Abstract
Five Toxoplasma gondii isolates (TgPgBr1–5) were isolated from hearts and brains of pigs freshly purchased at the market of Campos dos Goytacazes, Northern Rio de Janeiro State, Brazil. Four of the five isolates were highly pathogenic in mice. Four genotypes were identified. Multi-locus PCR-DNA sequencing showed that each strain possessed a unique combination of archetypal and novel alleles not previously described in South America. The data suggest that different strains circulate in pigs destined for human consumption from those previously isolated from cats and chickens in Brazil. Further, multi-locus PCR-RFLP analyses failed to accurately genotype the Brazilian isolates due to the high presence of atypical alleles. This is the first report of multi-locus DNA sequencing of T. gondii isolates in pigs from Brazil.
Keywords: Toxoplasma gondii, Pigs, Isolation, Genetic typing, Brazil
1. Introduction
Early studies in Europe and North America showed that Toxoplasma gondii strains were remarkably clonal and possessed very little genetic variability. These archetypal isolates were classified into three lineages designated Types I, II and III (Dardé et al., 1992; Howe and Sibley, 1995; Ajzenberg et al., 2002 a,b). Recent studies indicate that the isolates from Brazil are biologically and genetically different from the ones found in USA and Europe (Dubey et al., 2002; Lehmann et al., 2006; Belfort-Neto et al., 2007; Dubey et al., 2007a,b,c; Dubey et al., 2008). Archetypal strains also predominate in chickens from Africa, with higher prevalence of types II and III (Velmurugan et al., 2008). The Type II lineage is rare in Brazil, but has recently been collected from the Brazilian island of Fernando de Noronha (Dubey et al., 2010). Instead, there is considerable genetic variation, with the presence of many atypical alleles inherited in new combinations not found in other regions of the globe (Howe and Sibley, 1995; Khan et al., 2006; Dubey et al., 2007a,c; Dubey et al., 2008; Dubey and Su, 2009).
Pigs are considered to be important sources of human T. gondii infections in many countries, including Brazil (Dubey, 2009a). Although T. gondii has been isolated from pork or pork products in Brazil (Frazão-Teixeira et al., 2006), there are only limited molecular data on T. gondii isolates from pigs in Brazil (dos Santos et al., 2005; Ferreira et al., 2006; Belfort-Neto et al., 2007). In the present paper we report detailed genetic analysis of T. gondii isolates infecting pork from local retail meat stores, including the report of new genetic types of T. gondii.
2. Materials and Methods
2.1 Isolation of viable T. gondii
Fresh hearts (n=16) and brains (n=19) of pigs from five butcherhouses in the main popular market of Campos dos Goytacazes, Northern Rio de Janeiro State, Brazil were purchased for this study. Each of these 35 tissues were digested in pepsin and their homogenates were inoculated in outbred albino mice (Dubey, 1998) at the Laboratório de Sanidade Animal (LSA), Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), Campos dos Goytacazes, RJ, Brazil. Viable T. gondii was isolated from five tissues and the strains were designated TgPgBr 1 to 5 (Table 1). To obtain tissue cysts, tissues of infected mice were sub-inoculated into another two mice at UENF. Sick mice received sulfadiazine (1mg/ml) in their water until the mice recovered from illness. Brains of these mice containing tissue cysts were sent to the Animal Parasitic Diseases Laboratory (APDL), United States Department of Agriculture (USDA), Beltsville, MD, USA for biologic and genetic characterization. At APDL, brains were homogenized in antibiotic saline and inoculated subcutaneously in groups of two outbred swiss webster mice and two C57Bl/6 IFNγ-knockout (KO) mice. Lung smears and brain squashes of dead mice were examined for the presence of T. gondii. All surviving mice were bled at six weeks and sera tested for the presence of anti-T. gondii antibodies by modified agglutination test (MAT, titer ≥ 25; Dubey and Desmonts, 1987). Infected lung tissues were stored at −20°C for genetic characterization.
Table 1.
Biological characteristics of Toxoplasma gondii isolates from fresh pork from a meat shop in Campos dos Goytacazes, RJ, Brazil
| Isolate designation | Pig tissue | Oocysts shed | Mortality in mice |
|||
|---|---|---|---|---|---|---|
| (DPI) | Tissue stages inoculatedd | Oocyst fedf | ||||
| 1 | 10 | 100 | ||||
| TgPgBr1 | braina | 67c (11) | 2/2 | 3/3 | 4/4 | 4/4 |
| TgPgBr2 | hearta | 20 (8) | 2/2 | 1/1 | 2/2 | 4/4 |
| TgPgBr3 | brainb | 13 (11) | 2/2 | 2/2 | 4/4 | 4/4 |
| TgPgBr4 | brainb | X (39) | 0/2e | 0/0g | 0/4e | 4/4 |
| TgPgBr5 | heartb | 24 (24) | 2/2 | 2/2 | 3/3 | 4/4 |
Samples obtained on August 1, 2008.
Samples obtained on August 25, 2008.
Cat number. The data in parenthesis are DPI=day post inoculation of mice before being fed to cat.
No. of mice dead out of two mice inoculated with tissue cysts.
Mice were seropositive and had tissue cysts in their brains.
No. of mice dead out of four mice orally inoculated with oocysts. The dose is based on the assumption that each viable oocyst is infective to mice.
Mice were seronegative and determined to be not infected.
2.2 Pathogenicity of tissue stages and oocysts of porcine T. gondii isolates to mice
In order to obtain oocysts for each T. gondii isolate, tissues of infected mice were each fed to one of five pathogen free cats at APDL as described (Dubey, 1995). Cats were fed a pool of tissues from mice inoculated with parasites from the second or third passages; day post inoculation of mice is indicated in Table 1. Oocysts were collected from feline feces, sporulated in 2% sulfuric acid at room temperature for seven days, and kept at 4° C until bioassayed in mice.
For bioassay in mice, oocysts were counted in a hemocytometer, the solution neutralized with sodium hydroxide and diluted ten-fold until the last dilution was estimated to have no oocysts. The last infective dilution was considered to have 1 viable oocyst. Aliquots from each oocyst dilution were inoculated orally into four mice. The mice were observed for six-eight weeks. Smears of tissues (mesenteric lymph nodes of mice that died during the first week, and lungs of mice that died during the second and third week after feeding oocysts) were examined microscopically for tachyzoites. All surviving mice were bled at six weeks and their sera (1:25 dilution) tested for MAT antibodies. All mice were killed eight weeks p.i. and their brains examined microscopically for tissue cysts (Dubey, 2009b).
2.3 Genetic characterization
Toxoplasma gondii DNA was extracted from lungs of infected mice using the DNeasy® Qiagen kit.
DNA from the five isolates was amplified using the polymerase chain reaction (PCR) at 11 genotyping markers: SAG1, SAG2, SAG3, BTUB, c22-8, c29-2, L358, PK1, Apico, GRA6 and B1 (Grigg and Boothroyd, 2001; Su et al., 2006; Dubey et al., 2008). PCR products were incubated for 15 minutes with ExoSAP-IT (USB Corporation, Cleveland, OH) prior to DNA sequencing. DNA sequencing was carried out by the RML Genomics Unit, Hamilton, MT. Results were compared against published archetypal strain sequences in GenBank using the DNA STAR SeqMan application.
3. Results
3.1 Biologic characterization of T. gondii isolates
Toxoplasma gondii was isolated from five of 35 pig tissues (3 brains; 2 hearts) and these isolates were designated TgPgBr1–5 (Table 1). When infected in mice, the tissue stages and oocysts of four isolates (TgPgBr1–3, TgPgBr5) were lethal to mice (Table 1). Outbred mice inoculated with tissue stages (tachyzoites or tissue cysts) died of acute toxoplasmosis within 20 days. Oral inoculation with as few as 1 sporulated oocyst of these strains was lethal; infected mice died of acute toxoplasmosis within 8–13 days p.i. (Table 1). The fifth isolate (TgPgBr4), however, was less pathogenic. Mice inoculated with tissue stages or 1–10 oocysts remained asymptomatic; but 100 oocysts were lethal (Table 1).
3.2.Genetic characterization
PCR-RFLP analysis at 10 genetic loci revealed four genotypes; one of the four genotypes was archetypal, and two isolates (TgPgBr1 and TgPgBr2) were indistinguishable from each other (Table 2).
Table 2.
Multi-locus genotypes of Toxoplasma gondii isolates from Brazil by PCR-RFLP analysis
| Genotypes | Isolates | Origin | Genetic markers |
References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c22-8 | C29-2 | L358 | PK1 | SAG1 | SAG2 | BTUB | GRA6 | SAG3 | APICO | ||||
| Chromosome | Ib | III | V | VI | VIII | VIII | IX | X | XII | plastid | |||
| Type I | RII88 | USA | I | I | I | I | I | I | I | I | I | I | |
| Type II | PTG | USA | II | II | II | II | II/III | II | II | II | II | II | |
| Type III | CTG | USA | III | III | III | III | II/III | III | III | III | III | III | Dubey et al. (2008) |
| BrI | TgCkBr123, 124, 55 79, 86, 87, 10, 98, 101, 102, 104, 144 | Brazil | Dubey et al. (2008) | ||||||||||
| TgCatBr42, 47, 53, 54, 55, 62, 71, 75 | u-1 | I | I | I | I | I | I | II | III | I | Pena et al. (2008) | ||
| BrII | TgCkBr57, 64, 97 | Brazil | Dubey et al. (2008) | ||||||||||
| TgCatBr39, 51, 52, 56, 61, 68, 77, 78 | I | III | I | II | I | II | III | III | III | III | Pena et al. (2008) | ||
| BrIII | TgCkBr11, 7, 17, 131, 132, 133, 134 | Brazil | Dubey et al. (2008) | ||||||||||
| TgCatBr58, 59, 60, 73, 74 | II | III | III | III | I | III | III | III | III | III | Pena et al. (2008) | ||
| BrIV | TgCkBr81, 147, 148, 151, 154, 160, 162, 163 | Brazil | u-1 | I | I | III | u-1 | II | III | III | III | I | Dubey et al. (2008) |
|
| |||||||||||||
| #1 | TgPgBr1, TgPgBr2 | Rio de Janeiro | I | I | I | I | I | I | I | II | III | I | this research |
| #2 | TgPgBr3 | II | I | I | I | u-1 | u-1 | I | II | III | I | this research | |
| Type III | TgPgBr4 | III | III | III | III | II/III | III | III | III | III | III | this research | |
| #4 | TgPgBr5 | I | I | III | III | u-1 | u-1 | III | II | III | III | this research | |
|
| |||||||||||||
| TgCkBr48, 88 | II | I | III | u-1 | II | III | III | III | III | Dubey et al. (2008) | |||
| TgCkBr75, 76, 92 | III | III | III | u-1 | II | III | III | III | I | Dubey et al. (2008) | |||
| BrIV | TgCkBr81 | u-1 | I | III | u-1 | II | III | III | III | I | Dubey et al. (2008) | ||
| TgCkBr41, 42, 49, 60, 62 | u-1 | I | I | I | u-1 | II | I | III | III | I | Dubey et al. (2008) | ||
| TgCkBr46 | II | I | I | I | u-1 | II | I | III | III | I | Dubey et al. (2008) | ||
| TgCkBr38, 27, 44, 51, 65, 66, 78, 80 | u-1 | I | III | III | u-1 | II | III | III | III | III | Dubey et al. (2008) | ||
| TgCkBr45 | II | I | III | III | u-1 | II | III | III | III | I | Dubey et al. (2008) | ||
| BrI | TgCkBr55, 79, 86, 87 | u-1 | I | I | I | I | I | I | II | III | I | Dubey et al. (2008) | |
| TgCkBr40, 47 | u-1 | I | I | I | I | I | III | II | III | I | Dubey et al. (2008) | ||
| TgCkBr54 | Rio de Janeiro | II | I | I | I | I | I | I | III | III | III | Dubey et al. (2008) | |
| TgCkBr59, 30, 34, 67 | II | I | III | I | I | I | I | III | III | III | Dubey et al. (2008) | ||
| TgCkBr74 | III | I | III | III | u-1 | III | III | III | III | III | Dubey et al. (2008) | ||
| TgCkBr82, 90 | III | I | III | III | I | III | III | III | III | III | Dubey et al. (2008) | ||
| TgCkBr31, 56 | III | III | III | III | II/III | III | III | III | III | III | Dubey et al. (2008) | ||
| TgCkBr26, 69 | II | I | III | III | I | III | III | III | III | I | Dubey et al. (2008) | ||
| TgCkBr28, 33, 50, 52, 58 | I | I | I | u-1 | I | III | III | III | III | I | Dubey et al. (2008) | ||
| TgCkBr57, 64 | I | III | I | II | I | II | III | III | III | III | Dubey etal. (2008) | ||
| TgCkBr61 | u-1 | I | I | III | I | II | III | II | I | I | Dubey et al. (2008) | ||
| TgCkBr32, 36, 84, 85 | u-1 | I | I | III | I | II | III | III | III | I | Dubey et al. (2008) | ||
| TgCkBr89 | u-1 | I | I | III | I | II | III | III | III | I | Dubey et al. (2008) | ||
“u” indicates a nonarchetpyal allele; I, II or III refers to an archetypal allele from a Type I, II or III strain
Isolates TgCkBr7–24 were collected in the state of Sao Paulo
Isolates TgCkBr26–92 were collected in the state of Rio de Janeiro
Isolates TgCkBr93–187 were collected in other states of Brazil (please see Dubey et al., 2008)
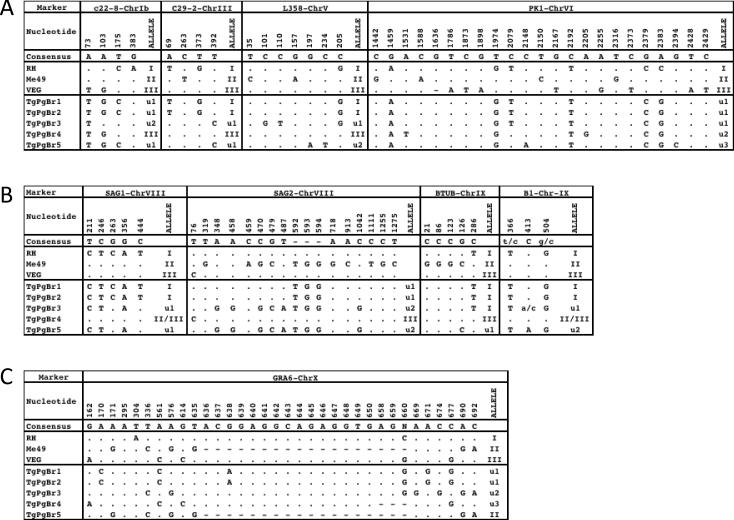
DNA sequencing confirmed that TgPgBr1 and TgPgBr2 were genetically identical. However, DNA sequencing was significantly more resolved and detected 15 atypical alleles across the 10 PCR-RFLP loci examined, which was substantially higher than the 2 detected by multi-locus PCR-RFLP analyses (Table 3). Further, DNA sequencing at an additional locus, B1, identified 2 more unique alleles that were circulating in pigs indigenous to Brazil (Table 3). Figure 1 shows the genetic polymorphisms encoded by each of the 17 unique alleles found in the pig isolates versus archetypal alleles expressed by clonal types I, II and III.
Table 3.
Multi-locus genotypes of Toxoplasma gondii isolates from pigs from Brazil, TgPgBr1–5, by PCR-DNA sequencing
| Isolates | Genetic markers |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| c22-8 | c29-2 | L358 | PK1 | SAG1 | SAG2 | BTUB | B1 | GRA6 | SAG3 | Apico plastid | |
| Chromosome | Ib | III | V | VI | VIII | VIII | IX | IX | X | XII | |
| TgPgBr1 | u-1 | I | I | u-1 | I | u-1 | I | I | u-1 | III | I |
| TgPgBr2 | u-1 | I | I | u-1 | I | u-1 | I | I | u-1 | III | I |
| TgPgBr3 | u--2 | u-1 | u-1 | u-1 | u-1 | u-2 | I | u-1 | u-2 | III | I |
| TgPgBr4 | III | III | III | u-2 | II/III | III | III | II/III | u-3 | III | III |
| TgPgBr5 | u-1 | u-1 | u-2 | u-3 | u-1 | u-2 | u-1 | u-2 | II | III | III |
Fig. 1.
Polymorphisms at 9 genetic markers by direct PCR-DNA sequencing of Toxoplasma gondii isolates from pigs from Brazil
DNA Sequence analysis at A) c22-8, c29-2, L358, PK1; B) SAG1, SAG2, BTUB, B1 and C) GRA6. Consensus sequence is defined as the nucleotide shared by at least two of the three archetypal Type I, II and III strain alleles. Numerical positions refer to sites in the published sequences (GenBank accession no. EU258475, EU258490, L42007, X14080, M33572, AY143127, AF179871 and F239283 for c22-8, L358, PK1, SAG1, SAG2, BTUB, B1 and GRA6 respectively). For c29-2, the amplicon was blasted (using the BlastN algorithm) at ToxoDB (www.toxodb.org) and the corresponding RH sequence used as the reference. “.” indicates identity with consensus; “-” indicates an insertion/deletion “u” indicates a nonarchetypal allele; I, II or III refers to an archetypal allelic sequence from a Type I, II or III strain.
4. Discussion
This study was undertaken to determine whether T. gondii strains infecting pigs destined for human consumption in the state of Rio de Janeiro, Brazil possessed the same genotypes as those already known to infect cats, chickens and people in Brazil. Previous multi-locus PCR-RFLP typing of cats and chickens has identified four main clonal genotypes, specifically Types BrI, BrII, BrIII and BrIV (Su et al., 2006; Dubey et al, 2008; Pena et al, 2008) causing patent infections in both animal groups, in addition to a large array of other nonarchetypal lines that apparently cluster geographically (Table 2). In contrast, none of the four pig genotypes resolved here, either by low resolution PCR-RFLP or high resolution PCR-DNA sequence typing, have been described previously in Brazil, perhaps identifying a role for natural selection in the pig intermediate host. By PCR-RFLP typing, the pig isolates possessed many of the same alleles present in other Brazilian genotypes, but the alleles had segregated differently across the loci examined. These data suggest several possible scenarios including: 1) the PCR-RFLP typing is not sufficiently resolved and that the lines are not natural recombinants expressing different combinations of the same alleles circulating in clonal BrI–IV lines, but rather exist as novel lines that possess a diversity of divergent alleles that reflect the tremendous genetic diversity indigenous to Brazil; or 2) that the pig isolates represent novel recombinants that arose by sexual recombination among extant Brazilian lineages, and were naturally selected for optimal expansion in the pig intermediate host. Ultimately, to resolve these possible scenarios will require additional sampling, and genotyping to proceed by direct DNA sequencing using at least 10 predominantly unlinked genetic markers.
Prior to genetically grouping North American and European T. gondii isolates into three clonal archetypes, commonly referred to as I, II and III, T. gondii strains were often classified based on their virulence in mice: either acutely virulent (LD100 = 1 parasite) or relatively avirulent (LD50 >1000 parasites); the latter strains being capable of establishing chronic, transmissible infections in mice. Type I isolates were later identified as a clonal lineage that is acutely virulent to mice, whereas types II and III were less so (Howe and Sibley, 1995; Howe et al., 1996). At present, no concrete evidence exists in Brazil correlating mouse pathogenicity and T. gondii strain genotype with clinical outcome during human infection in South America; most T. gondii strains isolated from asymptomatic animals are virulent in mice (Dubey et al., 2002; Pena et al., 2008). In this regard, four of the five isolates recovered from chronically infected pigs in the present study were highly pathogenic for mice, and 100 oocysts of the fifth isolate killed all inoculated mice.
With the introduction of multi-locus PCR-RFLP genotyping, population level analyses investigating the genetic structure of T. gondii strains circulating in North America and Europe were tremendously successful and established that the vast majority of infections in people and livestock were caused by just three genetically related, apparently recombinant lines that had expanded clonally only recently (Grigg et al, 2001; Su et al., 2003). When applied appropriately, this technique is very powerful for large-scale sampling, however, it is not as resolved as PCR-DNA sequencing methodology, and when applied in regions of the world where parasite genetic diversity is substantial, it fails to accurately resolve the true genetic “types” in, for instance, the strains isolated in this study from pigs from Campos dos Goytacazes, Rio de Janeiro State, Brazil. Indeed, Table 2 shows that many previous studies have reported an abundance of “recombinant” genotypes in South America that bear new combinations of archetypal alleles across the 10 PCR-RFLP loci examined (Dubey et al., 2008; Pena et al., 2008). However, it is likely that the majority of these I, II or III allele designations are not correct, and that the Brazilian isolates should not be classified as “recombinants” of archetypal strains, but rather as “atypical” because they possess altogether new combinations (or mixtures) of predominantly unique and some archetypal alleles. The high number of unique alleles identified by DNA sequencing among the 5 pig isolates genotyped in this study poignantly illustrates this fact (Table 3) and establishes that DNA sequencing is the preferred technique to infer the true genetic relationship and population structure of T. gondii strains circulating in Brazil.
Previous multi-locus PCR-RFLP analyses applied against isolates derived from chickens and cats identified four, widespread clonotypes, designated BrI, II, III and IV. None of the pig isolates in this study possessed a BrI-IV clonotype. This was not likely the result of any geographic bias, as clonotypes BrI and IV have been identified infecting chickens in Rio de Janeiro state (Table 2), and all four clonotypes have been identified infecting chickens and cats in neighboring São Paulo state (Dubey et al., 2008; Pena et al., 2008). Whether these strains infect swine, or humans, is currently unclear, chiefly because too few isolates have been examined from these two sources, and genotyping markers have not been consistently applied. Epidemiologic and environmental studies have suggested that the main routes of human infection are via ingestion of water from untreated sources or the barbecue eating habits among the Brazilian population (Bahia-Oliveira et al., 2003; Silva et al., 2003; Dubey et al., 2003; Frazão-Teixeira et al., 2006). Because pigs in this region of Brazil are bred free-ranging and are not kept under good hygiene conditions, this animal species may represent an ideal sentinel population to examine whether consumption of undercooked pork is contributing to the diversity of strains infecting people or represent a significant risk for causing human disease.
Unfortunately, the majority of studies performed to genotype T. gondii strains infecting people have used too few or different markers from the ones used in the animal studies, so comparative analyses to investigate sources of human infection cannot be drawn. In research performed by Khan et al. (2006), 17 specimens from clinical cases of human ocular toxoplasmosis in Brazil were characterized via multi-locus PCR-RFLP using the markers SAG2, SAG3, BTUB, and GRA6. Comparing their results with the multi-locus PCR-RFLP genotypes detected in our present work identified one human infection with the same multi-locus type as TgPgBr1 and 2. Vallochi et al. (2005) genotyped 11 human ocular toxoplasmosis patients at SAG2, all possessed a Type I allele by PCR-RFLP, the same as TgPgBr1 and 2. However, this does not prove that these strains were genetically identical to the ones infecting the pig samples indicating that further studies requiring larger datasets, and DNA sequencing using at least 10 genetic markers are required to interrogate whether consumption of undercooked pork is a risk factor for human disease in Brazil.
In the present study, four of the five T. gondii isolates from pigs were virulent to mice, but cats fed tissues of acutely infected mice shed oocysts, indicating that bradyzoites had formed within 8 days and so it is conceivable that these isolates could cycle between cats and mice in nature. The isolates were obtained from pig brains and hearts that were not individually identified at the butcher shop (Table 1), therefore we are uncertain if they belonged to 3 or 5 pigs. However, our genetic data suggests that they came from different pigs.
In conclusion, this is the first research study to DNA sequence T. gondii isolates acquired from pig tissues in Brazil at multiple genetic loci. Multi-locus DNA sequencing is more resolved than PCR-RFLP and is capable of detecting genetic differences nucleotide by nucleotide, which is required to accurately delineate the true genetic relationship among isolates circulating in regions of the world where tremendous genetic variation exists. While the multi-locus PCRRFLP technique is easy to perform, it was originally developed to resolve only those polymorphic sites that differentiate among extant, archetypal strains circulating in North America, Europe and Africa. As a result, it typically fails to detect unique polymorphisms naturally present in the majority of alleles circulating in strains isolated from South America (Pena et al., 2008). Hence, this technique should not be applied to interrogate the Toxoplasma population genetic structure in Brazil, where allelic diversity is substantial. Otherwise, the multi-locus genotype reported will be inaccurate and misinterpreted as either archetypal or possibly “recombinant”, which would not be correct. In this study, the predominance and inheritance pattern of atypical alleles resolved by multi-locus DNA sequencing indicated that the genotypes isolated from pigs destined for human consumption possessed new combinations of polymorphic alleles different from archetypal strains, and these genotypes possessed different multi-locus genotypes from those found circulating in chickens and cats in Brazil.
ACKNOWLEDGEMENTS
We thank Vera Lúcia Menezes Rosa, Jéssika de Oliveira Ventura, Leandra Ferreira, Yara Al Kappany, Oliver Kwok, Rajendran Chellaiah, and Robin Miller for their assistance with this research.
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, process # BEX 3409/08-2), Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), United States Department of Agriculture (USDA), and by the Intramural Research Program of the NIH and NIAID. MEG is a scholar of the Canadian Institute for Advanced Research (CIFAR) Program for Integrated Microbial Biodiversity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajzenberg D, Bañuls AL, Tibayrenc M, Dardé ML. Microsatellite analysis of Toxoplasma gondii shows considerable polymorphism structured into two main clonal groups. Int. J. Parasitol. 2002a;32:27–38. doi: 10.1016/s0020-7519(01)00301-0. [DOI] [PubMed] [Google Scholar]
- Ajzenberg D, Cogné N, Paris L, Bessières MH, Thulliez P, Filisetti D, Pelloux H, Marty P, Dardé ML. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J. Infect. Dis. 2002b;186:684–689. doi: 10.1086/342663. [DOI] [PubMed] [Google Scholar]
- Bahia-Oliveira LMG, Jones JL, Azevedo-Silva J, Alves CCF, Oréfice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro State, Brazil. Emerg. Infect. Dis. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort-Neto R, Nussenblatt V, Rizzo L, Muccioli C, Silveira C, Nussenblatt R, Khan A, Sibley LD, Belfort R. High prevalence of unusual genotypes of Toxoplasma gondii infection in pork meat samples from Erechim, Southern Brazil. An. Acad. Bras. Cienc. 2007;79:111–114. doi: 10.1590/s0001-37652007000100013. [DOI] [PubMed] [Google Scholar]
- Dardé ML, Bouteille B, Pestre-Alexandre M. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J. Parasitol. 1992;78:786–794. [PubMed] [Google Scholar]
- dos Santos CBA, de Carvalho ACFB, Ragozo AMA, Soares RM, Amaku M, Yai LEO, Dubey JP, Gennari SM. First isolation and molecular characterization of Toxoplasma gondii from finishing pigs from São Paulo State, Brazil. Vet. Parasitol. 2005;131:207–211. doi: 10.1016/j.vetpar.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Desmonts G. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Vet. J. 1987;19:337–339. doi: 10.1111/j.2042-3306.1987.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J. Parasitol. 1995;81:410–415. [PubMed] [Google Scholar]
- Dubey JP. Refinement of pepsin digestion method for isolation of Toxoplasma gondii from infected tissues. Vet. Parasitol. 1998;74:75–77. doi: 10.1016/s0304-4017(97)00135-0. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Graham DH, Blackston CR, Lehmann T, Gennari SM, Ragozo AMA, Nishi SM, Shen SK, Kwok OCH, Hill DE, Thulliez P. Biological and genetic characterisation of Toxoplasma gondii isolates from chickens (Gallus domesticus) from São Paulo, Brazil: Unexpected findings. Int. J. Parasitol. 2002;32:99–105. doi: 10.1016/s0020-7519(01)00364-2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Graham DH, Silva DS, Lehmann T, Bahia-Oliveira LMG. Toxoplasma gondii isolates of free-ranging chickens from Rio de Janeiro, Brazil: mouse mortality, genotype, and oocyst shedding by cats. J. Parasitol. 2003;89:851–853. doi: 10.1645/GE-60R. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sundar N, Gennari SM, Minervino AHH, Farias NAR, Ruas JL, dos Santos TRB, Cavalcante GT, Kwok OCH, Su C. Biologic and genetic comparison of Toxoplasma gondii isolates in free-range chickens from the northern Pará state and the southern state Rio Grande do Sul, Brazil revealed highly diverse and distinct parasite populations. Vet. Parasitol. 2007a;143:182–188. doi: 10.1016/j.vetpar.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Applewhaite L, Sundar N, Velmurugan GV, Bandini LA, Kwok OCH, Hill R, Su C. Molecular and biological characterization of Toxoplasma gondii isolates from free-range chickens from Guyana, South America, identified several unique and common parasite genotypes. Parasitology. 2007b;134:1559–1565. doi: 10.1017/S0031182007003083. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Gennari SM, Sundar N, Vianna MCB, Bandini LM, Yai LEO, Kwok OCH, Su C. Diverse and atypical genotypes identified in Toxoplasma gondii from dogs in São Paulo, Brazil. J. Parasitol. 2007c;93:60–64. doi: 10.1645/GE-972R.1. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Velmurugan GV, Chockalingam A, Pena HFJ, de Oliveira LN, Leifer CA, Gennari SM, Oliveira LMGB, Su C. Genetic diversity of Toxoplasma gondii isolates from chickens from Brazil. Vet. Parasitol. 2008;157:299–305. doi: 10.1016/j.vetpar.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Su C. Population biology of Toxoplasma gondii: what's out and where did they come from. Mem. Inst. Oswaldo Cruz. 2009;104:190–195. doi: 10.1590/s0074-02762009000200011. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis of animals and humans. Second edition CRC Press; Boca Raton, FL: 2009a. pp. 1–313. [Google Scholar]
- Dubey JP. Toxoplasmosis in pigs - the last 20 years. Vet. Parasitol. 2009b;164:89–103. doi: 10.1016/j.vetpar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Rajendran C, Costa DGV, Ferreira LR, Kwok OCH, Qu D, Su C, Marvulo MFV, Alves LC, Mota RA, Silva JCR. New Toxoplasma gondii genotypes isolated from free-range chickens from the Fernando de Noronha, Brazil: unexpected findings. J Parasitol. 2010;96:709–712. doi: 10.1645/GE-2425.1. [DOI] [PubMed] [Google Scholar]
- Ferreira AM, Vitor RWA, Grazzinelli RT, Melo MN. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multi-locus PCR-RFLP. Infect. Genet. Evol. 2006;6:22–31. doi: 10.1016/j.meegid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Frazão-Teixeira E, de Oliveira FCR, Pelissari-Sant'Ana V, Lopes CWG. Toxoplasma gondii em encéfalos de suínos comercializados no município de Campos dos Goytacazes, Estado do Rio de Janeiro, Brasil. Rev. Bras. Parasitol. Vet. 2006;15:33–36. [PubMed] [Google Scholar]
- Grigg ME, Bonnefoy S, Hehl AB, Suzuki Y, Boothroyd JC. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science. 2001;294:161–165. doi: 10.1126/science.1061888. [DOI] [PubMed] [Google Scholar]
- Grigg ME, Boothroyd JC. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J. Clin. Microbiol. 2001;39:398–400. doi: 10.1128/JCM.39.1.398-400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Howe DK, Summers BC, Sibley LD. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii. Infect. Immun. 1996;64:5193–5198. doi: 10.1128/iai.64.12.5193-5198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Jordan C, Muccioli C, Vallochi AL, Rizzo LV, Belfort R, Vitor RWA, Silveira C, Sibley LD. Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg. Infect. Dis. 2006;12:942–949. doi: 10.3201/eid1206.060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, Marcet PL, Graham DH, Dahl ER, Dubey JP. Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11423–11428. doi: 10.1073/pnas.0601438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena HFJ, Gennari SM, Dubey JP, Su C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol. 2008;38:561–569. doi: 10.1016/j.ijpara.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Silva DS, Bahia-Oliveira LMG, Shen SK, Kwok OCH, Lehmann T, Dubey JP. Prevalence of Toxoplasma gondii in chickens from an area in southern Brazil highly endemic to humans. J. Parasitol. 2003;89:394–396. doi: 10.1645/0022-3395(2003)089[0394:POTGIC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. Recent expansion of Toxoplasma through enhanced oral transmission. Science. 2003;299:414–416. doi: 10.1126/science.1078035. [DOI] [PubMed] [Google Scholar]
- Su C, Zhang X, Dubey JP. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int. J. Parasitol. 2006;36:841–848. doi: 10.1016/j.ijpara.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Vallochi AL, Muccioli C, Martins MC, Silveira C, Belfort R, Rizzo LV. The genotype of Toxoplasma gondii strains causing ocular toxoplasmosis in humans in Brazil. Am. J. Ophthalmol. 2005;139:350–351. doi: 10.1016/j.ajo.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Velmurugan GV, Dubey JP, Su C. Genotyping studies of Toxoplasma gondii isolates from Africa revealed that the archetypal clonal lineages predominate as in North America and Europe. Vet. Parasitol. 2008;155:314–318. doi: 10.1016/j.vetpar.2008.04.021. [DOI] [PubMed] [Google Scholar]