Abstract
There is emerging evidence of abnormal expression in human tumors of microRNAs (miRNAs) which have been assigned oncogenic and/or tumor suppressor functions. While some miRNAs commonly exhibit altered expression across tumors, more often, different tumor types express unique patterns of miRNAs, referable to their tissues of origin. The role of miRNAs in tumorigenesis underscores their value as mechanism-based therapeutic targets in cancer. Similarly, unique patterns of altered microRNA expression provide fingerprints that may serve as molecular biomarkers for tumor diagnosis, classification, prognosis of disease-specific outcomes, and prediction of therapeutic responses.
Keywords: Micro RNA, biomarkers, cancer, diagnosis, prognosis, prediction, individualized therapy
Cancer is a leading cause of mortality in the United States, with ~25% of deaths attributable to neoplasia [1, 2]. Worldwide, cancer-related global mortality follows only cardiovascular and infectious diseases [3]. In this context of expanded incidence and growing prevalence, clinical oncology is poised for unprecedented innovation. Harnessing discoveries in disease pathobiology, increasingly propelled by the development of high-throughput technologies including genomics, proteomics and metabolomics, modern cancer biology offers previously unavailable diagnostic and therapeutic paradigms tailored to meet the needs of individuals and populations [4]. Transforming clinical management is predicated on translation of the new science into application of advanced markers and targets for personalized cancer prediction, prevention, diagnosis, and treatment [4–6].
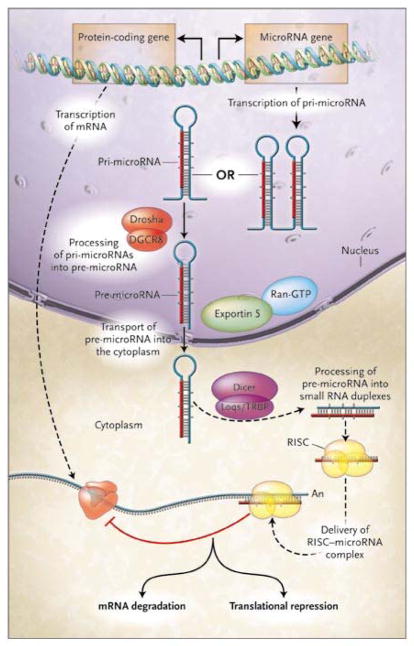
Indeed, the epigenetic, genetic, and post-genetic circuits restricting cell destiny are increasingly decoded, and their dysfunction linked to lineage-dependence underlying tumorigenesis [2, 7]. Critical in cell fate specification is post-transcriptional regulation of gene expression by microRNAs (Fig. 1) [8]. These molecules arise as transcripts from cognate genes in non-coding regions of chromosomes. MicroRNA undergo nuclear and cytoplasmic processing [8, 9] producing the targeting core of a multimeric complex hybridizing with mRNA molecules resulting in their sequestration or degradation, and thereby define the genes available for lineage commitment [10, 11]. This is the most recent addition to the hierarchical spectrum of molecular mechanisms defining nuclear-cytoplasmic information exchange [12], and forms the interface between transcriptional, translational, and post-translational regulation [13] Significantly, microRNAs represent a regulatory, rather than structural, mechanism that coordinates normal gene expression and whose dysregulation underlies neoplastic transformation [8, 10, 11].
Figure 1. MicroRNA generation and gene regulation [9].
Mature microRNAs of about 22 nucleotides originate from primary microRNA (pri-microRNA) transcripts. Nuclear pri-microRNAs of hundreds to thousands of base pairs are converted into stem-loop precursors (pre-microRNA) of about 70 nucleotides by Drosha, an RNase III endonuclease, and Pasha, a homologue of the human DiGeorge syndrome critical region gene 8 (DGCR8). Pre-microRNAs undergo cytoplasmic translocation mediated by exportin 5 in conjunction with Ran-GTP and subsequently processed into RNA duplexes of about 22 nucleotides by Dicer, an RNase III enzyme, and Loqacious (Loqs), a double-stranded RNA-binding–domain protein that is a homologue of the HIV transactivating response RNA-binding protein (TRBP). The functional strand of the microRNA duplex guides the RNA-induced silencing complex (RISC) to the mRNA target for translational repression or degradation. Figure reproduced from (9).
MicroRNAs and cancer
The essential nature of this novel mechanism indelibly patterning gene expression in cell lineage specification [8], in the context of the established model of cancer as a genetic disease in which pathobiology recapitulates cell and tissue ontogeny [14, 15], naturally implicates microRNAs in neoplastic transformation. In fact, altered microRNA expression is a defining trait of tumorigenesis [16, 17]. While the expression of some microRNAs are universally altered in tumors, more often unique patterns of microRNA expression reflect the lineage-dependence of tumors, referable to their tissues of origin [16–22]. Similarly, fundamental processes underlying tumorigenesis, including genomic instability, epigenetic dysregulation, and alterations in expression or function of regulatory proteins directly alter the complement of microRNAs expressed by cancer cells [8]. Additionally, microRNAs regulate key components integral to tumor initiation and progression, including tumor suppressors and oncogenes [8, 17, 23]. Further, microRNA signatures are a more informative source for classification of tumor taxonomy than genomic profiling [16]. Moreover, microRNAs can serve as unique targets for diagnostic imaging in vivo for taxonomic classification of tumors [24]. The emerging role of microRNAs in neoplasia highlights their potential value as mechanism-based therapeutic targets and biomarkers for diagnosis, prognosis of disease-specific outcomes, and prediction of therapeutic responses [25]. While there are numerous detailed reviews of this field, the purpose of this minireview is to provide, in overview, a summary of the potential application of microRNAs as diagnostic and therapeutic targets in cancer.
MicroRNAs as mechanism-based therapeutic targets in cancer
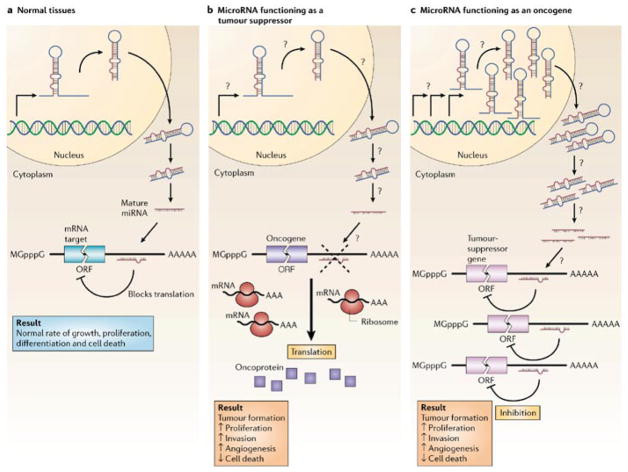
The case for microRNAs as tumor suppressors and oncogenes reflects their loss or gain, respectively, as a function of neoplastic transformation, their dysregulation in different tumors, the relevance of their mRNA targets to mechanisms underlying tumorigenesis, and their ability to directly alter tumorigenesis in model cells and organisms (Fig. 2; Table 1) [8, 26, 27]. Typically, microRNAs that serve as oncogenes are characterized by a gain of expression which inhibits levels of genes encoding tumor suppressors. Conversely, tumor suppressor microRNAs exhibit a loss of expression in cancer producing over-expression of transcripts encoded by oncogenes.
Figure 2. MicroRNA oncogenes and tumor suppressors [26].
a. Normally, microRNA (miRNA) binding to target mRNA represses gene expression by blocking protein translation or inducing mRNA degradation, contributing to homeostasis of growth, proliferation, differentiation and apoptosis. b. Reduced miRNA levels, reflecting defects at any stage of mirRNA biogenesis (indicated by question marks), produce inappropriate expression of target oncoproteins (purple squares). The resulting defects in homeostasis increase proliferation, invasiveness or angiogenesis or decrease levels o f apoptosis or differentiation, potentiating tumor formation. c. Conversely, overexpression of an oncogenic miRNA eliminates the expression of tumor-suppressor genes (pink), leading to cancer progression. Increased levels of mature miRNA could reflect amplification of the miRNA gene, a constitutively active promoter, increased efficiency in miRNA processing or increased stability of the miRNA (indicated by question marks). ORF, open reading frame. Figure reproduced from (26).
Table 1.
MicroRNAs in tumorigenesis
| MicroRNA | Gene Locus | Tumor Types | Gene Targets | Ref |
|---|---|---|---|---|
|
Suppressors
| ||||
| mir-15a, 16-1 | 13q14 | CLL, prostate, mantle cell lymphoma, multiple myeloma | BCL-2 | 28–32 |
| let-7 | 8 clusters | lung, gastric | RAS | 22, 23, 26, 34, 35 |
|
| ||||
|
Oncogenes
| ||||
| mir-17 cluster | 13q31–32 | B-CLL, follicular lymphoma, mantle cell lymphoma, cutaneous B cell lymphoma, colon, lung, breast, pancreas, prostate | PTEN TGFβ RII E2F1 |
17, 36–38, 40–43 |
| mir-21 | 17q23.2 | breast, colon, lung, prostate, gastric, endocrine pancreas, glioblastomas, leiomyomas | PTEN BCL-2 Tropomyosin I |
17, 44–50, 54 |
MicroRNA Tumor Suppressors (Fig. 2, Table 1)
The best characterized tumor suppressor microRNAs are miR-15a and miR-16-1. B cell chronic lymphocytic leukemia (CLL) is the most common adult leukemia in developed countries and universally associated with loss of chromosomal region 13q14 [8, 27, 28]. Within this deletion is a ~30 kb region in which resides miR-15a and miR-16-1, which are lost in ~70% of patients with CLL [29]. Similarly, loss of chromosomal region 13q14, including miR-15a and miR-16-1, occurs in prostate cancer, mantle cell lymphoma, and multiple myeloma [29, 30]. Tumor suppression by miR-15a and miR-16-1, in part, reflects inhibition of the expression of the anti-apoptotic oncogenic protein Bcl-2, which is characteristically over-expressed in CLL, promoting survival of leukemia cells [31]. Indeed, there is a reciprocal relationship between expression of miR-15a and miR-16-1 and Bcl-2, and heterologous expression of these microRNAs suppresses Bcl-2 levels [32]. Suppression is specifically mediated by complimentary binding sites for those microRNAs in the 3′ untranslated region of the Bcl-2 transcript [32]. Further, heterologous expression of miR-15a and miR-16-1 produces apoptosis in leukemia cell lines [32]. Moreover, mouse models of spontaneous CLL possess a mutation in the 3′ untranslated region of miR-16-1, identical to mutations in patients with CLL, associated with decreased expression of that microRNA [33]. Heterologous expression of miR-16-1 in CLL cells derived from those mice alters the cell cycle, proliferation and apoptosis of these tumor cells [33].
The microRNA let-7, a phylogenetically conserved gene product that regulates the transition of cells from proliferation to differentiation in invertebrates [34], also serves as a tumor suppressor [27]. There are twelve let-7 homologs in humans forming eight distinct clusters of which four are localized to chromosomal regions lost in many malignancies [35]. In that context, downregulation of let-7 family members in lung cancer is associated with poor prognosis [22]. A role for these microRNAs in growth regulation and the expression of the tumorigenic phenotype is highlighted by the ability of heterologous let-7 expression in lung cancer cells in vitro to inhibit colony formation [36]. Key downstream targets for let-7 include the human Ras family of proteins, oncogenes that are commonly mutated in many human tumors [23]. Indeed, KRas and NRas expression in human cells is regulated by let-7 family members [27]. Moreover, loss of let-7 expression in human tumors correlates with over-expression of Ras proteins [23].
MicroRNA Oncogenes (Fig. 2, Table 1)
The miR-17 cluster comprises a group of six miRNAs (miR-17–5p, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92) at 13q31–32, a chromosomal region amplified in large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma and primary cutaneous B-cell lymphoma [37]. Consistent with their functions as oncogenes, over-expression of this microRNA cluster is associated with amplification of the 13q31–32 genomic region in lymphoma cells in vitro [37, 38]. These miRNAs are over-expressed in many types of tumors, including lymphoma, colon, lung, breast, pancreas and prostate [17, 38, 39]. Interestingly, expression of the miR-17 cluster is induced by c-Myc, an oncogene over-expressed in many tumors. Heterologous expression of c-Myc up-regulates expression of the miR-17 cluster, mediated by direct binding of that transcription factor to the chromosomal region harboring those microRNAs [40]. In turn, the miR-17 cluster appears to regulate several downstream oncogene targets. Thus, miR-19a and miR-19b may regulate phosphatase and tensin homolog (PTEN), a tumor suppressor with a broad mechanistic role in human tumorigenesis, through interactions with complimentary sites in the 3′ untranslated region of this transcript [41]. Similarly, miR-20a may reduce the expression of transforming growth factor (TGF)-β receptor II (TGFBR2), a tumor suppressor frequently mutated in cancer cells which regulates the cell cycle, imposing growth inhibition [17]. The best characterized target of the miR-17 cluster is the E2F1 transcription factor whose expression is regulated by miR-17–5p and miR-20a [42]. In turn, E2F1 regulates cell cycle progression by inducing genes mediating DNA replication and cell cycle control [43]. Beyond the regulation of key targets contributing to transformation, the miR-17 cluster directly induces the tumorigenic phenotype. Heterologous expression of the miR-17 cluster increased proliferation in lung cancer cells in vitro [39]. Moreover, components of this cluster accelerate the process of lymphomagenesis in mice [44].
The microRNA miR-21 is over-expressed in many solid tumors including breast, colon, lung, prostate, stomach, and endocrine pancreas tumors, glioblastomas, and uterine leiomyomas [17, 45–47]. This microRNA is encoded at chromosome 17q23.2, a genetic locus which is frequently amplified in many tumors. The tumorigenic effects of miR-21 are mediated, in part, by targeting a number of mediators in critical cell survival pathways. Thus, in glioblastoma cells in vitro miR-21 modulates the expression of the common tumor suppressor PTEN, a central regulator of cell growth, proliferation, and survival mediated by the PI3 kinase-Akt pathway [48]. Also, miR-21 regulates breast cancer cell growth by reciprocally regulating apoptosis and proliferation, in part reflecting regulation of the anti-apoptotic protein Bcl-2 [49]. Moreover, miR-21 controls expression of the tumor suppressor tropomyosin 1 whose over-expression in breast cancer cells suppresses anchorage-independent growth [50]. Beyond signaling analyses, elimination of miR-21 expression in glioblastoma cells induces caspase-dependent apoptosis, underscoring the importance of this microRNA in mediating the survival phenotype [51]. Similarly, antisense oligonucleotides to miR-21 suppressed the growth of breast cancer cells in vitro and in xenografts in mice [48].
MicroRNAs as biomarkers in cancer
Their fundamental role in development and differentiation, and their pervasive corruption in lineage-dependent mechanisms underlying tumorigenesis suggest that microRNAs may be a particularly rich source of diagnostic, prognostic and predictive information as biomarkers in cancer [8, 26, 52]. Differential expression of microRNAs compared to their normal adjacent tissue counterparts is a characteristic of every type of tumor examined to date [8, 52], a feature that could be particularly useful in diagnosing incident cancers in otherwise normal tissues. Indeed, this approach discriminates normal and neoplastic tissues in various cancer types, including CLL, breast cancer, glioblastoma, thyroid papillary carcinoma, hepatocellular carcinoma, lung cancer, colon cancer and endocrine pancreatic tumors [8, 17–22, 26, 45, 52–54]. Similarly, microRNA expression profiles provide a powerful source of molecular taxonomic information, with an accuracy for classifying tumors according to their developmental lineage and differentiation state that surpasses mRNA expression profiling [16, 17]. These observations suggest the utility of microRNA expression profiling for identifying metastatic tumors of unknown origin, which represent ~5% of all malignancies worldwide [16, 17, 52]. Also, differential microRNA expression patterns are associated with disease prognosis [8, 52]. Specific patterns of microRNA expression identified patients with pancreatic cancer who survived >24 months, compared to those who survived <24 months [53]. In addition, the expression of specific microRNAs predicted overall poor survival in patients with pancreatic cancer [53]. Similarly, over-expression of specific microRNAs was an independent prognostic variable associated with advanced disease stage and decreased survival in patients with colon cancer [54]. Beyond diagnosis and prognosis, microRNA expression patterns predict responses to therapy, and over-expression of oncogenic microRNAs was associated with improved survival following adjuvant chemotherapy in patients with colon cancer [54]. These observations highlight the potential of microRNAs as biomarkers for diagnosis, taxonomic classification, prognosis, risk stratification, and prediction of therapeutic responses in patients with cancer.
Corruption of microRNA expression in cancer
The genetic basis of cancer, in part, reflects chromosomal rearrangements encompassing translocations, deletions, amplifications, and exogenous episomal integrations which alter gene expression. The essential role of microRNAs in tumorigenesis predicts coincidence between the location of their encoding genes and those cancer-associated chromosomal regions. Indeed, more than half of microRNA genes are located in cancer-associated genomic regions in a wide array of tumors including lung, breast, ovarian, colon, gastric, liver, leukemia, and lymphoma [28, 35]. Conversely, chromosomal regions harboring microRNAs are sites of frequent genomic alterations involved in cancer [28, 55]. Additionally, the impact of chromosomal remodeling on gene copy number directly translates to altered microRNA expression [19, 28, 55]. Beyond structural reorganization, epigenetic remodeling of chromosomal regions harboring microRNA loci contributes to transformation, and tumor-suppressing microRNAs silenced by CpG island hypermethylation result in dysregulation of essential proteins accelerating the cell cycle, including cyclin D and retinoblastoma [56, 57]. Moreover, alterations in the machinery responsible for processing microRNA contributes to tumorigenesis, and impairment of Dicer enhances lung tumor development in experimental mouse models and is associated with poor prognosis in patients with lung cancer [58–60].
Therapeutic targeting of microRNAs
The causal role of microRNAs in molecular mechanisms underlying transformation and the contribution of specific microRNA species to lineage-dependent tumorigenesis suggest that these molecules could serve as therapeutic targets to prevent and treat cancer [61]. In the context of established therapeutic paradigms in medical oncology, individualized therapy with microRNAs could re-establish the expression of silenced microRNA tumor suppressors, while antisense oligonucleotides could silence over-expressed oncogenic microRNAs [8, 28, 52, 61]. Indeed, antisense oligonucleotides targeted to microRNA sequences (AMOs) with modified RNA backbone chemistry resistant to nuclease degradation irreversibly eliminates over-expression of oncogenic microRNAs [61]. Similarly, locked nucleic acid analogs resist degradation and stabilize the microRNA target-antisense duplex required for silencing [62]. Moreover, single stranded RNA molecules complimentary to oncogenic microRNAs, termed antagomirs, silence microRNA expression in mouse models in vivo [63]. The specificity of targeting inherent in nucleic acid base complimentarity coupled with their mechanistic role in neoplastic transformation make microRNAs attractive therapeutic targets for future translation.
Summary
MicroRNAs represent one fundamental element of the integrated regulation of gene expression underlying nuclear-cytoplasmic communication. Disruption of these regulatory components in processes underlying tumor initiation and promotion contributes to the genetic basis of neoplasia. Beyond molecular mechanisms underlying pathophysiology that constitute therapeutic targets, unique patterns of microRNA expression characterizing lineage dependent tumorigenesis offer unique opportunities to develop biomarkers for diagnostic, prognostic, and predictive management of cancer. These novel discoveries are positioned to launch a transformative continuum linking innovation to patient management. Advancement of these novel paradigm-shifting concepts into patient application will proceed through development and regulatory approval to establish the evidence basis for integration of microRNA-based diagnostics and therapeutics into clinical practice
Acknowledgments
Authors are supported by grants from the NIH (SAW, AT), Targeted Diagnostic and Therapeutics, Inc (SAW), and the Marriott Foundation (AT). SAW is the Samuel M.V. Hamilton Endowed Professor of Thomas Jefferson University. AT is the Marriott Family Professor of Cardiovascular Research at the Mayo Clinic. SAW is a paid consultant to Merck.
Contributor Information
Scott A. Waldman, Email: scott.waldman@jefferson.edu.
Andre Terzic, Email: terzic.andre@mayo.edu.
References
- 1.Cancer statistics 2006. American Cancer Society; 2006. [Google Scholar]
- 2.Dalton WS, Friend SH. Cancer biomarkers--an invitation to the table. Science. 2006;312:1165–1168. doi: 10.1126/science.1125948. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson C, Schulz S, Waldman SA. Biomarker development, commercialization, and regulation: individualization of medicine lost in translation. Clin Pharmacol Ther. 2007;81:153–155. doi: 10.1038/sj.clpt.6100088. [DOI] [PubMed] [Google Scholar]
- 5.Wagner J, Williams S, Webster C. Biomarkers and surrogate endpoints for development and regulatory evaluation of new drugs. Clinical Pharmacology & Therapeutics. 2007;81:104–107. doi: 10.1038/sj.clpt.6100017. [DOI] [PubMed] [Google Scholar]
- 6.Waldman SA, Terzic MR, Terzic A. Molecular medicine hones therapeutic arts to science. Clin Pharmacol Ther. 2007;82:343–347. doi: 10.1038/sj.clpt.6100360. [DOI] [PubMed] [Google Scholar]
- 7.Garraway LA, Sellers WR. Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer. 2006;6:593–602. doi: 10.1038/nrc1947. [DOI] [PubMed] [Google Scholar]
- 8.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 9.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 10.Harfe BD. MicroRNAs in vertebrate development. Curr Opin Genet Dev. 2005;15:410–415. doi: 10.1016/j.gde.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Pasquinelli AE, Hunter S, Bracht J. MicroRNAs: a developing story. Curr Opin Genet Dev. 2005;15:200–205. doi: 10.1016/j.gde.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Faustino RS, Nelson TJ, Terzic A, Perez-Terzic C. Nuclear transport: target for therapy. Clin Pharmacol Ther. 2007;81:880–886. doi: 10.1038/sj.clpt.6100141. [DOI] [PubMed] [Google Scholar]
- 13.Waldman SA, Terzic A. MicroRNA signatures as diagnostic and therapeutic targets. Clin Chem. 2008;54:943–944. doi: 10.1373/clinchem.2008.105353. [DOI] [PubMed] [Google Scholar]
- 14.Bishop JM. Molecular themes in oncogenesis. Cell. 1991;64:235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg RA. Tumor suppressor genes. Science. 1991;254:1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 17.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos RT, Croce CM, de la Chapelle A. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 20.Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–2545. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 21.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, Croce CM. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 22.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Hwang DW, Lee DS. MicroRNA: Bioimaging of microRNA biogenesis and regulation. FEBS Journal. 2009 doi: 10.1111/j.1742-4658.2009.06935.x. This issue. [DOI] [PubMed] [Google Scholar]
- 25.Waldman SA, Terzic A. Translating MicroRNA discovery into clinical biomarkers in cancer. JAMA. 2007;297:1923–1925. doi: 10.1001/jama.297.17.1923. [DOI] [PubMed] [Google Scholar]
- 26.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 27.Kent OA, Mendell JT. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene. 2006;25:6188–6196. doi: 10.1038/sj.onc.1209913. [DOI] [PubMed] [Google Scholar]
- 28.Calin GA, Croce CM. Chromosomal rearrangements and microRNAs: a new cancer link with clinical implications. J Clin Invest. 2007;117:2059–2066. doi: 10.1172/JCI32577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong JT, Boyd JC, Frierson HF., Jr Loss of heterozygosity at 13q14 and 13q21 in high grade, high stage prostate cancer. Prostate. 2001;49:166–171. doi: 10.1002/pros.1131. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Beato M, Sanchez-Aguilera A, Piris MA. Cell cycle deregulation in B-cell lymphomas. Blood. 2003;101:1220–1235. doi: 10.1182/blood-2002-07-2009. [DOI] [PubMed] [Google Scholar]
- 32.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raveche ES, Salerno E, Scaglione BJ, Manohar V, Abbasi F, Lin YC, Fredrickson T, Landgraf P, Ramachandra S, Huppi K, Toro JR, Zenger VE, Metcalf RA, Marti GE. Abnormal microRNA-16 locus with synteny to human 13q14 linked to CLL in NZB mice. Blood. 2007;109:5079–5086. doi: 10.1182/blood-2007-02-071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 35.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 37.Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, Yoshida Y, Seto M. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 38.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17–92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 40.Pajic A, Spitkovsky D, Christoph B, Kempkes B, Schuhmacher M, Staege MS, Brielmeier M, Ellwart J, Kohlhuber F, Bornkamm GW, Polack A, Eick D. Cell cycle activation by c-myc in a burkitt lymphoma model cell line. Int J Cancer. 2000;87:787–793. doi: 10.1002/1097-0215(20000915)87:6<787::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc- regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 43.Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Hemann MT, Fridman JS, Zilfou JT, Hernando E, Paddison PJ, Cordon-Cardo C, Hannon GJ, Lowe SW. An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat Genet. 2003;33:396–400. doi: 10.1038/ng1091. [DOI] [PubMed] [Google Scholar]
- 45.Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM, Farace MG. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–1358. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 46.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 48.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 49.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 50.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 51.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 52.Fabbri M, Croce CM, Calin GA. MicroRNAs. Cancer J. 2008;14:1–6. doi: 10.1097/PPO.0b013e318164145e. [DOI] [PubMed] [Google Scholar]
- 53.Bloomston M, Frankel W, Ptrocca F, Volinia S, Alder H, Liu C, Bhat D, Taccioli C, Jenkins H, Croce C. MicroRNA expression patterns differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007 doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 54.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, Liu CG, Calin GA, Croce CM, Harris CC. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O’Brien-Jenkins A, Katsaros D, Hatzigeorgiou A, Gimotty PA, Weber BL, Coukos G. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci U S A. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setien F, Casado S, Suarez-Gauthier A, Sanchez-Cespedes M, Git A, Spiteri I, Das PP, Caldas C, Miska E, Esteller M. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- 57.Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 58.Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karube Y, Tanaka H, Osada H, Tomida S, Tatematsu Y, Yanagisawa K, Yatabe Y, Takamizawa J, Miyoshi S, Mitsudomi T, Takahashi T. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–115. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 61.Weiler J, Hunziker J, Hall J. Anti-miRNA oligonucleotides (AMOs): ammunition to target miRNAs implicated in human disease? Gene Ther. 2006;13:496–502. doi: 10.1038/sj.gt.3302654. [DOI] [PubMed] [Google Scholar]
- 62.Naguibneva I, Ameyar-Zazoua M, Nonne N, Polesskaya A, Ait-Si-Ali S, Groisman R, Souidi M, Pritchard LL, Harel-Bellan A. An LNA-based loss-of-function assay for micro-RNAs. Biomed Pharmacother. 2006;60:633–638. doi: 10.1016/j.biopha.2006.07.078. [DOI] [PubMed] [Google Scholar]
- 63.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]