (mdMCI). All subjects underwent PET imaging within 3 months of initial evaluation. Scans of 80 age matched, normal subjects studied previously in our center were used as control data (Supplementary Table 1 at http://sitemaker.umich.edu/albinsuppldata). PET Imaging: All subjects underwent [11C]DTBZ and [18F]FDG PET either in the same imaging session or on consecutive days if they were not able to tolerate the 4-hr procedure. DTBZ scans were performed on a Siemens ECAT Exact HR+ scanner (Knoxville, TN), while FDG scans were performed on a Siemens BioGraph Classic PET/CT scanner (Knoxville, TN). Subjects were positioned supine and awake in a quiet dimly lit room with eyes open. For [11C]DTBZ, 666 ± 66 MBq (18 ± 1.8 mCi) of [11C]DTBZ were administered intravenously. An equilibrium protocol was used, infusing 55% of the dose over 30 s, followed by continuous infusion of the remaining 45% over 60 min64. [18F]FDG studies were performed as a single 20-min scan acquired 30 min after intravenous injection of 296 ± 29 MBq (8.0 ± 0.8 mCi) of [18F]FDG. All scans were acquired in 3-dimensional (3D) mode. Measured attenuation correction was performed using a 5-min 2-dimensional (2D) transmission scan followed by segmentation and reprojection. Scatter correction was performed on all scans. After Fourier rebinning (FORE) of the 3D projection sinograms into 2D datasets, images were reconstructed using ordered-subset expectation-maximization (OSEM), with 4 iterations and 16 subsets with no additional smoothing, providing both in-plane and axial image resolution of ~5.5 mm full-width at half-maximum.
Image Processing and Data Analysis
The single 20-min [18]FDG image acquired starting 30-min post-injection provided an index of CMRglc. For the [11C]DTBZ scan, the average of the first 4 min of uptake provided our index of ligand transport, K1. PET images for both measures for each subject were reoriented to a common coordinate system based on the stereotactic atlas of Talairach and Tournoux65. After reorientation, all images underwent linear scaling and nonlinear warping66. A single transformation based on the individual’s summed [18F]FDG and [11C]DTBZ K1 images was calculated for each subject and then applied to both image sets.
All transaxial levels of the Talairach and Tournoux atlas have been digitized, and a set of standardized cortical and subcortical volumes of interest (VOIs) defined on the atlas images59. A subset of 34 cortical VOIs (17 per hemisphere) was selected for analysis and applied automatically to all images for all mild dementia and MCI subjects, and the 15 control subjects use for correlation analysis of DTBZ K1 and FDG CMRglc. This set of regions included cortical areas hypothesized to provide the best differentiation between groups. We expected frontal regions, particularly medial, to be affected more in FTD than AD or DLB and posterior parietal/posterior cingulate regions to be more affected in AD and DLB than FTD. We expected the occipital region to be more affected in DLB than AD or FTD, and the region that best differentiates DLB from AD. Sensorimotor cortex was included as a cortical region likely to be affected least in these dementias. FDG and DTBZ K1 images were normalized to the mean VOI value obtained from the pons and cerebellar vermis67. These two regions were selected as the normalizing factor because they are structures known to be minimally involved in these disorders, enhancing the ability to detect regional cortical deficits by removal of the more variable global factor in these measures.
Statistical Analysis
Both within-subject and across-subject correlations were performed. Within-subject correlations were calculated for each of the 50 patient subjects and 15 controls who received both FDG and DTBZ scans. VOI FDG CMRglc and DTBZ K1 normalized values were correlated for all 34 selected cortical regions. Across-subject correlations were calculated for each of the 34 regions separately for each of the 50 patient and 15 control subjects.
SSP
The use of stereotactic surface projections (SSP) is a standard method for processing FDG CMRglc data to produce easily interpretable parametric images68. In this voxel-based method, gray matter activity is projected to the cortical surface in an anatomically standardized manner. The resulting map of brain activity is compared with a database of appropriate matched subjects to generate a Z-score map of deviations from normal. The Z-score maps permit ready visual interpretation of CMRglc deficit patterns. We utilized the Neurostat program and a control database of the 27 FDG controls to generate Z-score maps of FDG CMRglc deficits for all subjects. The SSP approach was used also for processing of DTBZ K1 images. We constructed a control database from 68 subjects of similar age without clinical evidence of any neurologic disease. These 68 individuals were studied previously in our center using the same scan protocol used in this project.
Results
Subjects
50 subjects with dementia or MCI participated. Mean age was 70 years (SD 10; range 51-91; 28 female; 22 male). There were 19 AD, 5 DLB, and 9 FTD. Sixteen subjects were classified as MCI with 9 aMCI, 4 naMCI, and 3 mdMCI. One subject refused psychometric evaluation and is classified as indeterminate. Of the 80 control subjects scanned, 27 received FDG (mean age 71, SD 9, range 55-88; 13 female, 14 male), and 68 received DTBZ (mean age 68, SD 8, range 55-86, 38 Female, 37 Male). Fifteen control subjects received both FDG and DTBZ scans (mean age 70, SD 9, range 55-86, 8 Female, 7 Male).
FDG CMRglc and DTBZ K1 Correlation Analysis
There was excellent correspondence between regional cerebral metabolism assessed with FDG and regional cerebral blood flow assessed with DTBZ K1. Regional deficits averaged across all patient subjects, reported as percent of normal control mean, were similar for the two PET measures in most regions examined (Figure 1). Frontal cortical regions did exhibit slightly greater deficits in FDG than in DTBZ K1, reaching significance both laterally and medially. In other regions, both the magnitude of the declines and the variability across patients were comparable. Individual within-subject correlations between normalized FDG and DTBZ K1 (across the regions examined; left and right hemisphere for the 17 regions shown in Fig. 2) averaged 0.88±0.07 for the 50 patient subjects (range 0.73-0.96; Figure 2). Within-subject correlations for controls were lower due to the smaller range of values, averaging 0.78±0.08 (range 0.64-0.88). Besides the similarity in image pattern between the measures, the magnitude of the deficits correlated highly. Within-subject correlations of the Z-scores relative to controls average 0.74±0.14 (range 0.37-0.96; Supplementary Figure 1 at http://sitemaker.umich.edu/albinsuppldata). Z-score correlations for controls averaged 0.54±0.25 (range 0.05-0.82). The much broader range of Z-score correlations is due to the nature of the Z-score measure. Control as well as patient subjects who have image patterns similar to the normal mean pattern will have low correlations due to the limited range of data values.
Figure 1. Regional Metabolic and Perfusion Deficits.
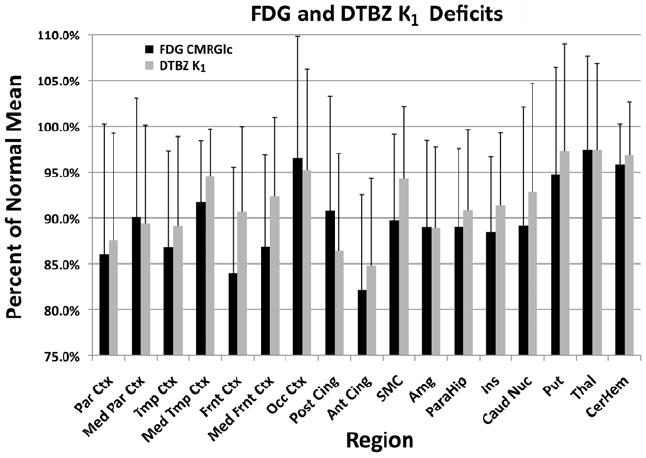
Bar graphs of regional metabolic and perfusion deficits, expressed as percent of control mean, for 50 subjects with dementia or MCI. Magnitudes of the deficits observed by either FDG CMRglc or DTBZ K1 vary similarly across regions. Error bars represent 1 standard deviation of the percent of normal mean for all patient subjects.
Figure 2. Within-Subjects Correlations.
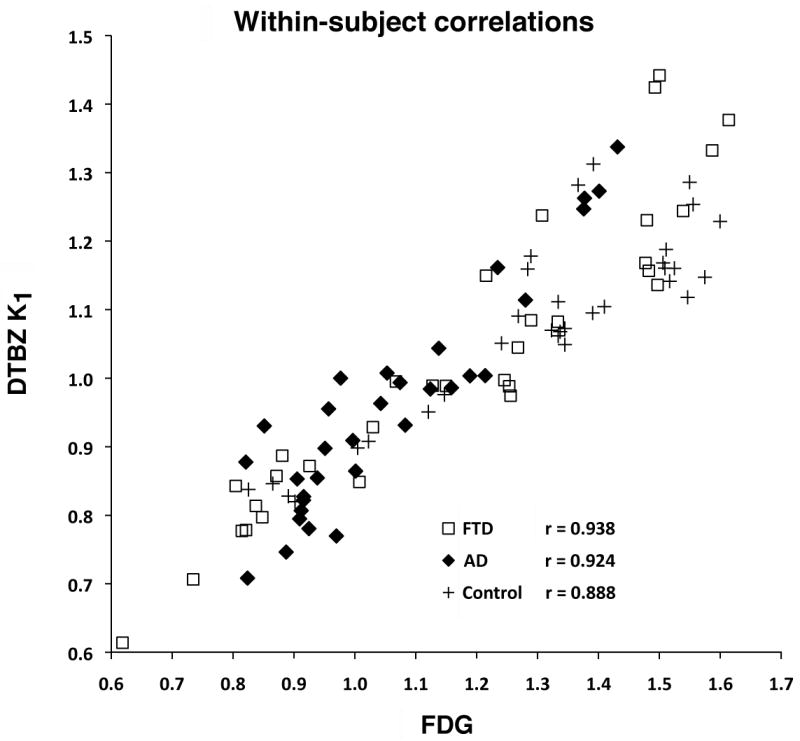
Representative within-subjects correlations (across the 34 regions examined) between FDG and DTBZ K1 for an individual subject with AD, one with FTD, and one control subject. FDG and DTBZ K1 values are each normalized to the average of pons and cerebellar vermis. Each data point represents the normalized K1 and FDG values for one of the 34 regions.
Across-subjects correlations between FDG and DTBZ K1 varied considerably across the regions examined. Correlations across the 34 regions average 0.78±0.14 (range 0.51-0.94) in patients and 0.76±0.13 (range 0.48-0.91) in controls. Correlations across the patient subjects were uniformly high (r>0.83) in cortical regions that show deficits in these patient groups (parietal, frontal cortex, posterior cingulate), while correlations in regions not primarily involved in disease (subcortical structures) were typically much lower. Correlations for a highly affected region, parietal cortex, are seen in Figure 3.
Figure 3. Across-Subject Correlations of Z-scores for Parietal Cortex.
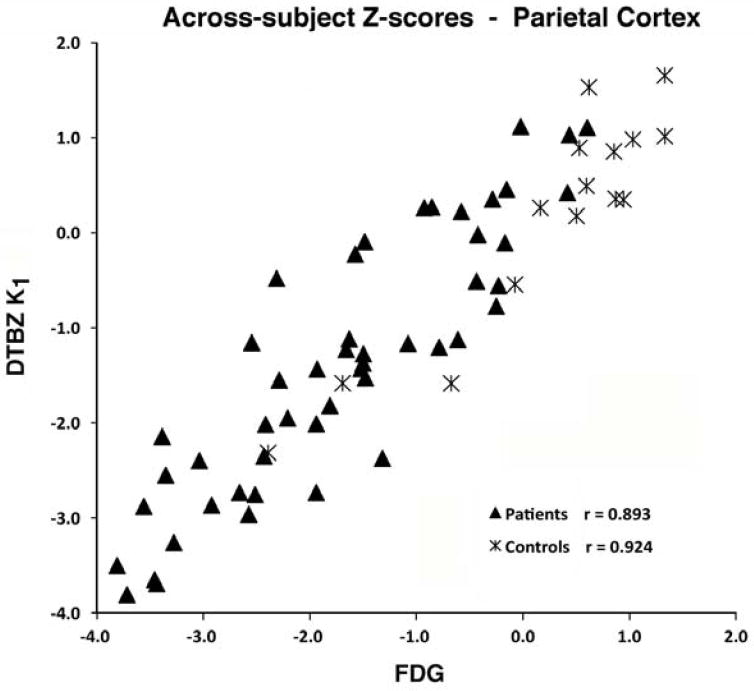
Across-subject correlations of Z-scores between FDG CMRglc and DTBZ K1 in the parietal cortex for patients (all demented and MCI subjects) and for control subjects. Each data point represents the Z-scores from normalized K1 and FDG values for a single subject.
SSP Analysis
SSP analysis was applied to FDG CMRglc and DTBZ K1 images for each subject as described above. The resulting Z-score maps were compared for qualitative similarity. FDG and DTBZ K1 Z-score maps were similar for all patients, regardless of diagnosis (Figure 4). In general, DTBZ K1 Z-score maps tended to identify a smaller number of abnormal surface map pixels. This is due primarily to the slightly higher noise at the pixel level of DTBZ K1 image than FDG images. For all surface pixels, the coefficient of variation of DTBZ K1 control subjects averaged 10.4% and that of FDG was 9.6%. In all cases, however, DTBZ K1 Z-score maps were readily identifiable as abnormal and qualitatively identical to FDG-PET CMRglc Z-score maps. The number of surface map pixels found to be abnormal (more than 2.5 standard deviations below the normal mean) in the most affected regions was higher for FDG than DTBZ K1 (Figure 5). The total of surface map pixels in the most affected areas (posterior cingulate, lateral parietal, lateral temporal, lateral and medial frontal) was 8920 (Supplementary Figure 2 at http://sitemaker.umich.edu/albinsuppldata). In the most severe patients, over the half the surface pixels have z-scores deficits of greater than 2.5. The regression line of DTBZ K1 on FDG shows a slope less than unity (0.863) indicating fewer significant pixels for DTBZ. Many of the pixels found to be significant in FDG but not DTBZ K1 are located in frontal cortex.
Figure 4. SSP Projection Images.
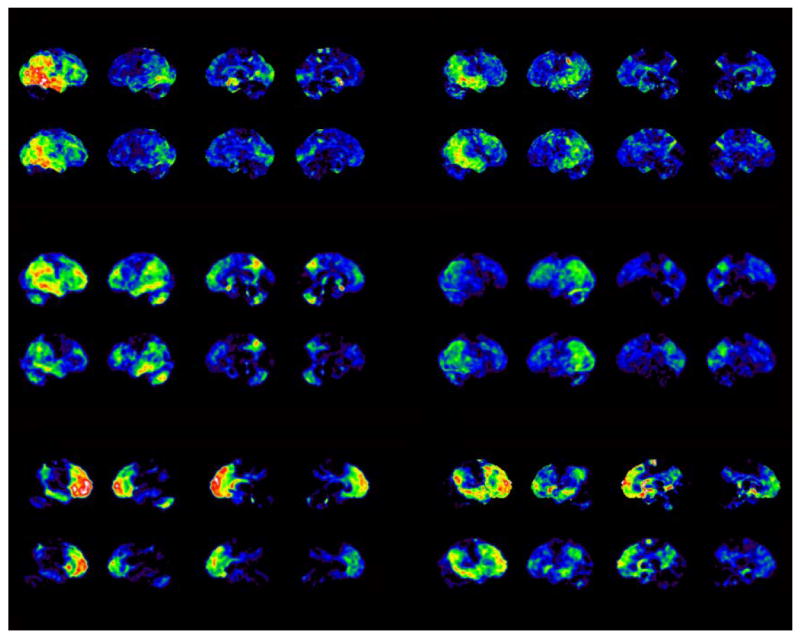
SSP projection images of Z-scores of FDG CMRglc and DTBZ K1 deviations relative to control datasets for six representative patient subjects. The four surface projections from left to right for each of the subjects represent right lateral, left lateral, right medial, and left medial views. Each data set is scaled to show a range of deficits from 0.0 to 5.0 (standard deviations below control mean). The patterns of deviation from normal are markedly similar.
Figure 5. Extent of Deficits Affected Cortex.
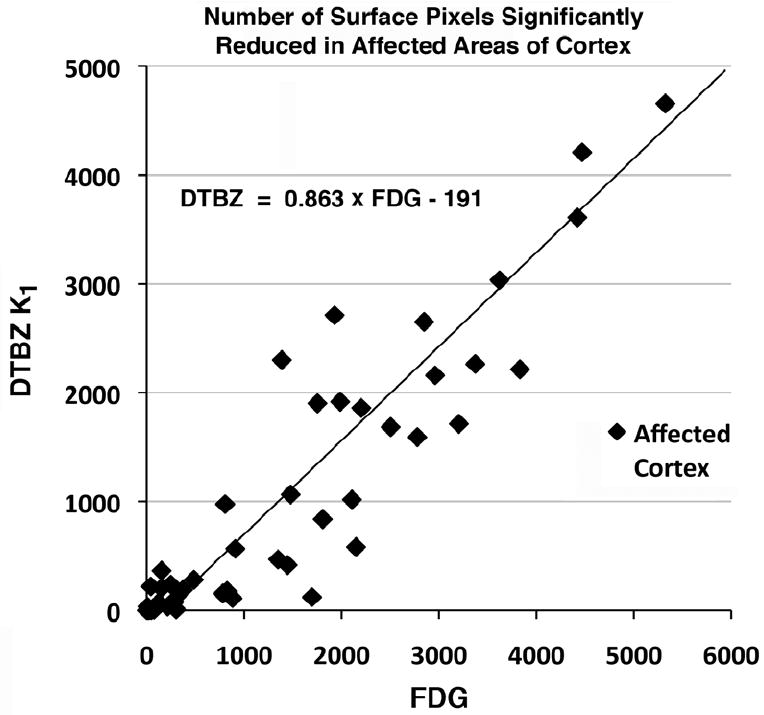
Number of SSP surface map pixels in affected cortex with greater than a 2.5 standard deviation decrease from normal control. Each point on the plot represents one of the 50 patient subjects. (see Supplementary Fig. 2 for description of affected cortex.)
Comment
Our results indicate similar properties of FDG-PET assessment of CMRglc and DTBZ-PET K1 assessment of regional cerebral blood flow to identify regional cerebral metabolic deficits in subjects with MCI and early, mild dementia. Regional cerebral metabolic deficits are characteristic of dementias and specific patterns of deficits are associated with each major form of degenerative dementia6-15,29-32,33-37. We did find that DTBZ K1 assessments tended to be somewhat less sensitive in SSP analysis because of higher variation in surface pixel values. This was most noticeable in frontal regions. This could reduce sensitivity of DTBZ K1 assessments in FTDs and related disorders.
Differentiation of dementias, based on clinical criteria and using structural imaging and laboratory studies to exclude other causes of dementia, has relatively poor specificity1. FDG-PET is suggested to improve diagnosis of dementias and may be cost effective in evaluation of patients with early, mild dementia69,70,71. Recent work demonstrates that FDG-PET with SSP analysis differentiates early dementia and controls in a clinically representative population of early dementia and MCI72. The “gold standard” for diagnosis of dementias remains histopathologic evaluation. Small series with FDG-PET evaluations followed by histopathologic analysis indicate that FDG-PET evaluation significantly improves specificity without sacrificing sensitivity8,9,31,73. These results are consistent with studies in which FDG-PET results were compared with systematic, longitudinal clinical evaluations69. Differentiation of dementias at initial evaluation is particularly difficult. Jagust et al. found that initial clinical evaluation of a series of mildly demented patients who were followed to autopsy had relatively poor sensitivity (76%) and specificity (56%) for diagnosis of pathologically confirmed AD, and that diagnostic precision improved significantly with addition of FDG-PET imaging data. Diagnostic sensitivity and specificity of FDG-PET evaluation was comparable to clinical evaluation after several years of followup.
While characteristic metabolic deficits occur in many individuals with dementia, confounding features can reduce accuracy of FDG-PET. There is considerable overlap between AD and DLB. Not all DLB subjects exhibit the typical visual cortex abnormalities; the sensitivity and specificity of FDG-PET for differentiating AD and DLB are not significantly better than application of clinical criteria32,74. Pathologic studies indicate that a high percentage of individuals with focal cortical syndromes typical of FTDs exhibit AD pathology75. These AD focal cortical syndromes are likely to be classified as FTDs by both clinical criteria and FDG-PET criteria76,77. PET tracers aimed at characteristic pathologic features of dementias are likely to be useful in overcoming the limitations of FDG-PET. PET and SPECT ligands identifying the characteristic nigrostriatal dopaminergic deficit of DLB improve differentiation of AD and DLB. Similarly, [11C]PIB is likely to be useful in identifying dementias characterized by substantial Aβ amyloid deposition such as AD and cases of DLB with substantial fibrillary amyloid burden.
Ligand binding results and assessment of regional cerebral metabolic deficits may be complementary. DTBZ-PET studies, for example, could identify nigrostriatal dopaminergic deficits in both DLB and some cases of FTDs78. The pattern of regional cerebral metabolic deficits would be useful in differentiating these different syndromes. [11C]DTBZ itself is not likely to become a clinically used ligand but fluorinated DTBZ analogues or another tracer quantifying nigrostriatal terminal density may become available for clinical use. Obtaining both ligand binding and regional cerebral metabolic deficit data from a single PET study will be advantageous in terms of cost, safety, and patient convenience. PET ligands with high brain to blood extraction fractions yield K1 parametric image data comparable to that obtained with FDG-PET and K1 parametric image data can be represented as SSP maps. Use of K1 parametric image data from PET ligands aimed at characteristic pathologic features of dementias is likely to enhance the clinical utility of PET methods for characterization of MCI and early, mild dementias.
Supplementary Material
Acknowledgments
Funding/Support: Supported by AG08671 & NS15655. The sponsors had no role in data collection, analysis, interpretation, or manuscript preparation.
Footnotes
Author Contributions: Dr. Albin had full access to all the data in the study and takes full responsibility for data integrity and data analysis. Study concept and design: Albin & Frey. Acquisition of data: Albin, Frey, Giordani, Koeppe, Kilbourn. Analysis and interpretation of data: Albin, Frey, Koeppe, Giordani, Burke. Drafting of the manuscript: Albin & Koeppe. Critical revision of the manuscript for important intellectual content: Albin, Koeppe, Burke, Giordani, Kilbourn, Gilman, Frey. Statistical analysis: Koeppe. Obtained funding: Albin, Frey, & Gilman. Administrative, technical, and material support: Koeppe & Kilbourn. Study supervision: Albin & Frey.
Financial Disclosure: None reported.
References
- 1.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review) Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Ballard CG, Perry RH, Ince PG, O’Brien JT, Neill D, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54:1050–1058. doi: 10.1212/wnl.54.5.1050. [DOI] [PubMed] [Google Scholar]
- 3.Lopez OL, Litvan I, Catt KE, Stowe R, Klunk W, Kaufer DI, et al. Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias. Neurology. 1999;53:1292–1299. doi: 10.1212/wnl.53.6.1292. [DOI] [PubMed] [Google Scholar]
- 4.Varma AR, Snowden JS, Lloyd JJ, Talbot PR, Mann DM, Neary D. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatr. 1999;66:184–188. doi: 10.1136/jnnp.66.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen HJ, Hartikainen KM, Jagust W, Kramer JH, Reed BR, Cummings JL, et al. Utility of clinical criteria in differentiating frontotemporal lobar degeneration (FTLD) from AD. Neurology. 2002;58:1608–1615. doi: 10.1212/wnl.58.11.1608. [DOI] [PubMed] [Google Scholar]
- 6.Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007:S160–S167. doi: 10.1259/bjr/97295129. [DOI] [PubMed] [Google Scholar]
- 7.Silverman DHS, Mosconi L, Ercoli L, Chen W, Small GW. Positron emission tomography scans obtained for the evaluation of cognitive dysfunction. Sem Nuc Med. 2008;38:251–261. doi: 10.1053/j.semnuclmed.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. Eur J Nuc Med. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 9.Silverman DHS, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 10.de Leon MJ, Ferris SH, George A, Reisberg B, Christman DR, Kricheff II, et al. Computed tomography and positron emission transaxial tomography evaluations of normal aging and Alzheimer’s disease. J Cereb Blood Flow Metab. 1983;3:391–394. doi: 10.1038/jcbfm.1983.57. [DOI] [PubMed] [Google Scholar]
- 11.Foster NL, Chase TN, Mansi L, Brooks R, Fedio P, Patronas NJ, et al. Cortical abnormalities in Alzheimer’s disease. Ann Neurol. 1984;16:649–654. doi: 10.1002/ana.410160605. [DOI] [PubMed] [Google Scholar]
- 12.Friedland RP, Brun A, Budinger TF. Pathological and positron emission tomographic correlations in Alzheimer’s disease. Lancet. 1985;26:228. doi: 10.1016/s0140-6736(85)92074-4. [DOI] [PubMed] [Google Scholar]
- 13.Koss E, Friedland RP, Ober BA, Jagust WJ. Differences in lateral hemispheric asymmetries of glucose utilization between early- and late-onset Alzheimer-type dementia. Am J Psychiatr. 1985;142:638–660. doi: 10.1176/ajp.142.5.638. [DOI] [PubMed] [Google Scholar]
- 14.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 15.Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 16.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatr. 2002;159:738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 17.Small GW, Ercoli LM, Silverman DHS, Huang SC, Komo S, Bookheimer S, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996 Mar 21;334(12):752–8. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 21.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–7. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 22.Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2003;54:343–51. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- 23.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 24.Arnaiz E, Jelic V, Almkvist O, Wahlund L-O, Winblad B, Valind S, et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport. 2001;12:851–5. doi: 10.1097/00001756-200103260-00045. [DOI] [PubMed] [Google Scholar]
- 25.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–7. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 26.Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–13. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 27.Mosconi L, Sorbi S, Nacmias B, De Cristofaro MTR, Fayyazz M, Cellini E, et al. Brain metabolic differences between sporadic and familial Alzheimer’s disease. Neurol. 2003;61:1138–40. doi: 10.1212/01.wnl.0000086816.30011.75. [DOI] [PubMed] [Google Scholar]
- 28.Herholz K, Nordberg A, Salmon E, et al. Impairment of neocortical metabolism predicts progression to Alzheimer’s disease. Dement Geriatr Cogn Disord. 1999;10:494–504. doi: 10.1159/000017196. [DOI] [PubMed] [Google Scholar]
- 29.Herholz K. PET studies in dementia. Ann Nuc Med. 2003;17:79–89. doi: 10.1007/BF02988444. [DOI] [PubMed] [Google Scholar]
- 30.Albin RL, Minoshima S, D’Amato C, Frey KA, Kuhl DE, Sima AAF. Fluoro-Deoxyglucose Positron Emission Tomography in Diffuse Lewy Body Disease. Neurology. 1995;47:462–466. doi: 10.1212/wnl.47.2.462. [DOI] [PubMed] [Google Scholar]
- 31.Minoshima S, Foster NL, Sima AAF, Frey KA, Albin RL, Kuhl DE. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50:358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- 32.Higuchi M, Tashiro M, Arai H, et al. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp Neurol. 2000;162:247–256. doi: 10.1006/exnr.2000.7342. [DOI] [PubMed] [Google Scholar]
- 33.Salmon E, Garraux G, Delbueck X, et al. Predominant ventromedial frontopolar metabolic impairment in frontotemporal dementia. NeuroImage. 2003;20:435–440. doi: 10.1016/s1053-8119(03)00346-x. [DOI] [PubMed] [Google Scholar]
- 34.Diehl-Schmid J, Grimmer T, Drzezga A, et al. Decline of glucose cerebral metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging. 2007;28:42–50. doi: 10.1016/j.neurobiolaging.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improve accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130:2616–2635. doi: 10.1093/brain/awm177. [DOI] [PubMed] [Google Scholar]
- 36.Ishii K, Sakamoto S, Sasaki M, et al. Cerebral glucose metabolism in patients with frontotemporal dementia. J Nucl Med. 1998;39:1875–73. [PubMed] [Google Scholar]
- 37.Jeong Y, Cho SS, Park JM, et al. 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J Nucl Med. 2005;46:233–239. [PubMed] [Google Scholar]
- 38.Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weiner MW, Chui HC, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerrouche N, Herholz K, Mielke R, Holthoff V, Baron JC. 18FDG PET in vascular dementia: differentiation from Alzheimer’s disease using voxel-based multivariate analysis. J Cereb Blood Flow Metab. 2006;26:1213–1221. doi: 10.1038/sj.jcbfm.9600296. [DOI] [PubMed] [Google Scholar]
- 40.Piggott MA, Marshall EF, Thomas N, et al. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain. 1999;122:1449–1468. doi: 10.1093/brain/122.8.1449. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki, Desmond TJ, Albin RL, Frey KA. Striatal monoaminergic terminals in Lewy body dementia and Alzheimer disease. Ann Neurol. 2002;51:767–771. doi: 10.1002/ana.10186. [DOI] [PubMed] [Google Scholar]
- 42.Walker, Costa DC, Walker RWH, et al. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry. 2002;73:134–140. doi: 10.1136/jnnp.73.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilman S, Koeppe RA, Little R, et al. Striatal monoaminergic terminals in Lewy body dementia and Alzheimer’s disease. Ann Neurol. 2002;55:774–780. doi: 10.1002/ana.20088. [DOI] [PubMed] [Google Scholar]
- 44.McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, Padovani A, Giubbini R, Bonuccelli U, Volterrani D, Holmes C, Kemp P, Tabet N, Meyer I, Reininger C DLB Study Group. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–313. doi: 10.1016/S1474-4422(07)70057-1. [DOI] [PubMed] [Google Scholar]
- 45.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 46.Villemagne VL, Fodero-Tavoletti MT, Pike KE, et al. The ART of loss: Aβ imaging in the evaluation of Alzheimer’s disease and other dementias. Mol Neurobiol. 2008;38:1–15. doi: 10.1007/s12035-008-8019-y. [DOI] [PubMed] [Google Scholar]
- 47.Verhoeff NP, Wilson AA, Takeshita S, Trop L, Hussey D, Singh K, Kung HF, Kung MP, Houle S. In-vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 48.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 49.Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, Dekosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;11:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 50.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2007;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, Chetty S, Patel P, Pagliaro TA, Klunk WE, Mathis CA, Rosen HJ, Miller BL, Jagust WJ. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 52.Engler H, Santillo AF, Wang SX, Lindau M, Savitcheva I, Nordberg A, Lannfelt L, Langstrom B, Kilander L. In vivo amyloid imaging with PET in frontotemporal dementia. Eur J Nucl Med Mol Imaging. 2007;35:100–106. doi: 10.1007/s00259-007-0523-1. [DOI] [PubMed] [Google Scholar]
- 53.Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, et al. Imaging of amyloid plaques ad cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage. 2007;39:619–633. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 54.Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Mathis CA, Elmaleh DR, Shoup T, Fischman AJ, Hyman BT, Growdon JH, Johnson KA. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi L, Rinne J, Mosconi L, et al. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer’s disease. Eur J Nucl Mel Mol Imaging. 2008;35:2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy CS, Sherrington CS. On the regulation of the blood supply of the brain. J Physiol. 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sokoloff L. Relationships among local functional activity, energy metabolism, and blood flow in the central nervous system. Fed Proc. 1981;40:2311–2316. [PubMed] [Google Scholar]
- 58.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 59.Koeppe RA, Gilman S, Joshi A, Liu S, Little R, Junck L, Heumann M, Frey KA, Albin RL. [11C]DTBZ and [18F]FDG PET measures in differentiating dementias. J Nuc Med. 2005;46:936–944. [PubMed] [Google Scholar]
- 60.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer disease: report of NINCDS-ADRDAwork group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 61.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 62.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 63.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 64.Koeppe RA, Frey KA, Kume A, Albin R, Kilbourn MR, Kuhl DE. Equilibrium versus compartmental analysis for assessment of the vesicular monoamine transporter using (+)-alpha-[11C]dihydrotetrabenazine (DTBZ) and positron emission tomography. J Cereb Blood Flow Metab. 1997;17:919–931. doi: 10.1097/00004647-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Talariach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System—An Approach to Cerebral Imaging. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- 66.Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med. 1994;35:1528–1537. [PubMed] [Google Scholar]
- 67.Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr. 1995;19:541–547. doi: 10.1097/00004728-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- 69.Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology. 2007;69:871–877. doi: 10.1212/01.wnl.0000269790.05105.16. [DOI] [PubMed] [Google Scholar]
- 70.Silverman DHS. Brain 18F-FDG PET in the diagnosis of neurodegenerative dementias: comparison with perfusion SPECT and with clinical evaluations lacking nuclear imaging. J Nucl Med. 2004:594–607. [PubMed] [Google Scholar]
- 71.Silverman DH, Gambhir SS, Huang HW, Schwimmer J, Kim S, Small GW, Chodosh J, Czernin J, Phelps ME. Evaluating early dementia with and without assessment of regional cerebral metabolism by PET: a comparison of predicted costs and benefits. J Nucl Med. 2002:253–266. [PubMed] [Google Scholar]
- 72.Mosconi L, Tsui WH, Herholz K, et al. Multicenter, standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N, Coleman RE. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000;41:1920–1928. [PubMed] [Google Scholar]
- 74.McKeith IG, Ballard CG, Perry RH, Ince PG, O’Brien JT, Neill D, et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54:1050–1058. doi: 10.1212/wnl.54.5.1050. [DOI] [PubMed] [Google Scholar]
- 75.Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 76.Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, et al. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, Chetty S, Patel P, Pagliaro TA, Klunk WE, Mathis CA, Rosen HJ, Miller BL, Jagust WJ. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 78.Rinne JO, Laine M, Kaasinen V, Norvasuo-Heilä MK, Någren K, Helenius H. Striatal dopamine transporter and extrapyramidal symptoms in frontotemporal dementia. Neurology. 2002;58:1489–1493. doi: 10.1212/wnl.58.10.1489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


