Abstract
Introduction
Limited evidence suggests that focal neurological injury (e.g., acute flaccid paralysis) caused by infection with the West Nile virus (WNV) is more common in older patients. We re-evaluate this association in a series of patients who were infected with the WNV during the 2002 epidemic.
Methods
We performed a retrospective chart review of 34 patients who were hospitalized for treatment of serologically confirmed WNV infection. Measurements included the patient’s demographic characteristics, baseline medical diagnoses, the occurrence of symptoms and exam findings, the results of various diagnostic tests, and the patient’s clinical outcome.
Results
Patients infected with the WNV who developed focal neurological injury were found to be comparable to patients who did not develop focal neurological injury both in terms of patient age and the number of medical conditions the patient had prior to infection. This is in contrast to WNV-infected patients who developed an encephalitis-like clinical course, or who died or were institutionalized after their hospitalization; such patients tended to be older and–in cases with a poor outcome–have more medical conditions prior to WNV infection.
Conclusions
In our patient group, focal neurological injury caused by WNV infection was not related to advanced patient age or to the number of medical conditions the patient had prior to infection. Our findings bring into question commonly held views about the development of focal neurological injury caused by WNV infection, and they raise concerns about the management of future WNV epidemics and the testing and use of potential antiviral treatments against this infection.
Keywords: Age, Medical condition, Meningitis, Encephalitis, Focal neurological injury
1. Introduction
It has been well documented that advanced age [1-6] and poor premorbid health [2,3] increase the risk of death following infection with the West Nile virus (WNV). One retrospective review [3] also associated advanced patient age with an encephalitis-like clinical course of the WNV infection. Subsequently, these associations have been extrapolated to the context of focal neurological injury (e.g., poliomyelitis-like acute flaccid paralysis) caused by WNV infection (see Ref. [7]). During the epidemic in the summer of 2002, however, the neurology service of the Northwestern Memorial Hospital encountered many patients with focal neurological injury caused by WNV infection who did not fit this high-risk profile. In order to reevaluate the aforementioned associations, we performed a retrospective review of patients with WNV infection who were hospitalized in the Northwestern University hospital system during that epidemic, and we report the results of our review herein.
2. Methods
Hospitalized patients infected with the WNV (family Flavivirus, genus Flavivirus Japanese Encephalitis Antigenic Complex, species West Nile virus) were identified by means of a positive serological test (described below). After obtaining Institutional Review Board approval and ensuring HIPAA compliance, the medical records from WNV-infected patients hospitalized between April and October, 2002, in either of the two major Northwestern University-affiliated hospitals (the Northwestern Memorial Hospital in downtown Chicago and Evanston Hospital in Evanston, Illinois) were evaluated with a preformed checklist of symptoms and exam findings. This checklist was compiled during a review of other case reports of WNV infection [1-6,8-11]. The documented presence or absence of each symptom or exam finding was noted during a complete reading of the patient’s medical record. The patient’s basic demographic information (e.g., age, sex, race), his or her medical conditions at the time of hospital admission, the course of treatment, and the outcome from the infection (e.g., discharged home, dead, etc.) were also noted. Additionally, the use of, and the results from, blood, cerebrospinal fluid, electrodiagnostic, and radiological tests were noted during the evaluation of the medical record.
Serological tests for the WNV were performed by the laboratory services of the Illinois Department of Public Health. Blood and/or cerebrospinal fluid samples were assessed by means of an enzyme-linked immunosorbant assay (ELISA) for the WNV. The presence of IgM for the WNV in either blood or cerebrospinal fluid samples was considered indicative of active WNV infection.
A total of 34 hospitalized patients with serologically confirmed WNV infection were retrieved by the medical records service. These patients were alternately grouped according to (a) the type of clinical course (i.e., encephalitis, meningitis, or nonspecific febrile illness), (b) the development of focal neurological injury, and (c) the outcome from the infection. Statistical comparisons were done with unpaired two-tailed t-tests, and measures of statistical power are provided for each comparison. Data pertaining to symptoms and exam findings were analyzed as fractions of the total number of cases and separately as the number of cases in which the specific symptom or exam finding was mentioned in the medical records. In no instance were these two means of expressing the data notably different, so we present all data here as the fraction of the total number of cases.
All data are reported as the mean ± S.E.M., or else as the mean and range as in Table 2.
Table 2.
Results of cerebrospinal fluid studies from lumbar puncture in serologically confirmed, hospitalized WNV patients
| Cerebrospinal fluid test | Nonspecific febrile illness (n = 9) | Meningitis (n = 10) | Encephalitis (n = 7) | No focal neurological injury (n = 13) | Focal neurological injury (n = 13) | Discharged to home (n = 18) | Not discharged home (n = 8) |
|---|---|---|---|---|---|---|---|
| WBC count | 183 (9 – 683) | 241 (51 – 750) | 73 (11 – 162) | 164 (11 – 683) | 186 (13 – 750) | 195 (9 – 750) | 133 (11 – 250) |
| RBC count | 25 (2 – 50) | 32 (0 – 167) | 9 (0 – 25) | 20 (0 – 50) | 31 (1 – 167) | 27 (0 – 167) | 14 (0 – 40) |
| Glucose | 56 (43 – 66) | 65 (49 – 107) | 67 (48 – 93) | 64 (43 – 107) | 59 (49 – 93) | 61 (43 – 107) | 65 (49 – 93) |
| Protein | 91 (42 – 185) | 79 (50 – 107) | 88 (44 – 168) | 73 (42 – 126) | 105 (71 – 185) | 86 (42 – 195) | 93 (44 – 140) |
Data are displayed as the average and range.
3. Results
3.1. Patient characteristics, and general symptoms and exam findings of WNV infection
The average age of the patients with serologically confirmed WNV infection was 55.5 ± 3.0 (range: 19–86) and 62% were women. Race was reported for 25 patients, 80% of whom were white and 17% of whom were black.
Most of the common symptoms of WNV infection were non-specific for a mild febrile illness (i.e., headache {85%}, nausea {71%}, myalgia {65%}, chills {47%}). On exam, fever was measured in 94% of the cases with a mean maximum temperature of 102.2 °F (range: 100.8–104.2 °F). Rash and conjunctival erythema were much less common (38% and 9%, respectively).
3.2. Meningitis and encephalitis
Symptoms and exam findings suggestive of meningitis and encephalitis were frequent in patients infected with the WNV. Of the 18 patients complaining of neck pain or stiffness, 11 also exhibited meningismus upon examination; 1 of these 11 patients also complained of confusion and exhibited an altered mental status and so was included in the encephalitis group (described below) for further analysis. In contrast to neck pain or stiffness, the complaint of confusion (n = 9 patients, including the aforementioned patient who complained of both neck stiffness and confusion) was uniformly accompanied by the finding of altered mental status during physical examination. Using the most inclusive definitions of meningitis (i.e., fever and meningismus) and encephalitis (i.e., fever and altered mental status), 10 patients exhibited meningitis (29%) and 9 patients exhibited encephalitis (27%). Of the remaining 15 patients who had neither meningismus nor altered mental status, all presented with the nonspecific febrile illness described in the previous section.
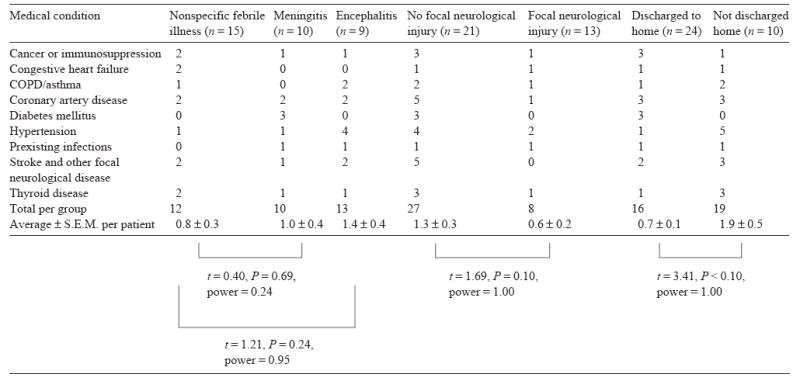
Patients who developed meningitis appeared to be about the same age as patients who had only a nonspecific febrile illness (meningitis=47.2 ± 3.3 years; nonspecific febrile illness=50.1 ± 4.1 years; t = 0.51, P = 0.61, power=0.13). However, the average age of patients who developed encephalitis (67.7 ± 6.5 years) appeared to be greater than that of patients who had a nonspecific febrile illness (t = 2.41, P <0.05, power=0.71). Furthermore, in comparisons of the average number of medical conditions per patient, there did not appear to be an obvious difference between the patients who had meningitis- or encephalitis-like clinical courses and the patients who had only a nonspecific febrile illness (Table 1).
Table 1.
Distribution of major medical conditions diagnosed prior to hospital admission according to type of WNV infection
|
3.3. Focal neurological injury
Overall, 13 patients (38%) developed some sort of focal neurological injury. Of these 13 patients, 4 exhibited encephalitis and 5 exhibited meningitis, leaving 4 patients who did not exhibit either meningitis or encephalitis. Except for their focal neurological injury, this last group of patients would have had clinical courses consistent only with a nonspecific febrile illness.
The most common form of focal neurological injury was an acute weakness that developed in seven patients (21%). The weakness was distributed either in one limb (n = 3) or bilaterally in both upper extremities or lower extremities (n = 4). In all cases the weakness was described as acute in onset and was found to be flaccid and areflexic upon examination. Three patients who noted weakness in the extremities also complained of facial weakness, and one of these patients ultimately complained of diplopia (due to an abducens nerve palsy). Overall, four patients (12%) reported diplopia (excluding the complaint of blurred vision): three of these patients exhibited extraocular muscle weakness while the remaining patient had normal exam findings. Upon examination, six patients (18%) exhibited dysfunction of a cranial nerve: n = 2 with unilateral facial weakness, n = 2 with unilateral abducens nerve palsy, n = 1 with unilateral sensorineural hearing loss, and n = 1 with both unilateral facial weakness and an abducens nerve palsy.
Three patients (9%) reported vertigo (excluding the complaint of lightheadedness) that was believed by the treating physicians to be caused by dysfunction of the central nervous system. However, only one of these patients exhibited another exam finding (i.e., a unilateral facial weakness) that would support the diagnosis of an intrinsic brain injury; this patient and the other two with vertigo had normal neuroimaging studies. We included these patients in the focal neurological injury group despite the possibility that some of them were suffering from dysfunction of the inner ear.
Patients who developed focal neurological injury were on average 52.1 ± 4.9 years-of-age. Those patients who did not develop focal neurological injury were on average 57.1 ± 3.8 years-of-age. Comparing these two groups showed them to be statistically indistinguishable given the sample size (t = 0.79, P = 0.43, power=0.20). Additionally, patients who developed focal neurological injury after WNV infection did not appear to have more medical conditions prior to the infection than did patients without focal neurological injury (Table 1).
3.4. Outcome from WNV infection
The length of hospitalization for all patients averaged 9.5 days, being as short as a visit to the emergency room (n = 1 patient) and lasting as long as 30 days. During hospitalization, no patient was treated with immunomodulatory therapy, plasmapharesis, or intravenous immunoglobulin. No patient required vasopressor support, but three patients who developed encephalitis required intubation and mechanical ventilation (two of whom were later converted to tracheostomy for long-term ventilation).
Following hospitalization, 24 patients were discharged home, 5 patients required inpatient physical rehabilitation, and 4 patients were newly placed into a nursing facility. One patient died during his hospitalization apparently as a direct consequence of WNV encephalitis. Of the 10 patients with meningitis only 1 was discharged to a rehabilitation facility whereas the remaining 9 were discharged home. Conversely, of the nine patients with encephalitis only three went home from the hospital; of the remaining six, n = 1 went to inpatient physical rehabilitation, n = 4 were placed in a nursing facility, and n = 1 died. Eight of the 13 patients with focal neurological injury were discharged home after hospitalization (of the remaining, n = 3 went to rehab, n = 1 to a nursing home, and n = 1 died), in comparison to 16 of the 21 patients without focal neurological injury (of the remaining, n = 2 went to rehab and n = 3 to a nursing home).
Patients who were discharged home after their hospitalization were younger than patients who required further institutionalization or who died as a result of WNV infection (discharged home = 47.4 ± 3.3 years; not discharged home=67.0 ± 5.0 years; t = 3.35, P <0.005, power=0.93). Furthermore, patients who were institutionalized or who died as a result of WNV infection had more medical conditions prior to infection than those who were discharged home following hospitalization (Table 1).
3.5. Diagnostic testing
All 10 patients who had meningitis and 7 of the 9 patients who had encephalitis were examined with lumbar puncture. Nine other patients who did not exhibit meningitis or encephalitis (i.e., who presented with a nonspecific febrile illness) were also evaluated with lumbar puncture, uniformly for the evaluation of headache. All patients evaluated by lumbar puncture demonstrated a central nervous system inflammatory response, including those patients with only a nonspecific febrile illness (Table 2). Thus, cerebrospinal fluid analysis did not appear to differentiate between the groups of WNV-infected patients. White blood cell differential counts in the cerebrospinal fluid were highly variable, with lymphocytes accounting for 8–80% of the total white blood cell count in the meningitis group and for 24–96% in the encephalitis group. Evaluation of patients groups according to the presence or absence of focal neurological injury, or grouped according to outcome, similarly did not disclose any obvious difference in terms of cerebrospinal fluid studies (Table 2).
Brain imaging was the most common type of diagnostic test performed in patients with WNV infection. Fourteen patients (41%) were evaluated with CT of the head, 13 patients with brain MR (38%), and 4 patients with both imaging modalities. The CT scans identified significant abnormalities in only two studies. One CT scan identified a parasagittal meningioma, and another identified chronic ischemic changes in the frontal lobes (this was not further evaluated, however, and the patient had a normal examination); these findings were not thought to be related to the clinical condition of the patients. Brain MRI identified a small, subcortical frontal lobe white matter infarct with petechial hemorrhage in a patient who complained of contralateral lower extremity weakness, yet the location of this lesion was not thought to account for the patient’s weakness. Three other patients had abnormal MRI findings: n = 2 showed diffusely increase T2 signal in the cortex suggestive of encephalitis, and n = 1 showed meningeal enhancement. Interestingly, no alteration of mental status was noted in the patients who had encephalitis-like MRI abnormalities, and no meningismus was noted in the patient who had meningeal enhancement.
Only four nerve conduction studies were performed in our patient group. All were performed within 6–10 days of hospitalization. Two nerve conduction studies were consistent with motoneuron disease, whereas one demonstrated a mild sensory-motor polyneuropathy that was documented to be a preexisting condition due to alcohol abuse. All three of these patients exhibited focal neurological injury involving weakness of one or more limbs. A fourth patient who did not exhibit focal weakness was evaluated with both a nerve conduction study and electromyography for the complaints of diffuse myalgias and weakness; these studies were read as being consistent with a demyelinating polyneuropathy such as the Guillain–Barre syndrome. No follow-up electrophysiological studies were performed on these patients.
4. Conclusions
The principle finding of our study is that the development of focal neurological injury after WNV infection was not readily associated with increased patient age. Focal neurological injury has been associated with advanced age in other studies, most notably that of Nash et al. [2] who evaluated 59 patients infected with WNV during the 1999 epidemic in New York. They identified 20 cases of “encephalitis with muscle weakness”, which appeared to be more common in patients greater than 70 years-of-age. Although the weakness they describe was hyporeflexic, it is not clear if it was consistent in any other way with the poliomyelitis-like syndrome of WNV infection (i.e., rapid onset, focal distribution, and flaccid in nature). It also does not appear that the 20 patients who comprised their “encephalitis with muscle weakness” group represented all of the patients who exhibited weakness in that case series: specifically, at least 13 other patients who had weakness but not encephalitis were excluded from that group. As we and others [8,9] have found, the poliomyelitis-like acute flaccid paralysis caused by WNV infection does not always occur in the setting of the general symptoms and signs of encephalitis. Furthermore, it does not appear that Nash et al. evaluated patients who had other possible focal neurological injuries as vigorously as they did cases of “encephalitis with muscle weakness”. In their study, there were apparently 16 cases of WNV infection that involved tremor, slurred speech, or “focal sensory change” (as they listed in Table 2 of their manuscript), 13 cases that involved “abnormal cranial nerve function”, and 6 cases that involved “flaccid paralysis” (Table 4 of their manuscript); how such cases overlap with each other and those that involved “encephalitis with muscle weakness” is not clear. With such limitations it is difficult to accept that their WNV-infected patients who had “encephalitis with muscle weakness” necessarily suffered from acute flaccid paralysis or to believe that this group represents all the WNV-infected patients who had focal neurological injury.
Our observation that focal neurological injury was not necessarily more common in older patients also would appear to be contrary to the case series of Emig and Apple [1], who retrospectively evaluated 44 patients infected with the WNV during the 2002 outbreak. They found that out of 25 patients with WNV encephalitis who were less than 65 years-of-age, 5 (20%) developed a mono-, para-, or quadriparesis. In comparison, of the 17 patients with WNV encephalitis who were greater than 65 years-of-age in that case series, 5 developed such symptoms (29%). Despite the apparent difference between age groups, comparing these two proportions with a two-tailed Fisher’s exact test suggests that they are unlikely to be different (P=0.71). Furthermore, certain features of the study of Emig and Apple may have distorted its evaluation of focal neurological injury caused by WNV infection. First, as with the report of Nash et al. [2], the case series of Emig and Apple limited its definition of focal neurological injury to limb weakness. Second, all of their patients infected with WNV were classified as having either a meningitis- or encephalitis-like clinical course, and none appeared to have only a nonspecific febrile illness. This may have occurred either by the design of the study or else by chance, although the latter seems very unlikely. Excluding patients with a nonspecific febrile illness caused by WNV infection may have eliminated some patients who nonetheless had focal neurological injury, which could be a biasing influence on their analysis. WNV-infected patients who develop focal neurological injury in the presence of only a nonspecific febrile illness are not necessarily rare, either, as they accounted for 4 of the 13 patients who had focal neurological injury in our case series.
We did confirm that WNV encephalitis was more likely to occur in the elderly. This is despite a surprisingly high rate of WNV encephalitis in younger patients during the 2002 epidemic. In the case series of Emig and Apple [1], 16 of 25 patients (64%) who were less than 65 years-of-age had an encephalitis-like clinical course in comparison with 13 of 17 patients (76%) who were greater than 65 years of age, but the incidence does not appear to be different in these two groups (Fisher’s exact test, P=0.81). Our finding does agree with that of Chowers et al. [3], who analyzed 233 Israelis infected with WNV during the 2000 epidemic and demonstrated a higher incidence of encephalitis in patients greater than 70 years-of-age. Furthermore, in our study we confirmed the previously reported association between age [1-6] and the number of premorbid medical conditions [2,3] with the overall outcome from WNV infection.
The interpretation of our data is hindered by the number of patients in our case series, which unfortunately is a necessary limitation of this kind of research. Key statistically comparisons in our study are hampered by low statistical power that would be insurmountable outside of a large, multicenter case series. For example, to adequately demonstrate the absence of an age difference between patients with and without focal neurological injury (assuming a minimum power of 0.8), almost 200 patients per group would have to be analyzed. Thus, we can conclude only that we were unable to show a difference between the groups. Additionally, our patient sample is undoubtedly biased by the retrospective nature of our study that involves only patients who were hospitalized because of WNV infection. However, these limitations are also shared by those case series that suggest focal neurological injury is more common in older WNV-infected patients. Thus, any attempts–current or previous–to associate patient age and premorbid health with the neurological complications of WNV infection should be regarded cautiously.
We suggest that with the available data it may be inappropriate to consider that the focal neurological injury caused by WNV infection is a concern principally for the elderly and infirm. Such a conclusion is not only consistent with the observations we reported here, but it is also prudent considering the limitations inherent in studies that would suggest otherwise. Furthermore, it may also prove to be misleading to test or administer as therapy antiviral medications only in patients who are old and in poor premorbid health assuming that they are more susceptible to focal neurological injury caused by WNV infection. It is our hope, then, that the observations we note herein will serve as the impetus to develop a prospective system for evaluating the clinical course of WNV infection so that those patients who are susceptible to focal neurological injury may be adequately and appropriately identified.
References
- 1.Emig M, Apple DJ. Severe West Nile virus disease in healthy adults. Clin Infect Dis. 2003;38:289–92. doi: 10.1086/380458. [DOI] [PubMed] [Google Scholar]
- 2.Nash D, Mostashari F, Fine A, Miller J, O’Leary D, Murray K, et al. The outbreak of West Nile virus infection in the New York City area in 1999. NEJM. 344:1807–14. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 3.Chowers MY, Lang R, Nassar F, Ben-David D, Giladi M, Rubinstein E, et al. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis. 2001;7:675–8. doi: 10.3201/eid0704.010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767–71. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 5.Platonov AE, Shipulin GA, Shipulina OY, Tyutyunnik EN, Frolochkina TI, Lanciotti RS, et al. Outbreak of West Nile virus infection, Volgograd Region, Russia, 1999. Emerg Infect Dis. 2001;7:128–32. doi: 10.3201/eid0701.010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberger M, Pitlik SD, Gandacu D, Lang R, Nassar F, Ben David D, et al. West Nile fever outbreak, Israel, 2000: epidemiologic aspects. Emerg Infect Dis. 2001;7:686–91. doi: 10.3201/eid0704.010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med. 2002;137:173–9. doi: 10.7326/0003-4819-137-3-200208060-00009. [DOI] [PubMed] [Google Scholar]
- 8.Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, van Gerpen JA, et al. Neurological manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–5. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 9.Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile virus infection: a new acute paralytic illness. Neurology. 2003;61:55–9. doi: 10.1212/01.wnl.0000073617.08185.0a. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh BM, Jupp PG, dos Santos I, Meenehan GM. Epidemics of West Nile and Sindbis viruses in South Africa with Culex (Culex) univittatus Theobald as vector. S Afr J Sci. 1976;72:295–300. [Google Scholar]
- 11.Weiss D, Carr D, Kellachan J, Tan C, Phillips M, Bresnitz E, et al. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg Infect Dis. 2001;7:654–8. doi: 10.3201/eid0704.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]


