“Man should always strive to have his intestines relaxed all the days [of his life] and that [bowel function] should approximate diarrhea. This is a fundamental principle in medicine, [namely] whenever the stool is withheld or is extruded with difficulty, grave illnesses result.”
Moses ben Maimon (Maimonides)
Chronic constipation is one of the most common sources of morbidity affecting health care expenditures and quality of life in the developed world.1 In striking contrast, diarrheal disease continues to be a leading cause of morbidity and mortality in under-developed countries.2 In a metamorphosis from pathogenetic mechanism to therapeutic paradigm, linaclotide, a first-in-class 14 amino acid peptide homologous to bacterial heat-stable enterotoxins (STs; Fig. 1A) contributing to global endemic diarrhea, is emerging as a promising agent to treat chronic constipation. In this issue of Gastroenterology, a phase IIb U.S. multi-center, double-blind, placebo-controlled trial, revealed that linaclotide was well-tolerated and improved symptoms, bowel function and disease-relevant quality-of-life measures in patients with chronic constipation.1
Figure 1.
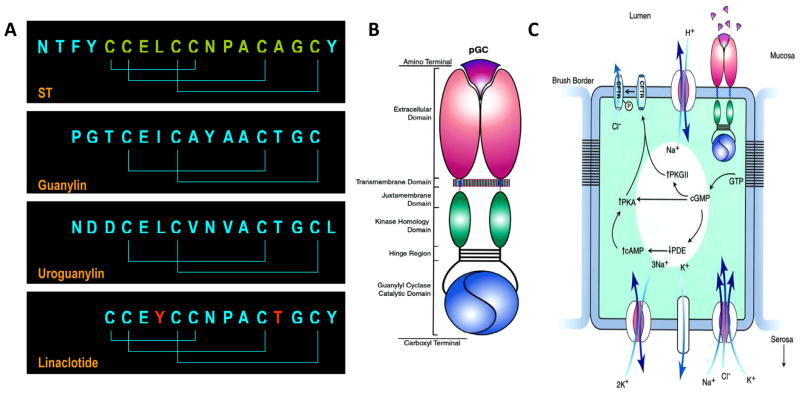
Guanylyl cyclase C ligands and intestinal secretion. (A) Homologous peptide ligands that activate GCC include the diarrheagenic bacterial heat-stable enterotoxins (ST) and the paracrine hormones guanylin, produced in the colon, and uroguanylin, produced in the small intestine. Linaclotide is a substituted (red residues) ST analog preserving the 3 disulfide bonds delimiting the 13 amino acid biologically active core ST peptide (green) required for maximum activity and stability. (B) GCC is one isoform of the seven member (GCA - GCG) subfamily of membrane-bound particulate guanylyl cyclases (pGC) which are homo-oligomers comprising extracellular ligand binding, short transmembrane, cytoplasmic juxtamembrane, regulatory kinase homology, oligomerization (hinge), and catalytic domains. (C) Regulation of intestinal secretion by GCC.6 Cognate peptides in the intestinal lumen ligate the extracellular domain of GCC, expressed in brush border membranes of epithelial cells. Ligand-receptor interaction is translated across the plasma membrane into activation of the cytoplasmic catalytic domain producing accumulation of intracellular cyclic GMP. This cyclic nucleotide directly activates cyclic GMP or cyclic AMP-dependent protein kinase (PKG or PKA, respectively). Also, cyclic GMP inhibits cyclic AMP-specific phosphodiesterase (PDE), increasing intracellular cyclic AMP concentrations, which can activate PKA. The ultimate result of cyclic GMP accumulation is phosphorylation of the cystic fibrosis transmembrane conductance regulator (CFTR) which is co-localized with GCC in brush border membranes. CFTR is a chloride channel and its phosphorylation by protein kinase results in a persistent open state, permitting chloride to flow down a concentration gradient from intracellular to the extracellular compartments. Other ion channels and transporters maintain electroneutrality of cyclic GMP-dependent chloride efflux. Vectoral water flux from basolateral to the apical membranes is driven by these ionic conductances, resulting in accumulation of fluid and electrolytes in the intestine and, in the case of STs, secretory diarrhea.
STs, and by extension linaclotide, exemplify molecular mimicry wherein pathogenic bacteria enhance survival strategies by exploiting host physiological systems through convergent evolution. STs are 3-disulfide homologs of the endogenous 2-disulfide paracrine hormones uroguanylin in the small intestine and guanylin in the colon (Fig. 1A).3, 4 The 3-disulfide structure remarkably enhances potency for their only identified receptor, guanylyl cyclase C (GCC; Fig. 1B), one isoform of the larger family of guanylyl cyclases that includes receptors for nitric oxide and natriuretic peptides.5, 6 In contrast to those other receptors, which are broadly expressed in almost all tissues, GCC is selectively expressed in brush border membranes of intestinal mucosa cells from the duodenum to the rectum.6 Ligation of the extracellular binding domain of GCC activates the intracellular catalytic domain, converting GTP to cyclic guanosine monophosphate (cyclic GMP), inducing downstream effectors that phosphorylate the cystic fibrosis transmembrane conductance regulator (CFTR; Fig. 1C).6 This phosphorylation-dependent activation opens the CFTR chloride channel, producing a net efflux of ions and water into the intestinal lumen. Regulation by endogenous paracrine hormones presumably coordinates intestinal fluid and electrolyte homeostasis.7 In contrast, local nutrient depletion by colonizing enterotoxigenic bacteria engages a survival program triggering synthesis and secretion of STs,8 which produces diarrhea, facilitating dissemination of pathogenic organisms to new resource-rich hosts9.
Linaclotide exploits these unique (patho)physiological mechanisms, offering a previously unanticipated pro-secretory therapeutic solution for chronic constipation. Linaclotide is a substituted homolog of STs, incorporating the 3 disulfide bonds characterizing the toxin structure producing maximum potency.5, 10, 11 Amino acid substitutions enhance pharmacokinetic stability by producing a 13 amino acid core peptide (Fig. 1A) that retains maximal biological activity while resisting proteolysis.10, 11 Moreover, linaclotide is a tissue-specific therapeutic, targeted to GCC which is selectively expressed in intestine,6 with minimal oral bioavailability to the systemic compartment,10 eliminating mechanism (GCC)-independent and off-target (extra-intestinal) adverse effects.
In a phase IIa placebo-controlled study of 2 weeks duration, linaclotide improved symptoms and intestinal transit in patients with chronic constipation.12 In the present phase IIb trial, 310 patients with chronic constipation were randomized to placebo or 75, 150, 300 or 600 mcg of linaclotide once daily for 4 weeks. Trial design considered the Food and Drug Administration’s (FDA’s) recent recommendations to transition from global (e.g., overall relief) to symptom-based primary endpoints.13 Thus, endpoints here included spontaneous bowel movements (SBMs), which are not preceded by a laxative, enema, or suppository in the prior 24 hours, and complete spontaneous bowel movements (CSBMs), which are accompanied by the subjective sensation of complete evacuation, underscoring the contribution of features other than stool frequency to satisfaction with bowel habits. Linaclotide responders were defined as patients who had ≥3 SBMs or CSBMs weekly and an increase of ≥1 weekly units relative to baseline.
In this multi-dose study, linaclotide produced durable dose-dependent increases in the mean weekly rate of SBMs across the 4-week treatment interval, with maximum improvements of ~1.75-fold at the 600 mcg dose compared with placebo. Similar increases in the mean weekly rate of CSBMs were observed, although with a more modest dose-dependence. Improved intestinal transit was associated with a more favorable stool consistency and decreased straining at defecation, presumably reflecting linaclotide’s primary pharmacological mechanism of increasing secretion and stool water content. In the context of these functional enhancements in intestinal transit, linaclotide achieved the primary study endpoints, improving the percent of patients responding with SBMs (~2-fold) and CSBMs (a maximum of ~4-fold). Objective improvements in therapeutic endpoints were associated with enhanced subjective attributes of bowel function, including abdominal discomfort, bloating, constipation severity, and global constipation relief. Moreover, subjective improvements in bowel function were linked to enhanced quality-of-life indicators for constipation. Diarrhea, the principle mechanism-based adverse event, affecting ~14% of patients at the 600 mcg dose, was generally mild and did not require discontinuation of therapy.
These observations suggest that linaclotide is positioned to become a new addition to the armamentarium of agents available to treat chronic constipation. However, it is noteworthy that fewer than 35% of patients with chronic constipation responded to linaclotide with increased SBMs and CSBMs.1 While the precise mechanisms underlying this substantial heterogeneity in therapeutic efficacy of GCC ligands in constipation remain to be defined, contributions from variations in pathophysiology, pharmacokinetics and pharmacodynamics deserve consideration. For example, anorectal manometry and colonic transit were not evaluated as part of study entry criteria to exclude patients with a pathophysiological basis for constipation that might be refractory to pro-secretory agents. Indeed, some non-responders may reflect, respectively, chronic constipation due to defecatory disorders, which may be more appropriately treated with biofeedback, or slow transit constipation secondary to colonic motor dysfunction, which may respond better to colonic prokinetic agents.14, 15 Moreover, colonic motor dysfunction could blunt or eliminate therapeutic responses to linaclotide, reflecting the prodigious organ-specific capacity for water reabsorption in, coupled with prolonged transit through, the colon in that disorder.16 These considerations suggest that the dose-dependent effects of secretagogues like linaclotide may substantially vary in the context of slow transit and normal transit constipation. They suggest that future studies may benefit from stratifying patients based on the pathophysiological mechanisms underlying chronic constipation, to better identify those who may be optimally responsive to pro-secretory agents like GCC ligands. The therapeutic efficacy of GCC ligands also is directly related to the pharmacokinetic stability defining mean residence time of the active peptide at the site of action in the intestinal lumen. The 14 amino acid biologically active parent drug, linaclotide, has a half-life of ~3 min in intestine reflecting proteolysis by carboxypeptidase A, which removes the C-terminal tyrosine residue, producing the core 13 amino acid 3 disulfide pharmacophore which retains full biological activity (Fig. 1A).11 In turn, this active metabolite has a half-life in intestine of ~10 min.11 While the precise metabolic sequence underlying elimination of the active metabolite from the intestinal lumen remains to be confirmed, peptide degradation appears to occur in two phases.11 In the unfolding phase, the core 13 amino acid pharmacophore undergoes enzymatic reduction of the 3 disulfide bonds by free lumenal glutaredoxin, employing lumenal glutathione as a source of reducing equivalents, requiring robust regeneration of reduced glutathione by free lumenal glutathione reductase to support peptide unfolding. In the degradation phase, the reduced and unfolded peptide undergoes complete proteolytic elimination by pancreatin. Like many other therapeutic agents, heterogeneity in responses to linaclotide could reflect pharmacogenetic variability in the expression or activity of enzymes mediating metabolism and elimination of the pharmacophore. In the future, linaclotide non-responders may be identified as “rapid eliminators” based on metabolic phenotyping or genotyping of polymorphisms producing over-expression or hyperactivity of key enzymes shaping peptide absorption, distribution, metabolism and excretion.
Beyond pharmacokinetics, the therapeutic efficacy of linaclotide reflects the machinery in epithelial cells available to transmit signals mediating secretory responses. The proximal component initiating this cascade, GCC (Fig. 1C), exhibits an established temporal gradient of expression, greatest during infancy and decreasing with age thereafter.17 Moreover, there is 10- to 100-fold inter-individual variability in GCC mRNA expression in normal intestinal epithelial cells across the population (Waldman, unpublished data). These observations suggest that differences in responses to linaclotide could reflect inter-individual variability in the expression of GCC and, by extension, the downstream effectors of cyclic GMP signaling (Fig. 1C). Studies of linaclotide may benefit from analyzing response rates stratified by age, to indirectly assess the possible contribution of decreased GCC expression to heterogeneity in therapeutic sensitivity. As with pharmacokinetic variability, future studies may identify linaclotide-sensitive patients based on phenotypic or pharmacogenetic tests that quantify the integrity and capacity of GCC-dependent signaling circuits mediating intestinal secretory responses.
Linaclotide represents the first in a new class of pro-secretory agents exploiting mechanistic insights in the molecular pathophysiology of enterotoxigenic diarrhea to target GCC, increase intestinal water secretion, and improve bowel habits in chronic constipation and constipation-dominant irritable bowel syndrome. Further development of linaclotide and other agents in this class will focus on their utility across the spectrum of constipation disorders; therapeutic individualization to ensure that only patients responsive to GCC-targeted agents receive treatment; and evolving treatment strategies to provide durable long-term normalization of bowel function while minimizing adverse effects. In the context of this latter therapeutic objective, it is notable that lower doses of linaclotide, 133 and 266 mcg, were used in follow-on 12 week phase III trials in chronic constipation,18 presumably reflecting maximum improvement in objective and subjective measures of bowel function with minimum induction of diarrhea at comparable doses in the present study1.
Ultimately, GCC-targeted agents will assume a position on formularies with other emerging molecularly directed therapies for constipation, including the pro-kinetic serotoninergic 5-HT4 receptor agonist prucalopride,19–21 also in clinical development, and the FDA-approved pro-secretory CIC-2 channel agonist lubiprostone22. The challenge for the future will be to integrate these targeted agents with established approaches directing bulk water movement, including fiber supplementation and osmotic laxatives, and intestinal transit, including timed evacuation with stimulant suppositories, to define cost-effective therapeutic algorithms for chronic constipation.23–25 The precise sequencing of these therapies, and whether osmotic agents which may be more universally effective across broad populations of patients, precede molecularly targeted therapies in a stepped care approach, await the evidence base established by prospective trials. Similarly, the therapeutic synergy that could be achieved by co-administration of agents targeting different molecular mechanisms remains to be defined. Indeed, patients with colonic motor dysfunction might derive benefit from both a pro-secretory agent, like linaclotide or lubiprostone, that increases stool water content, and a pro-kinetic agent, like prucalopride, that increases transit through the colon minimizing the reabsorption of stool water antagonizing the actions of pro-secretory agents.
Linaclotide, and other GCC-targeted agents,26 expand the spectrum of molecularly based therapeutic options available to treat chronic constipation disorders. In the context of the emerging paradigm of individualized medicine, the objective to provide life-long relief will be met by matching the right combination of agents at the right doses with the right patient, to maximize therapeutic efficacy, safety, and cost-effectiveness.27
Acknowledgments
Support was provided by NIH grants P01 DK068055 and RO1 DK 78924 (AEB) and R01 CA75123, R01 CA95026, RC1 CA146033, and P30 CA56036 (SAW). SAW is the Samuel M.V. Hamilton Professor.
Footnotes
Conflicts of Interest. SAW is a consultant to Merck and CombiMab, Inc., the Chair of the Data Safety Monitoring Board for the C-Cure Trial™ sponsored by Cardio3 Biosciences; and the Chair (uncompensated) of the Scientific Advisory Board to Targeted Diagnostics and Therapeutics, Inc., from which he also receives research support. AEB reports no conflicts.
References
- 1.Lembo AJ, Kurtz CR, MacDougall JE, Lavins BJ, Currie MG, Fitch DA, Jeglinski BI, Johnston JM. Linaclotide is effective for patients with chronic constipation. Gastroenterology. 2010 doi: 10.1053/j.gastro.2009.12.050. In press. [DOI] [PubMed] [Google Scholar]
- 2.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bulletin of the World Health Organization. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 3.Forte LR. Guanylin regulatory peptides: structures, biological activities mediated by cyclic GMP and pathobiology. Regul Pept. 1999;81:25–39. doi: 10.1016/s0167-0115(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 4.Hamra FK, Eber SL, Chin DT, Currie MG, Forte LR. Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc Natl Acad Sci U S A. 1997;94:2705–10. doi: 10.1073/pnas.94.6.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpick BW, Gariepy J. The Escherichia coli heat-stable enterotoxin is a long-lived superagonist of guanylin. Infect Immun. 1993;61:4710–5. doi: 10.1128/iai.61.11.4710-4715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 7.Forte LR., Jr Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther. 2004;104:137–62. doi: 10.1016/j.pharmthera.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Alderete JF, Robertson DC. Repression of heat-stable enterotoxin synthesis in enterotoxigenic Escherichia coli. Infect Immun. 1977;17:629–33. doi: 10.1128/iai.17.3.629-633.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guttman JA, Finlay BB. Subcellular alterations that lead to diarrhea during bacterial pathogenesis. Trends Microbiol. 2008;16:535–42. doi: 10.1016/j.tim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Harris LA, Crowell MD. Linaclotide, a new direction in the treatment of irritable bowel syndrome and chronic constipation. Curr Opin Mol Ther. 2007;9:403–10. [PubMed] [Google Scholar]
- 11.Kessler M, Busby R, Wakefield J, Bartolini W, Bryant A, Tobin J, Cordero E, Fretzen A, Kurtz C, Currie M. Rat intestinal metabolism of linaclotide, a therapeutic agent in clinical development for the treatment of IBS-C and chronic constipation. 15th North American ISSX Meeting; San Diego. 2008. [Google Scholar]
- 12.Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, Foxx-Orenstein A, Kurtz CB, Sharma V, Johnston JM, Currie MG, Zinsmeister AR. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–8. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ. Green light from the FDA for new drug development in irritable bowel syndrome and functional dyspepsia. American Journal of Gastroenterology. 2009;104:1339–41. doi: 10.1038/ajg.2009.295. [DOI] [PubMed] [Google Scholar]
- 14.Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–64. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Ravi K, Bharucha AE, Camilleri M, Rhoten D, Bakken T, Zinsmeister AR. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.07.057. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74:698–703. [PubMed] [Google Scholar]
- 17.Cohen MB, Guarino A, Shukla R, Giannella RA. Age-related differences in receptors for Escherichia coli heat-stable enterotoxin in the small and large intestine of children. Gastroenterology. 1988;94:367–73. doi: 10.1016/0016-5085(88)90423-4. [DOI] [PubMed] [Google Scholar]
- 18.Ironwood And Forest Announce Positive Linaclotide Results From Two Pivotal Phase 3 Trials In Patients With Chronic Constipation. Ironwood Pharmaceuticals Press Release; Nov 2, 2009. [Last accessed: 12/15/09.]. http://www.ironwoodpharma.com. [Google Scholar]
- 19.Camilleri M, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterology & Motility. 2009;21:1256–e117. doi: 10.1111/j.1365-2982.2009.01398.x. [DOI] [PubMed] [Google Scholar]
- 20.Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation--a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:315–28. doi: 10.1111/j.1365-2036.2008.03884.x. [DOI] [PubMed] [Google Scholar]
- 21.Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009;58:357–65. doi: 10.1136/gut.2008.162404. [DOI] [PubMed] [Google Scholar]
- 22.Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. American Journal of Gastroenterology. 2008;103:170–7. doi: 10.1111/j.1572-0241.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 23.Bijkerk CJ, de Wit NJ, Muris JWM, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial BMJ. 2009;339:b3154. doi: 10.1136/bmj.b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dipalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am J Gastroenterol. 2007;102:1436–41. doi: 10.1111/j.1572-0241.2007.01199.x. [DOI] [PubMed] [Google Scholar]
- 25.Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebo-controlled study. Alimentary Pharmacology & Therapeutics. 2006;23:1479–88. doi: 10.1111/j.1365-2036.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 26.Shailubhai K, Gerson W, Talluto C, Jacob G. Digestive Disease Week. San Diego: 2008. A randomized, double-blind, placebo-controlled, single-, ascending-, oral-dose safety, tolerability and pharmacokinetic study of SP-304 in healthy adult human male and female volunteers. [Google Scholar]
- 27.Waldman SA, Terzic A. Therapeutic targeting: a crucible for individualized medicine. Clin Pharmacol Ther. 2008;83:651–4. doi: 10.1038/clpt.2008.65. [DOI] [PubMed] [Google Scholar]